Abstract
Langerin/CD207 is expressed by a subset of dendritic cells (DC), the epithelial Langerhans cells. However, langerin is also detected among lymphoid tissue DC. Here, we describe striking differences in langerin-expressing cells between inbred mouse strains. While langerin+ cells are observed in comparable numbers and with comparable phenotypes in the epidermis, two distinct DC subsets bear langerin in peripheral, skin-draining lymph nodes of BALB/c mice (CD11chigh CD8αhigh and CD11clow CD8αlow), whereas only the latter subset is present in C57BL/6 mice. The CD11chigh subset is detected in mesenteric lymph nodes and spleen of BALB/c mice, but is virtually absent from C57BL/6 mice. Similar differences are observed in other mouse strains. CD11clow langerin+ cells represent skin-derived Langerhans cells, as demonstrated by their high expression of DEC-205/CD205, maturation markers, and recruitment to skin-draining lymph nodes upon imiquimod-induced inflammation. It will be of interest to determine the role of lymphoid tissue-resident compared to skin-derived langerin+ DC.
Keywords: Langerhans cells, lymphoid organs, phenotype
Introduction
Dendritic cells (DC) are specialized antigen-presenting cells, derived from bone marrow leucocyte progenitors and present in most peripheral tissues.1 They capture self and non-self antigens in situ and transport them to regional draining lymph nodes (LN), where their degradation products are presented to specific T cells, leading to immunity or tolerance.2,3
Langerhans cells (LC) constitute a DC subset that is present in basal and suprabasal layers of the epidermis, and in stratified mucosal epithelia.4 As a result of their location, LC represent the first immune barrier to the external environment. They express several specialized cell-surface receptors, including the C-type lectins CD205/DEC-205 and CD207/langerin.5 Langerin features an extracellular carbohydrate recognition domain which binds ligands in a Ca2+-dependent manner, with specificity for mannose, N-acetyl-glucosamine and fucose.5,6 Expression of langerin induces the formation of Birbeck granules, the hallmark organelles of LC.7–9 Langerin is not necessary, though, for the generation of LC and their settlement in the epidermis, as indicated by C57BL/6 langerin null mice that have normal numbers of epidermal LC.10 Epidermal LC are, however, absent in some genetically modified mice, i.e. transforming growth factor-β1 knockout mice and inhibitor of DNA binding 2 (Id2) knock-out mice.11,12
In addition to its presence in resident skin and epithelial LC, langerin expression is detected in most murine lymphoid organs.13 Furthermore, we have recently shown that, in BALB/c mice, langerin is displayed by two distinct DC subsets in lymphoid organs.14 A similar observation was also made in mice that had been genetically engineered to express green fluorescent protein (GFP) under the control of the langerin promoter.15 This prompted us to have a systematic look at the expression of langerin in the spleen and LN of various inbred mouse strains.
Materials and methods
Mice
Mice of inbred strains 129/Sv, BALB/c, C57BL/6, CBA and DBA2 were purchased from Charles River Laboratories (Sulzfeld, Germany) and used at 2–6 months of age. Mice were euthanasied by cervical dislocation or CO2 inhalation. Samples (ear skin or lymphoid organs) were collected as required and animals were disposed of by incineration.
Epidermal sheet stainings
Ears were cut off and split into two halves. Epidermis from the dorsal side was separated from the dermis using ammonium thiocyanate.16 Epidermal sheets were fixed in acetone, rinsed in staining buffer, and immunolabelled with anti-major histocompatibility complex (MHC) class II (anti-I-A/Ediverse, clone 2G9; BD-Pharmingen, San Diego, CA) or anti-langerin (clone 929F3; Dendritics, Dardilly, France).17 Primary antibodies were detected with goat anti-rat immunoglobulin G (IgG; H + L) coupled to Alexa Fluor 488™ or Alexa Fluor 594™ (Invitrogen-Molecular Probes, Eugene, OR). Specimens were viewed on a Zeiss Axioscop® epifluorescence microscope. Pictures were taken using an Optronics MagnaFire® Digital Camera (Optronics, Goleta, CA).
