Abstract
To examine heterogeneity in dendritic cell (DC) antigen presentation function, murine splenic DCs were separated into CD4+ and CD8+ populations and assessed for the ability to process and present particulate antigen to CD4+ and CD8+ T cells. CD4+ and CD8+ DCs both processed exogenous particulate antigen, but CD8+ DCs were much more efficient than CD4+ DCs for both major histocompatibility complex (MHC) class II antigen presentation and MHC class I cross-presentation. While antigen processing efficiency contributed to the superior antigen presentation function of CD8+ DCs, our studies also revealed an important contribution of CD24. CD8+ DCs were also more efficient than CD4+ DCs in inducing naïve T cells to acquire certain effector T-cell functions, for example generation of cytotoxic CD8+ T cells and interferon (IFN)-γ-producing CD4+ T cells. In summary, CD8+ DCs are particularly potent antigen-presenting cells that express critical costimulators and efficiently process exogenous antigen for presentation by both MHC class I and II molecules.
Keywords: dendritic cells, antigen presentation/processing, costimulation
Introduction
The ability of a dendritic cell (DC) to process and present antigen depends on three major factors: the maturity of the DC, its tissue of origin, and the DC subpopulation. Immature tissue-derived DCs have high endocytic activity but express low levels of major histocompatibility complex (MHC) and costimulatory molecules.1–3 Mature DCs have lower endocytic activity but higher expression of MHC and costimulatory molecules, enhancing their efficiency for antigen presentation.4. DCs also change their expression of chemokine receptors during maturation. Immature DCs express CC chemokine receptor (CCR)1, CCR2 and CCR6, while maturing DCs express CCR7.5–7 Thus, a DC that encounters antigen and a maturation stimulus will first internalize and process the antigen, then shift its pattern of chemokine receptor expression, migrate from the site of antigenic challenge to T-cell areas in lymphoid tissue, and finally present processed antigen and costimulatory molecules that are necessary for effective stimulation of T cells.
In addition to the maturation state of the DC, the outcome of DC–T-cell interaction depends on the tissue where this encounter occurs. Both splenic and Peyer's patch (PP) DCs express MHC class II (MHC-II) molecules and costimulatory molecules, but the outcomes of the interaction between T cells and DCs from these two sites are different. Spleen-derived DCs generally activate T cells that produce interferon (IFN)-γ, whereas PP-derived DCs generate T cells that predominately produce interleukin (IL)-10 and transforming growth factor (TGF)-β.8 Liver-derived DCs induce apoptosis of T cells, and this apoptosis is reversible by caspase inhibitor, leading to proliferation and production of IL-10, IFN-γ and TGF-β, but not IL-2 or IL-4.9 Differences in antigen-presenting cell (APC) function in different tissues may account for the development of immune privileged sites.10,11
The final level of functional heterogeneity in DC populations occurs with distinct subpopulations of DCs identified within a specific tissue. Splenic DCs have been divided into CD8+ (CD8+ CD4– CD11b–), CD4+ (CD8– CD4+ CD11b+) and double-negative (DN) DCs (CD8– CD4– CD11b+).12–15 More recent reports have also identified spleen-derived plasmacytoid (B220+ Ab clone (GR)-1+) DC populations.16 Heterogeneity has also been described within the liver and PP.17–19 These different DC subpopulations may play distinct roles in regulating the immune response.17–21
The precise functions of different splenic DC subsets remain uncertain. Most studies have divided splenic DCs into CD8+ and CD8– subsets, but CD8– DCs can be divided into three other subsets (CD4+ CD8– CD11b+, CD4– CD8– CD11b+ and B220+). Thus, many studies fail to fully characterize splenic DC subpopulations. Despite these inconsistencies, several studies have identified differences in the ability of splenic DC subpopulations to generate T-cell responses. In some reports, freshly isolated splenic CD8– DCs were more effective than CD8+ DCs for presentation of soluble antigen to naïve CD4+ T cells.22–25 In contrast, other studies have reported that CD8+ DCs present antigen to CD4+ T cells, are a prime source of IL-12 and IFN-γ, and promote T helper type 1 (Th1)-type responses, while CD8– DCs produce IL-10 and induce Th2-type responses.22,26–32 Overall, the type of antigen that is being processed and the activation state of the DC must be taken into account when determining the fate of the immune response.
In the results presented here, we used a procedure that does not engage CD11c to isolate CD4+ and CD8+ DCs. We were able to characterize the ability of two populations of splenic DCs to process and present particulate antigen to T cells, and to discern differences in both antigen processing and costimulatory functions. CD8+ DCs were more efficient in antigen processing via both the ‘alternate MHC-I’ or ‘cross processing’ pathway and the classical MHC-II pathway. Increased efficiency of antigen processing resulted in better antigen presentation to naïve T cells, as assessed by increased proliferation at lower antigen doses and more efficient generation of more CD8+ cytotoxic T lymphocytes (CTLs). In addition, CD8+ DCs expressed higher levels of CD24, which functions as an essential costimulatory molecule for both CD4+ and CD8+ T cells. Superior antigen processing efficiency and costimulator expression may make CD8+ DCs more effective than CD4+ DCs for initiation of T-cell responses.