Cytofluorimetric analyses of epidermal cells
Ears were cut and split into two halves. Epidermal cells were isolated from the dorsal side by standard trypsinization.18 Cells were stained for CD11c (clone HL3, hamster IgG; BD-Pharmingen) on their surface, and for intracellular langerin using a Cytofix/Cytoperm™ kit (BD-Pharmingen) according to the manufacturer’s recommendations. Flow cytometric analysis was performed on a FACSCalibur™ (BD Biosciences, Mountain View, CA) with CellQuest™ software (BD Biosciences).
Cytofluorimetric analyses of lymph node and spleen cells
Spleen, mesenteric and retro-auricular LN were collected and digested with 0·5 mg/ml collagenase P (Roche, Mannheim, Germany). Cells were directly labelled, without further enrichment, with anti-CD11c, anti-CD8α (clone 53-6.7; BD-Pharmingen), anti-DEC-205 (clone NLDC145; BMA Biomedicals, Augst, Switzerland), CD11b (clone M1/70; BD-Pharmingen), CD40 (clone 3/23; BD-Pharmingen) and CD86 (clone GL1; BD-Pharmingen) on their surface, and with anti-langerin intracellularly, using a Cytofix/Cytoperm™ kit.
Induction of local skin inflammation with imiquimod cream
Mice were treated on both ears with 5% imiquimod cream (Aldara™; 3M, Perchtoldsdorf, Austria), or left untreated. After 4 days, retro-auricular LN were harvested and digested with collagenase. Cells were counted and labelled with anti-CD11c and anti-CD86 on their surface, and with anti-langerin intracellularly, using a Cytofix/Cytoperm™ kit.
Statistical analyses
All experiments were performed at least three times with similar results. Error bars represent standard error of the mean. P-values are from two-tailed Student’s t-tests.
Results
Langerin expression in the epidermis
BALB/c and C57BL/6 mice differ by obvious genetic traits such as coat or eye colour. Regarding the epidermis, BALB/c mice have very few dendritic epidermal γδ+ T cells in contrast to the C57BL/6 strain.19,20
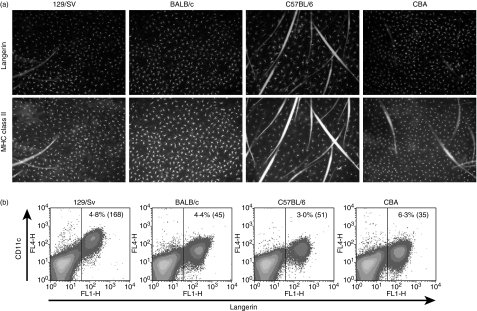
As shown in Fig. 1a, a dense network of LC, co-expressing langerin and MHC class II, was revealed by immunofluorescence on epidermal sheets. We confirmed earlier publications, which found a lower density of LC in C57BL/6 mice, as compared to BALB/c.21,22 In addition, the epidermal LC network in 129/Sv and CBA mice resembled what was observed in BALB/c.
Figure 1.
Similar expression of langerin in epidermal Langerhans cells (LC) from different mouse strains. Ear skin from 129/Sv, BALB/c, CBA and C57BL/6 was harvested for immunofluorescence and fluorescence-activated cell sorter (FACS) analysis. (a) Epidermal sheets were fixed in acetone and labelled with anti-langerin and fluorescein isothiocyanate-coupled anti-major histocompatibility complex class II. Anti-langerin antibodies were detected with chicken anti-rat immunoglobulin G coupled with Alexa Fluor 594™. (b) Epidermal cells were isolated by trypsinization, stained for CD11c (y-axes) and intracellular langerin (x-axes), and analysed by FACS. The percentage of CD11c+ langerin+ LC among all epidermal cells, and the mean fluorescence intensity of CD11c staining (between brackets) are indicated. Results shown here are representative of five independent experiments.
When isolated, CD11c+ epidermal cells displayed equal intensity of langerin, qualifying them as LC (Fig. 1b). The intensity of CD11c in LC from 129/Sv mice was higher than in the other inbred mouse strains tested. Furthermore, LC from all strains were CD11b+, DEC-205+ and CD8α− (data not shown), as previously described.14,23–25 The distribution of langerin and the phenotype of epidermal LC were therefore very similar in 129/Sv, BALB/c, C57BL/6 and CBA mice. We extended these observations to C3H and DBA2 mice, to ascertain that LC in all these strains expressed langerin (data not shown).