Materials and methods
Cell isolation
Spleens from C57BL/6 J mice (Jackson Laboratories, Bar Harbor, ME) were minced and incubated in digestion buffer containing 150 U/ml collagenase (Worthington, Lakewood, NJ) and 30 U/ml DNase (Sigma, St Louis, MO) at 37° with mixing for 1 hr. Spleen single-cell suspensions were prepared by passage through a 70-µm cell strainer (Becton Dickinson Laboratories, Franklin Lake, NJ) and incubation in Gey's solution (0·85% NH4Cl with 10 mm KHCO3) for 5 min to lyse red blood cells. Cells were washed, and CD4+ or CD8+ DC subsets were isolated using a magnetic bead isolation kit (Miltenyi Biotec Inc., Auburn, CA). Isolated CD4+ and CD8+ DCs were 80–85% pure, with less than 10% T cells, no MHC+ CD11c– cells, and with no detectable cross-contamination of CD4+ DCs in the CD8+ DC population or vice versa (data not shown). To isolate T-cell populations from OT-I mice (Jackson Laboratories) or OT-II mice (kind gift from Dr Judith Kapp, Emory University, Atlanta, GA), splenocytes were prepared, erythrocytes were lysed, and cells were washed with magnetic antibody cell sorting (MACS) buffer [phosphate-buffered saline (PBS)−0·1% bovine serum albumin (BSA) and 2 mm ethylenediaminetetraacetic acid (EDTA)]. Cells were then incubated with antibody cocktail and magnetic beads to enrich for CD8+ or CD4+ T-cell populations by negative selection (Miltenyi Biotec Inc.). This procedure generated T cells that were > 85% pure (data not shown). Case Western Reserve University School of Medicine's Institutional Animal Care and Use Committee have approved all studies involving animals.
Antigen processing and presentation assays
To examine antigen processing, 2 × 104 DCs were added to 96-well flat-bottom plates (Becton Dickinson Laboratories) with various concentrations of latex-ovalbumin (L-OVA; ovalbumin non-covalently linked to a latex particle). L-OVA was generated by incubating 2-µm polystyrene latex beads (Polyscience, Warrington, PA) with 10 mg/ml ovalbumin (Worthington) in citrate buffer (0·2 m citric acid, pH 4·2) for 48 hr at 4°. To ensure antigen loading, DCs and L-OVA were centrifuged at 500 g for 10 min at 37° and incubated at 37° for an additional 1 hr. T hybridoma cells (CD8OVA1·3 for detection of MHC-I antigen processing; DOBW for detection of MHC-II antigen processing) were added (105 cells/well) and the culture was incubated for 20–24 hr at 37°.33,34 After 24 hr, culture supernatants were assessed for IL-2 production using a CTLL-2 bioassay and spectrophotometric readout with Alamar blue (Alamar Bioscience, Inc., Sacramento, CA). All antigen-processing reactions were performed in triplicate. For antigen presentation experiments, DCs (2 × 104 cells/well) were cultured with L-OVA for 1 hr, OT-I or OT-II T cells were added (2 × 105 cells/well), cultures were incubated for 96 hr, and 1 µCi 3H-Thy (Perkin-Elmer, Boston, MA) was added for the last 8 hr of culture. For antibody blocking experiments, DCs were first cultured with L-OVA for 1 hr at 37° and then antibody was added at 1 µg/ml for an additional 1 hr. When cells were washed after antibody incubation, there was no significant decrease in T-cell inhibition, suggesting that interaction with DCs was sufficient to inhibit the T-cell response. Cells were collected using a Tomtec cell harvester (Tomtec, Hamden, CT) and the amount of 3H-Thy incorporation was determined using a Wallac beta-plate reader (Perkin-Elmer).