Langerin expression in the lymph nodes and spleen
In peripheral, skin-draining LN (i.e. retro-auricular), DC from BALB/c mice featured more than twice as many langerin+ cells than DC from C57BL/6 mice (14 791 ± 3240 versus 5482 ± 227 per LN, respectively; n = 3) (Fig. 2a). This could be a result of the lower density of epidermal LC in the latter strain. Strikingly, in BALB/c LN, langerin was expressed both by CD11clow and CD11chigh subsets, whereas in C57BL/6 LN langerin was almost exclusively detected on CD11clow DC.
Figure 2.
Differential langerin expression in lymphoid organs from different mouse strains. Spleen, mesenteric, and retro-auricular (skin-draining) lymph nodes (LN) were collected and digested with collagenase. Cells were labelled with anti-CD11c and anti-CD8α on their surface, and with anti-langerin intracellularly. (a) CD11c (y-axes) and langerin (x-axes) stainings of total lymphoid organ cells. Gates highlight the two populations (CD11clow and CD11chigh) of langerin+ cells. (b) CD8α expression of langerin+ CD11clow and langerin+ CD11chigh populations in skin-draining LN. Langerin+ cells were gated as shown in the upper row. CD11chigh cells are depicted by grey dots in the bottom row (upper quadrants). Results shown here are representative of at least six independent experiments.
In mesenteric LN of C57BL/6 mice, langerin+ cells were completely absent (Fig. 2a). In striking contrast, mesenteric LN from BALB/c mice contained a sizeable population of CD11chigh langerin+ cells. Thus, our results explain previous contradictory observations whereby a positive signal for langerin was detected in mesenteric LN from BALB/c,13,26 but not from C57BL/6 mice.27
We also found an outstanding difference between these two mouse strains in the spleen. In BALB/c mice, ∼0·25% of total spleen cells expressed intracellular langerin, as well as high levels of CD11c (Fig. 2a). In marked contrast, CD11chigh langerin+ cells represented < 0·03% of total spleen cells from C57BL/6 mice and they only expressed very low levels of langerin.
We then further phenotyped the two langerin+ subsets in skin-draining LN of four different mouse strains. In BALB/c mice, low levels of CD8α were revealed in CD11clow langerin+ DC, while this molecule was present at higher levels in CD11chigh langerin+ DC (Fig. 2b). On the other hand, in 129/Sv mice, in which two subsets of langerin+ DC could also be distinguished based on differential expression of CD11c, the CD8α marker was displayed at similar levels in both populations. Finally, the myeloid marker CD11b could not be used to discriminate between the two langerin+ subsets because they both expressed it at equivalent low levels (data not shown).
We found such clear-cut discrepancies in langerin expression between BALB/c and C57BL/6 mice that we compared spleen cells from other inbred mouse strains. In 129/Sv mice, we consistently found DC expressing langerin and high levels of CD11c, which were undetectable in CBA (Fig. 3a) or DBA2 (data not shown) mice. Interestingly, the phenotype of splenic langerin+ DC was similar in 129/Sv and BALB/c mice, i.e. they did not express the activation markers CD40 and CD86, and only low levels of DEC-205 and of CD11b (Fig. 3b). CD8α expression was weaker in 129/Sv mice compared to BALB/c mice, correlating with our observations of the CD11chigh langerin+ subset in skin-draining LN. This dissimilarity for CD8α was also apparent in mesenteric LN (data not shown).
Figure 3.
Langerin expression in spleens from different mouse strains. Spleens from 129/Sv, BALB/c, C57BL/6 and CBA mice were digested with collagenase, and total splenic cells were labelled for flow cytometry analysis. (a) CD11c (y-axes) and intracellular langerin (x-axes) expression. (b) Cell surface expression of CD8α, CD11b, DEC-205, CD40 and CD86 (y-axes), and intracellular langerin (x-axes) expression. Numbers in the upper row indicate the percentage of langerin+ CD8α+ and langerin+ CD8α− cells among total spleen cells. Results are representative of five independent experiments.