Flow cytometry analysis of DC populations
DCs were added to 96-well V-bottom plates (105 cells/well) and incubated in PBS with 0·1% BSA and 10% normal mouse serum [fluorescence-activated cell sorting (FACS) buffer] for at least 20 min at 4°. For characterization of lineage markers and costimulatory signals, cells were stained with fluorochrome-conjugated rat anti-mouse CD11b (clone M1/70; BD Pharmingen, San Diego, CA), hamster anti-mouse CD11c (clone N418; eBioscience, San Diego, CA), rat anti-mouse DEC-205 (clone NLDC-145; AbD Serotec, Raleigh, NC), rat anti-mouse 33D1 (clone 33D1; eBioScience), rat anti-mouse CD24 (clone M1/69; BD Pharmingen), hamster anti-mouse CD80 (clone 16-10A1; BD Pharmingen), rat anti-mouse CD86 (clone GL1; BD Pharmingen) or isotype control antibody (rat IgG2a, hamster IgG or biotin-conjugated rat-IgG2b; BD Pharmingen) in the presence of 10% normal mouse serum. Cells incubated with biotinylated antibodies were then washed two times in FACS buffer and then incubated for an additional 20 min in FACS buffer containing PE-conjugated streptavadin (eBioscience). Cells were washed in PBS, and fixed in 2% paraformaldehyde in PBS for flow cytometry. To examine intracellular cytokine production, DCs (2 × 104 cells/well) were cultured for 4 days with OT-II T cells (2 × 105 cells/well). DCs cells were removed with anti-CD11c+ beads, and remaining T cells were incubated for 5–6 hr in the presence of 1 µg/ml brefeldin in plates coated with anti-CD3 (coated with 100 µg/ml anti-CD3 and washed). Cells were fixed with BD Cytofix/CytoPerm solution (BD Pharmingen) containing formaldehyde and then stained with anticytokine antibodies in BD Perm/Wash buffer (BD Pharmingen) containing saponin for cell permeablization. Cells were then examined within 4 hr with a FACScan flow cytometer (Becton Dickenson Immunocytometry Systems, San Jose, CA). Approximately 1–2 × 104 events were acquired and analysed using flowjo software (Tree Star Inc., Ashland, OR). The mean fluorescent value (MFV) for isotype control antibody was subtracted from the MVF with specific antibody to determine specific MFV.
Mouse cytokine antibody array
Conditioned media from 96-hr cultures containing L-OVA-pulsed CD4+ or CD8+ DCs and OT-II T cells were incubated on Cytokine Antibody Array Membrane (RayBiotech Inc., Norcross, GA) for 2 hr at 4°. Membranes were washed and biotin-conjugated anticytokine antibodies were added to the membrane for an additional 2 hr. After washing, horseradish peroxidase (HRP)-conjugated streptavidin was added to the membrane for another 2 hr. After final washes, detection buffer was added to the membrane for 2 min and then membrane was then photographed using a CCD camera (Versa Doc 3000 Imaging System; Bio-Rad, Hercules, CA) and analysed using QualityOne 4·4.0 software (Bio-Rad). The intensities of signal for all cytokines were compared to that for the positive control. To determine relative amounts of cytokine produced, these corrected values for T cells cultured with DC subsets were then compared with those for media that contained T cells alone.
Cytotoxicity assay
The just another method (JAM) assay was set up as previously described.35 Specifically, DCs (105 cells/well) were preincubated in 24-well plates with 1000 ng/ml L-OVA for 1 hr prior to the addition of OT-I T cells (2 × 106 cells/well) and then incubated for 5 days. Cultured OT-I T cells were washed extensively, added to 96-well plates and cultured for 4–5 hr with 5 × 103 EL4 target cells (which were previously cultured at 2 × 104 cells/ml overnight and labelled with 5 µCi/ml 3H-Thy for 4–5 hr in the presence or absence of OVA257-264). A single plate without T cells was harvested to determine [3H]thymidine release as a result of spontaneous lysis. In all experiments spontaneous lysis was less than 5%. Cells were harvested and the percentage of cytotoxicity was determined using the following formula: % cytotoxicity = [(S − E)/S] × 100, where E represents release in experimental wells and S represents spontaneous release from labelled EL4 cells in media only.