Inflammation-induced migration of langerin+ cells to skin-draining lymph nodes
We previously demonstrated that the CD11clow CD8αlow langerin+ population in LN of BALB/c mice is derived from the skin because only this population, but not CD11chigh CD8αhigh langerin+ cells, was labelled in fluorescein isothiocyanate painting experiments.14 To support and extend these observations with regard to possible strain-related differences, we used an alternative model of skin inflammation. Imiquimod is a synthetic ligand for Toll-like receptor 7 (TLR7), which is known to induce LC depletion from the skin when topically applied,28,29 although migration of LC to draining LN was never formally demonstrated with langerin-specific antibodies. Four days after application of imiquimod cream onto the ears of BALB/c or C57BL/6 mice, there was a threefold increase in CD11clow langerin+ cells present in the retro-auricular LN (Fig. 4a). By contrast, we did not observe any significant expansion of the CD11chigh subset.
Figure 4.
Topical skin inflammation increases numbers of CD11clow langerin+ cells in draining lymph nodes (LN). BALB/c or C57BL/6 mice were treated on both ears with 5% imiquimod cream (imiq.) or left untreated (n.t.). After 4 days, retro-auricular LN were harvested and digested with collagenase. Cells were counted and labelled with anti-CD11c and anti-CD86 on their surface, and with anti-langerin intracellularly. (a) Gates (as shown in Fig. 2a) were set to determine the percentage of langerin+ CD11clow and langerin+ CD11chigh cells, and these percentages were multiplied by the total number of LN cells. Bar graphs represent mean ± SEM of three independent experiments. (*P < 0·05; **P < 0·01). (b) Expression of CD86 by langerin+ cells, in the absence or presence of imiquimod treatment. Cells were gated as depicted in Fig. 2a. Numbers indicate the mean fluorescence intensity for CD11clow or CD11chigh cells. Results are representative of three independent experiments.
The dichotomy observed in BALB/c LN parallelled the expression of activation markers. Indeed, CD11chigh langerin+ cells expressed low levels of CD86, whereas CD11clow langerin+ cells displayed high levels of this costimulatory molecule, which is characteristic of mature DC (Fig 4b). Likewise, in C57BL/6 mice, the single CD11clow langerin+ cell subset was also in a phenotypically mature state. Interestingly, in both strains, expression of CD86 was considerably increased upon imiquimod treatment in CD11clow, but not CD11chigh, langerin+ cells (Fig. 4b). In three independent experiments, mean fluorescence intensities for CD86 rose from 177 ± 24 (untreated) to 569 ± 28 (imiquimod-treated) in BALB/c mice, and from 208 ± 53 (untreated) to 431 ± 64 (imiquimod-treated) in C57BL/6 mice. In contrast, CD86 expression on CD11chigh cells from BALB/c mice remained relatively stable (52 ± 10 versus 86 ± 13) in response to imiquimod. These results are consistent with a skin origin for the CD11clow langerin+ LN cells in both BALB/c and C57BL/6 strains.
Differential expression of CD205/DEC-205 in langerin+ cell subsets
To further investigate the origin of langerin+ CD11chigh and CD11clow subsets, we then compared their expression of DEC-205, another C-type lectin receptor. High expression levels of DEC-205 have thus far been considered as a marker of choice to identify and sort skin-derived LC in draining LN, as opposed to DEC-205int dermal DC.27 In peripheral LN of BALB/c mice, where both subsets of langerin+ cells can be observed in parallel, expression of DEC-205 was indeed higher in skin-derived CD11clow cells than in CD11chigh cells (Fig. 5a,b). However, and importantly, a substantial part of the CD11clow subset displayed intermediate levels of DEC-205 (Fig. 5a). Thus, we conclude that, in some mouse strains, DEC-205 alone is not a suitable marker for discrimination of an LC origin in peripheral LN.
Figure 5.