Results
Antigen processing by DC subsets
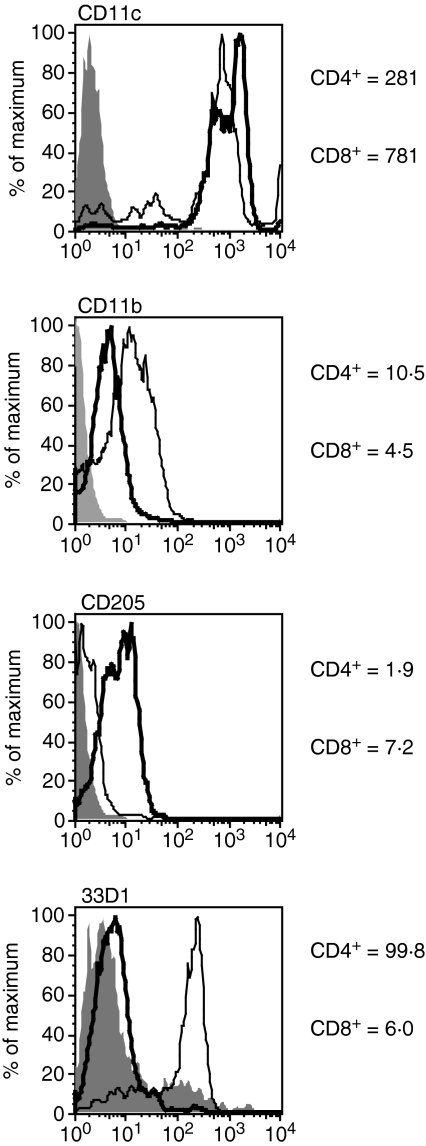
To test the ability of DC subsets to process and present antigen, CD4+ or CD8+ DCs were isolated by an immunomagnetic bead method that first depleted large populations of non-DCs and then isolated CD4+ or CD8+ DCs by positive selection (avoiding engagement of CD11c). To further define these CD11c+ DC subsets (specific MFV for CD4, 281; for CD8, 781), we examined them for expression of CD11b, DEC-205 and 33D1, markers known to be differently expressed on these DC populations.36 We found that, as expected, CD4+ DCs expressed higher levels of CD11b (specific MFV 10·5 versus 4·5), but lower levels of DEC-205 (specific MFV 1·9 versus 7·2) when compared with CD8+ DCs. More importantly, we found that 33D1 helped to distinguish CD4+ DCs from CD8+ DCs, with CD4+ DCs expressing high levels of 33D1 (specific MFV 99·8 versus 6·0) (Fig. 1). After exposure of DCs to L-OVA, cell-surface expression of peptide–MHC complexes was used as a measure of antigen processing and was assessed using MHC-I or MHC-II restricted T-cell hybridomas (CD8OVA1·3 or DOBW, respectively; see ‘Materials and methods’). CD8+ DCs were more efficient than CD4+ DCs at processing particulate antigen for presentation to both MHC-I and MHC-II restricted T cells (Fig. 2). Both populations of DCs were effective at MHC-II antigen processing and presented antigen to CD4+ T cells, but CD8+ DCs were more efficient. For MHC-I cross-processing and cross-presentation, only CD8+ DCs were effective, and CD4+ DCs did not cross-present L-OVA.
Figure 1.
Phenotypically distinct splenic dendritic cell (DC) populations. Freshly isolated CD4+ or CD8+ DCs were stained for expression of CD11c, CD11b, DEC-205 and 33D1 to distinguish between the two populations. CD4+ DCs expressed higher levels of CD11b and lower levels of DEC-205 when compared with CD8+ DCs. 33D1 was effective in differentiating CD4+ DCs from CD8+ DCs.
Figure 2.
CD8+ dendritic cells (DCs) are more efficient than CD4+ DCs for processing and presentation of latex-ovalbumin (L-OVA) to T hybridoma cells. CD4+ or CD8+ DCs (2 × 104) were cultured with L-OVA for 1 hr prior to addition of 105 CD8OVA1·3 [a, major histocompatibility complex class I (MHC-I)-restricted] or DOBW (b, MHC-II-restricted) T hybridoma cells. Cultures were then incubated at 37° for 24 hr, and supernatants were collected for a CTLL assay to assess interleukin (IL)-2 production. (a) CD8OVA1·3 responses. (b) DOBW responses. Data points are mean ± standard deviation (*, P < 0·01 by Student's t-test). When not visible, error bars are smaller than the symbol. Results are representative of five independent experiments.
Antigen presentation and the costimulatory molecule CD24
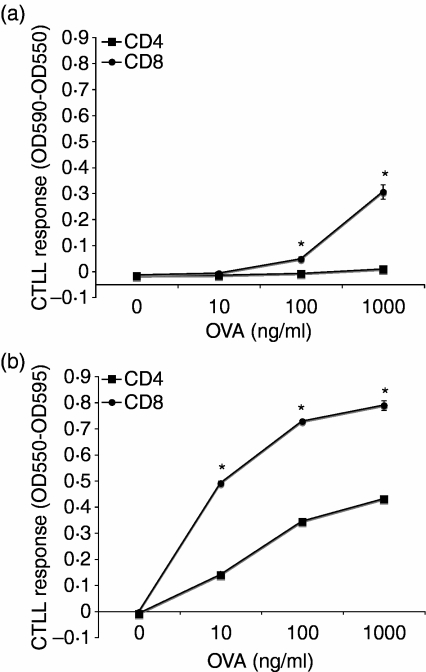
As T hybridoma cells respond to peptide–MHC complexes in the absence of costimulatory molecules that are required by primary T cells, they do not allow a full assessment of antigen presentation function. To more fully assess the antigen-presenting function of splenic DC subsets, we cultured CD4+ and CD8+ DCs with L-OVA and added T-cell receptor (TCR) transgenic, OVA-specific naïve MHC-I-restricted OT-I T cells or MHC-II-restricted OT-II T cells. These naïve T cells require both peptide–MHC complexes and additional costimulatory molecules to induce proliferation. Consistent with the results shown in Fig. 2, CD8+ DCs were more efficient than CD4+ DCs at stimulating OT-I and OT-II T cells (Fig. 3). For example, at 100 ng/ml L-OVA CD8+ DCs produced a 6-fold greater OT-I response than CD4+ DCs.