Expression of the C-type lectin DEC-205/CD205 by langerin+ cells in lymphoid organs. Cells from spleen, retro-auricular (skin-draining) or mesenteric lymph nodes (LN) of BALB/c or C57BL/6 mice were labelled with anti-CD11c on their surface, and intracellularly with anti-langerin. (a) Monoclonal antibody NLDC145 was used to detect DEC-205 expression on the cell surface. CD11c+ langerin+ cells were gated as shown in Fig. 2a. (b) Based on fluorescence-activated cell sorter analyses for each population in skin-draining LN, the mean fluorescence intensity of surface DEC-205 on langerin+ cells is depicted in bar graphs. Asterisks denote significant differences between CD11clow and CD11chigh cells: (***P < 0·01). Results represent mean ± SEM of at least five independent experiments.
Discussion
Langerin expression was, for a long time, considered as a hallmark of epidermal LC and skin-derived LC present in LN. However, langerin was recently revealed on a DC subtype14,15 unrelated to skin LC, and appearing in both skin-draining and non-skin-draining lymphoid organs. Our detailed analysis of langerin-expressing cells demonstrates that this subset, best defined as CD11chigh CD8αint/high CD11blow DEC-205low langerin+, is only present in selected mouse strains, i.e. BALB/c and 129/Sv. During the submission process of this article, another group published a comparison of langerin+ cells limited to C57BL/6 and BALB/c mice, and drew conclusions in accordance with our own analysis.30
Differences in the representation of DC populations between mouse strains have been described for the plasmacytoid DC (pDC) subset. Indeed, 129/Sv mice have more pDC in their lymphoid organs compared to C57BL/6 mice, which parallels a higher interferon-α production in response to viral infection.31 This exemplifies how the lack of a DC population with unique properties could result in variable susceptibility to infectious agents in different mouse strains. Indeed, disparities in the responses of BALB/c and C57BL/6 mice have been described in several infectious models, including murine cytomegalovirus,32Toxoplasma gondii,33Yersinia enterocolitica34 and murine gammaherpesvirus.35 For example, BALB/c mice are susceptible to Leishmania major infection, whereas C57BL/6 mice are resistant to this parasite.36 Interestingly, LC are known to be involved in the response against Leishmania, although their exact role is still controversial.37–40
Models of pathogen infection should thus be reconsidered in light of the differences in langerin-expressing cell subsets between these mouse strains. In this respect TLRs are key elements in pathogen recognition by immune cells, and are involved in the early stages of the development of immune responses.41 Recently, it has been shown that murine LC express TLR2, TLR4 and TLR9.42 This particular study was, however, performed in BALB/c mice, and, in view of our present results, it would be of considerable interest to analyse expression of TLRs in the CD11chigh langerin+ population present in the lymphoid organs of this strain.
Further investigations into the properties of langerin+ cells in lymphoid organs could lead to interesting insights on the function of langerin. Indeed, even though this lectin is a highly efficient endocytic receptor, little is known about its role in antigen uptake and presentation. Human langerin has been described as a critical receptor for the uptake of mycobacterial glycolopids and their presentation on CD1a.43 It has recently been described that langerin may function as a natural barrier to human immunodeficiency virus infection of LC and subsequent transmission to T cells by routing the virus into Birbeck granules for degradation.44 In view of these reports, it can be hypothesized that langerin expression could confer DC with functions related to both antigen presentation and innate clearance of pathogen. For instance, it has been proposed that only CD8α+ DC are able to cross-present exogenous antigens to naïve CD8+ T cells, as opposed to CD8α− DC.45–49 Most studies on cross-presentation have been performed with DC from C57BL/6 mice, where these CD8α+ DC are devoid of langerin. In addition, a recent demonstration clearly stated that epidermal langerin+ LC cross-present antigens in vitro and ex vivo.50 Considering the possible role of langerin in antigen uptake, one might then question whether the splenic CD8α+ DC from BALB/c and 129/Sv mice, which include a subpopulation expressing high amounts of langerin, could be more potent than their counterparts from C57BL/6 mice for this particular function.