Figure 3.
CD8+ dendritic cells (DCs) are more efficient than CD4+ DCs for induction of naïve CD4+ and CD8+ T cell proliferative responses. CD4+ or CD8+ DCs (4 × 104) were cultured with latex-ovalbumin (L-OVA) for 1 hr prior to addition of 105 T cells. Cultures were then incubated at 37° for 96 hr with 1 µCi [3H]thymidine added for the last 8 hr. Cells were harvested and [3H]thymidine incorporation was assessed. (a) Major histocompatibility complex class I (MHC-I)-restricted OT-I T-cell responses. (b) MHC-II-restricted OT-II T-cell responses. Data points represent mean ± standard deviation (*, P < 0·01 by Student's t-test). When not visible, error bars are smaller than the symbol. Results are representative of six independent experiments. CPM, counts per minute.
Because naïve T-cell responses require the presence of costimulatory molecules as well as peptide–MHC complexes produced by antigen processing, we examined the expression of costimulatory molecules on the two DC populations. CD8+ DCs expressed slightly higher levels of CD80 and CD86 than CD4+ DCs (Fig. 4; specific MFV ratios of 42 : 36 and 46 : 16, respectively). In addition, CD8+ DCs exhibited much stronger CD24 staining than CD4+ DCs (Fig. 4; specific MFV ratio of 316 : 37). To determine the functional significance of CD80, CD86 and CD24 on CD8+ DCs, we cultured CD8+ DCs with L-OVA (1000 ng/ml) and either isotype control or specific blocking antibody against CD24, CD80 and/or CD86 for 1 hr prior to the incubation with OT-I or OT-II T cells. Addition of antibodies to DCs for 1 hr prior to the addition of T cells is effective for blockade of functional costimulatory activity on DCs, and washing the cells after this initial incubation does not decrease blockade of the DC costimulatory function (data not shown). OT-I T cell proliferation was partially inhibited by antibodies to CD80 or CD86 (inhibition of 33 or 50%, respectively), and a combination of these antibodies reduced OT-I proliferation by 93%(Fig. 5a). Thus, naïve OT-I T cells require both CD80 and CD86 for generation of a full response, confirming the importance of CD28 signalling in responses of these naïve CD8+ T cells to CD8+ DCs. Strikingly, anti-CD24 blocking antibody alone inhibited OT-I T cell proliferation by 75%, a far greater value than that for antibodies against either CD80 or CD86 alone, revealing an important costimulatory function for CD24 in driving responses of CD8+ T cells to CD8+ DCs. OT-II T-cell proliferation was less susceptible to blockade by a single antibody to CD80 or CD86 (which produced 0 and 3% inhibition, respectively; Fig. 5b), but addition of both antibodies inhibited OT-II T-cell proliferation by 71%. Addition of anti-CD24 substantially blocked OT-II T-cell proliferation (69% inhibition). Thus, CD24 is an essential costimulatory molecule that is required for CD8+ DCs to generate responses by CD8+ and CD4+ T cells. Although CD80 and CD86 provided overlapping functions, particularly for OT-II cells, CD24 provided a distinct signal. These results suggest that CD24 functions as an important costimulatory molecule, and expression of CD24 by CD8+ DCs may be responsible, in part, for the particularly effective antigen presentation function of CD8+ DCs.
Figure 4.

CD24 expression distinguishes CD4+ and CD8+ dendritic cells (DCs). Splenic CD4+ and CD8+ DCs were stained for expression of costimulatory molecules CD24, CD80 and CD86 and assessed by flow cytometry. Bold line, CD8+ DCs; thin line, CD4+ DCs. Shaded histogram, staining of CD8+ DCs with isotype-matched negative control antibody (CD4+ DCs gave similar staining with control antibody). Results are representative of five independent experiments.
Figure 5.
CD24 functions as a costimulatory molecule for CD4+ and CD8+ T cells. CD8+ dendtritic cells (DCs) (4 × 104) were cultured for 1 hr with latex-ovalbumin (L-OVA) (1 µg/ml) with or without blocking antibodies (5 µg/ml) to CD24 (clone M1/69), CD80 (clone 16·10A1), and/or CD86 (clone GL1) for 1 hr. The isotype control is represented by rat immunoglobulin G2a (IgG2a) (clone R35-95). There was no change in T-cell proliferation when other isotype controls were used (rat IgG2b or Armenian hamster IgG). T cells (105) were then added and cultures were incubated for 96 hr, with 1·0 µCi [3H]thymidine added for the last 8 hr. Cells were then harvested and [3H]thymidine incorporation was assessed. (a) Major histocompatibility complex class I (MHC-I)-restricted OT-I responses. (b) MHC-II-restricted OT-II responses. Data points represent mean ± standard deviation (*, P < 0·01 by Student's t-test). The results are representative of three independent experiments. CPM, counts per minute.