Finally, our analysis raises concerns regarding the methods usually employed to identify or purify skin-derived DC from lymphoid organs. Indeed, these cells are generally defined as CD11c+ CD11b+ CD8α− DEC-205+, and subtle differences in expression of the endocytic receptor DEC-205 are often considered as sufficient to separate LC (DEC-205high) from dermal DC (DEC-205int).27,51–53 However, even though LC express the highest levels of DEC-205, in some mouse strains, a large proportion of DEC-205int DC does not come from the skin, but rather belong to the CD11chigh langerin+ population. In addition, our results also suggest that selection of skin-derived DC based on weak expression of CD8α may be delusive in mouse strains such as 129/Sv, where both subsets of langerin+ DC had similar levels of this marker. In summary, CD11c+ DC, which underwent maturation during migration from the skin to draining LN, should be defined by their high expression of surface MHC class II and maturation markers CD40 or CD86, thereby excluding the immature DC residing in lymphoid organs.54,55 Then, identification of LC should be preferentially based on langerin rather than on differential expression of DEC-205. This is of particular importance because many transgenic mice are bred on a mixed background between 129/Sv and C57BL/6 strains. Indeed, the LN-resident langerin+ DC population derived from 129/Sv may persist and influence the transgenic phenotype, especially because, in this strain, skin-derived DC are indistinguishable from LN-resident DC when CD8α expression is used as a marker.
In conclusion, we herein describe differences in langerin/CD207 expression by DC, which depend on the mouse strain analysed. Our data highlight that interpretation of immune responses obtained in such mice should be made with respect to inherent heterogeneity in DC subset composition and phenotype. In particular, it would be interesting to examine differences in antigen presentation and susceptibility toward a variety of pathogens in the context of our present findings.
Acknowledgments
We are very grateful to Dr Maria Sibilia and Martina Hammer, who kindly provided us with the CBA and 129/Sv mice. This work was supported by grants from the Austrian Science Fund/FWF (L120-B13 to V.F. and N.R.).
Abbreviations
- DC
dendritic cell
- LC
Langerhans cell
- LN
lymph node
- pDC
plasmacytoid dendritic cell
- TLR
Toll-like receptor
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells – changing views on their function in vivo. Immunol Lett. 2006;106:119–25. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 6.Stambach NS, Taylor ME. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–10. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- 7.Birbeck MS, Breathnach AS, Everall JD. An electron microscopic study of basal melanocytes and high level clear cells (Langerhans cell) in vitiligo. J Invest Dermatol. 1961;37:51–64. [Google Scholar]
- 8.Valladeau J, Dezutter-Dambuyant C, Saeland S. Langerin/CD207 sheds light on formation of Birbeck granules and their possible function in Langerhans cells. Immunol Res. 2003;28:93–107. doi: 10.1385/IR:28:2:93. [DOI] [PubMed] [Google Scholar]
- 9.Wolff K. The fine structure of the Langerhans cell granule. J Cell Biol. 1967;35:468–73. doi: 10.1083/jcb.35.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kissenpfennig A, Aït-Yahia S, Clair-Moninot V, et al. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol Cell Biol. 2005;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor β1 in Langerhans cell biology: The skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 13.Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, et al. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–92. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- 14.Douillard P, Stoitzner P, Tripp CH, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207+ dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–94. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 15.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Juhlin L, Shelley WB. New staining techniques for the Langerhans cell. Acta Derm Venereol Suppl (Stockh) 1977;57:289–96. [PubMed] [Google Scholar]
- 17.Stoitzner P, Holzmann S, McLellan AD, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against langerin/CD207. J Invest Dermatol. 2003;120:266–74. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 18.Koch F, Kämpgen E, Schuler G, Romani N. Isolation, enrichment and culture of murine epidermal Langerhans cells. In: Stagg AJ, Robinson S, editors. Dendritic Cell Protocols. Totowa, NJ: Humana Press; 2001. pp. 43–62. [DOI] [PubMed] [Google Scholar]
- 19.Bergstresser PR, Tigelaar RE, Dees JH, Streilein JW. Thy-1 antigen bearing dendritic cells populate murine epidermis. J Invest Dermatol. 1983;81:286–8. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- 20.Tschachler E, Schuler G, Hutterer J, Leibl H, Wolff K, Stingl G. Expression of Thy-1 antigen by murine epidermal cells. J Invest Dermatol. 1983;81:282–5. doi: 10.1111/1523-1747.ep12518326. [DOI] [PubMed] [Google Scholar]
- 21.Bergstresser PR, Toews GB, Streilein JW. Natural and perturbed distributions of Langerhans cells: responses to ultraviolet light, heterotopic skin grafting, and dinitrofluorobenzene sensitization. J Invest Dermatol. 1980;75:73–7. doi: 10.1111/1523-1747.ep12521261. [DOI] [PubMed] [Google Scholar]
- 22.Bergstresser PR, Fletcher CR, Streilein JW. Surface densities of Langerhans cells in relation to rodent epidermal sites with special immunologic properties. J Invest Dermatol. 1980;74:77–80. doi: 10.1111/1523-1747.ep12519909. [DOI] [PubMed] [Google Scholar]
- 23.Romani N, Stingl G, Tschachler E, Witmer MD, Steinman RM, Shevach EM, Schuler G. The Thy-1 bearing cell of murine epidermis. A distinctive leukocyte perhaps related to natural killer cells. J Exp Med. 1985;161:1368–83. doi: 10.1084/jem.161.6.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–46. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraal G, Breel M, Janse M, Bruin G. Langerhans’ cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–97. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahara K, Omatsu Y, Yashima Y, et al. Identification and expression of mouse langerin (CD207) in dendritic cells. Int Immunol. 2002;14:433–44. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- 27.Henri S, Vremec D, Kamath A, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–8. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 28.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–61. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Wang BH, Shivji GM, Toto P, Amerio P, Tomai MA, Miller RL, Sauder DN. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–41. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 30.Cheong C, Idoyaga J, Do Y, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asselin-Paturel C, Brizard G, Pin JJ, Brière F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 32.Webb JR, Lee SH, Vidal SM. Genetic control of innate immune responses against cytomegalovirus: MCMV meets its match. Genes Immun. 2002;3:250–62. doi: 10.1038/sj.gene.6363876. [DOI] [PubMed] [Google Scholar]
- 33.Lee YH, Kasper LH. Immune responses of different mouse strains after challenge with equivalent lethal doses of Toxoplasma gondii. Parasite. 2004;11:89–97. doi: 10.1051/parasite/200411189. [DOI] [PubMed] [Google Scholar]
- 34.Handley SA, Dube PH, Revell PA, Miller VL. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect Immun. 2004;72:1645–56. doi: 10.1128/IAI.72.3.1645-1656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg JB, Lutzke ML, Alfinito R, Rochford R. Mouse strain differences in the chemokine response to acute lung infection with a murine gammaherpesvirus. Viral Immunol. 2004;17:69–77. doi: 10.1089/088282404322875467. [DOI] [PubMed] [Google Scholar]
- 36.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 37.Blank C, Fuchs H, Rappersberger K, Röllinghoff M, Moll H. Parasitism of epidermal Langerhans cells in experimental cutaneous Leishmaniasis with Leishmania major. J Infect Dis. 1993;167:418–25. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 38.Moll H, Fuchs H, Blank C, Röllinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 39.Ritter U, Osterloh A. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med Microbiol Immunol (Berl) 2007;196:51–9. doi: 10.1007/s00430-006-0023-0. [DOI] [PubMed] [Google Scholar]
- 40.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–31. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 42.Mitsui H, Watanabe T, Saeki H, et al. Differential expression and function of Toll-like receptors in Langerhans cells: comparison with splenic dendritic cells. J Invest Dermatol. 2004;122:95–102. doi: 10.1046/j.0022-202X.2003.22116.x. [DOI] [PubMed] [Google Scholar]
- 43.Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–8. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Witte L, Nabatov A, Pion M, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 45.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 46.Den Haan JMM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–95. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 48.Schnorrer P, Behrens GMN, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–34. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch LS, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA. 2006;103:7783–8. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waithman J, Allan RS, Kosaka H, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179:4535–41. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 52.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 53.Wilson NS, Villadangos JA. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol Cell Biol. 2004;82:91–8. doi: 10.1111/j.1440-1711.2004.01216.x. [DOI] [PubMed] [Google Scholar]
- 54.Wilson NS, El Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–94. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 55.Ruedl C, Koebel P, Karjalainen K. In vivo-matured Langerhans cells continue to take up and process native proteins unlike in vitro-matured counterparts. J Immunol. 2001;166:7178–82. doi: 10.4049/jimmunol.166.12.7178. [DOI] [PubMed] [Google Scholar]