Effector function of T cells after encountering splenic DC subsets
The development of T-cell effector functions is another physiologically relevant measure of immune response. To evaluate the effector function of T cells, we examined the acquisition of cytotoxic function by CD8+ T cells and the induction of cytokine secretion by CD4+ T cells. To determine the development of CTLs, a JAM cytotoxicity assay with [3H]thymidine-labelled OVA-pulsed tumour cells was performed using OT-I T cells. CD4+ or CD8+ DCs were pulsed with 1000 ng/ml L-OVA for 1 hr prior to the addition of OT-I T cells and were further incubated for an additional 5 days. The T cells were then incubated for 5 hr with [3H]thymidine-labelled EL4 target cells in the presence or absence of OVA323-339. Despite their poor cross-presentation function, CD4+ DCs did generate CTLs, but they were much less efficient than CD8+ DCs (Fig. 6 and data not shown). One-hundred-fold less antigen was required to produce effective CTLs from OT-I T cells cultured with CD8+ DCs (data not shown). Thus, CD8+ DCs promote development of CTLs much more efficiently than CD4+ DCs.
Figure 6.
CD8+ dendritic cells (DCs) are more efficient at generating CD8+ cytotoxic T lymphocytes (CTLs). CD4+ or CD8+ DCs (2 × 105) were cultured with 2 × 106 OT-I CD8+ T cells in 24-well plates for 5 days. EL4 cells were incubated with [3H]thymidine and ovalbumin (OVA323-339), and labelled EL4 target cells (104 cells/well) were plated in a 96-well plate with the indicated ratio of cultured OT-I CD8+ T cells for 5 hr at 37°. The percentage cytotoxicity was determined as described in ‘Materials and methods’. Data points represent mean ± standard deviation (*, P < 0·01 by Student's t-test). When not visible, error bars are smaller than the symbol Results are representative of six independent experiments.
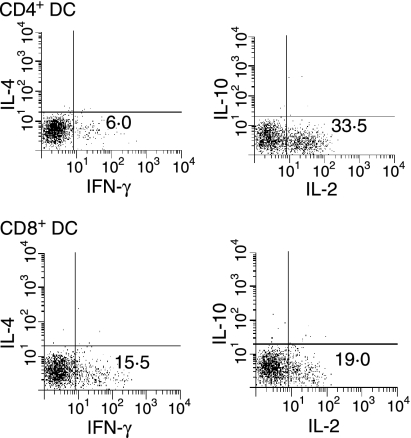
Specific cytokine production, another physiologically relevant measure of the T-cell immune response, was measured in OT-II CD4+ T cells. Culture of OT-II T cells with either CD4+ or CD8+ DCs and L-OVA induced production of cytokines, including IFN-γ and IL-2 (Fig. 7). CD8+ DCs effectively induced both IFN-γ and IL-2 responses, whereas CD4+ DCs induced production of IL-2 but only low amounts of IFN-γ by CD4+ T cells. As with the generation of CTLs, CD4+ DCs required higher antigen doses than CD8+ DCs to generate effector CD4+ T cells producing cytokines (data not shown). When we examined the production of cytokines in cultures using antibody-based cytokine arrays, we found that both DC populations could promote OT-II to produce IFN-γ and IL-2. The cytokine levels were always higher in OT-II T cells cultured with CD8+ DCs (Fig. 8). Thus, CD8+ DCs were more efficient than CD4+ DCs for generation of effector T-cell responses, including both cytotoxic function and cytokine secretion.
Figure 7.
CD4+ and CD8+ dendritic cells (DCs) both generate T helper type 1 (Th1)-like responses by CD4+ T cells. CD4+ or CD8+ DCs (4 × 104) were cultured for 1 hr with latex-ovalbumin (L-OVA) (1 µg/ml). OT-II cells were then added for 96 hr. Cells were then washed and added to 96-well plates coated with anti-CD3 in the presence of 1 µg/ml brefeldin A for 5–6 hr. Cells were washed, stained with anti-CD3, fixed with formaldehyde, labelled with anticytokine antibodies in the presence of saponin, and assessed by flow cytometry. Results are representative of four independent experiments. IFN, interferon; IL, interleukin.
Figure 8.
CD4+ and CD8+ dendritic cells (DCs) support production of interferon (IFN)-γ and interleukin (IL)-2 by ovalbumin (OVA)-specific CD4+ T cells. Latex-ovalbumin (L-OVA)-pulsed CD4+ or CD8+ T cells were cultured with OT-II T cells for 96 hr. (a) Cell supernatant was collected and production of cytokines was determined using a cytokine antibody array. (b) The relative levels of each cytokine were compared to those for T cells cultured in media alone, as described in ‘Material and methods’. CD8+ DCs promoted increased production of IL-2 and IFN-γ when compared with T cells cultured with CD4+ DCs (*, P < 0·01 by Student's t-test). Results are representative of three independent experiments.
Discussion
Earlier studies of splenic DCs divided them into two subpopulations based on expression of CD8α or CD11b defining lymphoid DCs (CD8α+CD11b–) or myeloid DCs (CD8α– CD11b+).12,37 Changes in the isolation procedure resulted in splenic DCs being divided into CD8α+ DCs (CD8α+ CD4– CD11b–), CD4+ DCs (CD8α– CD4+ CD11b+), or DN DCs (CD8α– CD4– CD11b+).12–15 These DC subpopulations are often classified as conventional DCs to distinguish them from B220+ plasmacytoid DCs.16,38,39 It is unclear whether or not these conventional DC subsets are developmentally related. While one study suggested that CD8+ DCs arise from the CD8– subset,40 other studies have identified unique precursor cells, suggesting that these subpopulations do not represent immature phenotypes of other DC subpopulations.13,21,41,42 Given the existence of phenotypically and developmentally distinct DC subpopulations, it is important to determine whether these subpopulations are functionally distinct.
For studies of the functional heterogeneity of splenic DC subpopulations, it is necessary to account for how the DC subpopulations are isolated, the type of antigen being processed (soluble versus particulate), and whether or not adjuvants are being used. Furthermore, it is important to isolate single defined DC populations. In some earlier studies examining CD8+ and CD8– DC populations, the CD8– population consisted of at least two subpopulations based on CD4+ expression. While some studies suggest that there are distinct functions for different DC subpopulations in their interactions with naïve T cells, these studies have often provided conflicting results as to T-cell type and outcome of response.22–32 Our studies support the role of CD8+ DCs as the predominant APC in the spleen when dealing with particulate antigen for both CD4+ and CD8+ T-cell responses, and CD8+ T-cell responses were particularly focused on this DC population.
A number of factors may contribute to differences in MHC-I and MHC-II antigen presentation by distinct DC populations. One factor is the ability to internalize antigen; our CD4+ and CD8+ DCs may differ to some degree in antigen uptake, but both DC subsets were able to process and present antigen to CD4+ T cells (Figs 2b and 3b), and the differences in MHC-II-restricted presentation were less than for MHC-I-restricted presentation. Another factor is the efficiency with which antigen enters the processing pathway and is processed to form peptide–MHC complexes. The greater ability of CD8+ DCs to present antigen to CD8+ T cells may be explained by greater access to antigen for MHC-I processing and greater cross-processing efficiency in CD8+ DCs. Finally, different costimulator expression may account for significant differences in the antigen presentation functions of different DC subsets. While many costimulatory molecules have been identified, the most recognized essential molecules are members of the B7 family, CD80 and CD86.43–45 The role of these molecules is to interact with CD28, which is constitutively expressed on T cells, to promote proliferation and survival upon interaction with APCs. In our studies, CD8+ DCs had a slightly higher expression of CD86 compared with CD4+ DCs, but it is unlikely that this small difference accounts for the differences in antigen presentation between the two populations of DCs. One costimulatory molecule that is differentially expressed on CD8+ and CD8– DCs is CD24.12–14,46–48 In CD28 knockout mice, CD24 plays an essential role as a costimulatory molecule, especially in the activation of CD4+ T cells and in isotype switching.47 While CD24 may not be essential for development of effector T cells, signalling through both CD24 and CD28 has been found to promote the development of memory T cells.48 In CD24 knockout mice, CD4+ T cells developed both effector and memory responses.49 As soluble antigen was used this is not surprising, as we would expect CD8– DCs to play a dominant role in activating CD4+ T cells. Because these cells tend to be CD24–, we would not expect to find a difference in T-cell response. In our studies, blocking antibodies against CD24 and CD80/CD86 showed that both pathways were essential for T-cell proliferation (Fig. 5).
In summary, our studies demonstrated that CD8+ DCs are more efficient at presenting particulate antigen to both CD4+ and CD8+ T cells, and CD8+ DCs were notably predominate in driving CD8+ T cell responses. These results differ from other studies that suggested a separation of function, with CD8+ DCs presenting antigen to CD8+ T cells and CD8– DCs (including CD4+ DCs) presenting antigen to CD4+ T cells.23,24,50,51 Differences in isolation technique may account for these differences. While the efficiency of antigen presentation depends on antigen processing, the presence of the costimulatory molecule CD24 on CD8+ DCs was a significant factor in determining the efficiency of antigen presentation. The expression of CD24 may also enhance the development of memory T cells, a key to maintaining an immune response against recall antigens. While these studies show heterogeneity of splenic DC populations when particulate antigen is used, these same differences may not be seen when soluble antigen is used (data not shown). In addition, our studies were carried out without the addition of adjuvants to enhance APC–T-cell interactions. One possible consequence of using adjuvants is to expand the repertoire of DC subpopulations that can interact with T cells and thus provide a more robust immune response. While we and other investigators have identified functional heterogeneity of CD4+ and CD8+ DCs, both of these DC subpopulations show a range of function that may be essential for the development of an effective immune response.
Acknowledgments
This work was supported by NIH grant AI35726. DA was supported by NIH training grant HL07889.
References
- 1.Pure E, Inaba K, Crowley MT, Tardelli L, Witmer-Pack MD, Ruberti G, Fathman G, Steinman RM. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990;172:1459–69. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–19. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch F, Heufler C, Kampgen E, Schneeweiss D, Bock G, Schuler G. Tumor necrosis factor alpha maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colonly-stimulating factor, without inducing their functional maturation. J Exp Med. 1990;171:159–71. doi: 10.1084/jem.171.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieu M-C, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Caux C, Vanbervliet B, Massacrier C, Ait-Yahia S, Vaure C, Chemin K, Dieu-Nosjean, Vicari A. Regulation of dendritic cell recruitment by chemokines. Transplantation. 2002;73(1 Suppl.):S7–11. doi: 10.1097/00007890-200201151-00005. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, Bonham CA, Liang X, et al. Liver-derived DEC205+B220+CD19– dendritic cells regulate T cell responses. J Immunol. 2001;166:7042–52. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- 10.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999;83:598–604. doi: 10.1136/bjo.83.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164:1346–54. doi: 10.4049/jimmunol.164.3.1346. [DOI] [PubMed] [Google Scholar]
- 12.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs. cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–73. [PubMed] [Google Scholar]
- 13.Kamath AT, Pooley J, O'Keeffe MA, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–70. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 14.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 15.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 16.Nakano H, Yanagita M, Gunn MD. CD11c(+) B220(+) Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–47. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian ZX, Okada T, He XS, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–30. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 19.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 20.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8alpha(+) B220(+) dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–90. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 21.Nikolic T, Dingjan GM, Leenen PJ, Hendriks RW. A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur J Immunol. 2002;32:686–92. doi: 10.1002/1521-4141(200203)32:3<686::AID-IMMU686>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.De Smedt T, Butz E, Smith J, Maldonado-Lopez R, Pajak B, Moser M, Maliszewski C. CD8alpha(–) and CD8alpha(+) subclasses of dendritic cells undergo phenotypic and functional maturation in vitro and in vivo. J Leukoc Biol. 2001;69:951–8. [PubMed] [Google Scholar]
- 23.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(–) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8– dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 25.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–39. [PubMed] [Google Scholar]
- 27.Maldonado-Lopez R, De Smedt T, Pajak B, et al. Role of CD8alpha+ and CD8alpha– dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–6. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(–) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345–50. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol. 2001;13:275–82. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 31.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–55. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 34.Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–3. [PubMed] [Google Scholar]
- 35.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Meth. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–31. [PubMed] [Google Scholar]
- 37.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–6. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 39.Hochrein H, O'Keeffe M, Wagner H. Human and mouse plasmacytoid dendritic cells. Hum Immunol. 2002;63:1103–10. doi: 10.1016/s0198-8859(02)00748-6. [DOI] [PubMed] [Google Scholar]
- 40.del Hoyo GM, Martin P, Arias CF, Marin AR, Ardavin C. CD8alpha(+) dendritic cells originate from the CD8alpha(–) dendritic cell subset by a maturation process involving CD8alpha, DEC-205, and CD24 up-regulation. Blood. 2002;99:999–1004. doi: 10.1182/blood.v99.3.999. [DOI] [PubMed] [Google Scholar]
- 41.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–41. [PubMed] [Google Scholar]
- 42.Dakic A, Shao QX, D'Amico A, O'Keeffe M, Chen WF, Shortman K, Wu L. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–27. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 43.Carreno BM, Collins M. The B7 family of ligands and its receptors. New pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 44.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 45.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Jones B, Aruffo A, Sullivan KM, Linsley PS, Janeway CA., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992;175:437–45. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Zhou Q, Zheng P, Liu Y. CD28-independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat-stable antigen. J Exp Med. 1998;187:1151–6. doi: 10.1084/jem.187.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;185:251–62. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Heusch M, Garze V, Maliszewski C, Urbain J, Liu Y, Moser M. The heat stable antigen (CD24) is not required for the generation of CD4+ effector and memory T cells by dendritic cells in vivo. Immunol Lett. 2004;94:229–37. doi: 10.1016/j.imlet.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Schnorrer P, Behrens GM, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–34. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(–) dendritic cells in vivo. J Exp Med. 2002;196:817–27. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]