Abstract
Lipocalin allergens, which contain most of the important animal-derived respiratory sensitizers, induce T helper type 2 (Th2) deviation, but the reasons for this are not clear. To explore the prospects for peptide-based allergen immunotherapy and to elucidate the characteristics of the immunodominant epitope of Bos d 2, BALB/c mice were immunized with a peptide containing the epitope, peptides containing its analogues, peptides from the corresponding regions of other lipocalin proteins, and peptides with a homologous sequence. We observed that murine spleen cells recognized the immunodominant epitope of Bos d 2, p127–142, in almost the same way as human Bos d 2-specific T cells did. Enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) analyses showed that p127–142 and a corresponding peptide from horse Equ c 1 induced a Th2-deviated cellular response, whereas a homologous bacterial peptide from Spiroplasma citri induced a Th0-type response. Interestingly, the spleen cell response to the bacterial peptide and p127–142 was cross-reactive, that is, able to induce reciprocally the proliferation and cytokine production of primed spleen cells in vitro. More importantly, the peptides were able to skew the phenotype of T cells primed with the other peptide. Our results suggest that modified peptides can be useful in allergen immunotherapy.
Keywords: allergen peptide, cross-reactivity, immunotherapy, priming, Th2 deviation
Introduction
Lipocalins are proteins that are present in humans,1 so it is noteworthy that almost all important respiratory allergens from mammals belong to this protein family.2 Although the cellular immune response to lipocalin allergens has not yet been fully characterized, it seems that in general it is weak.3–7 This is surprising, as humans mount a strong immunoglobulin E (IgE) response against these allergens.3,8,9 We have proposed that the presence of endogenous lipocalins might be a factor contributing to the T helper type 2 (Th2) deviation of the immune response against exogenous lipocalin allergens.10
In an attempt to unravel the factors involved in the allergenic capacity of lipocalin allergens, we observed that the spleen cell response of BALB/c mice was directed to a single immunodominant epitope in Bos d 2, a bovine lipocalin allergen.4 We had observed earlier that the peptide (p127–142) of the allergen that contained the epitope also contained an epitope recognized by T cells from individuals with cow dust asthma.3 Furthermore, the human T-cell response against the immunodominant epitope was Th2-deviated.3 This finding is consistent with our results with BALB/c mice, as their spleen cell response was interleukin (IL)-4-biased upon in vitro stimulation with the peptide.4 Detailed studies with human T-cell clones later showed that the immunodominant epitope of Bos d 2 was a suboptimal ligand.11 This is perhaps not surprising, as several studies have suggested that suboptimal stimulation through the T-cell receptor (TCR) can favour Th2-deviated immune responses.12–14
Conventional allergen immunotherapy, in which increasing subcutaneous doses of allergen extract are administered, is an efficient method of treating allergy.15,16 An alternative to conventional immunotherapy is to administer allergen peptides containing T-cell epitopes. Peptide treatment decreases the risk of anaphylaxis and circumvents new sensitizations associated with allergen extracts.17–19 Recent clinical trials have shown that peptide-based allergen immunotherapy has potential for treating allergy.19,20
It is still not clear why inert inhaled antigens induce Th2 deviation of the immune response and allergy. The primary purpose of this study was therefore to elucidate the immune characteristics of the dominant T-cell epitope of Bos d 2 in BALB/c mice. Another purpose of the study was to explore the prospects for peptide-based allergen immunotherapy. We report here that recognition of the immunodominant epitope in p127–142 of Bos d 2 by BALB/c mouse spleen cells closely resembled recognition of the immunodominant epitope by human Bos d 2-specific T cells. In particular, p127–142 was able to prime cells for a Th2-biased cellular response, whereas a homologous peptide from Spiroplasma citri favoured a more balanced response. Interestingly, p127–142 and the peptide from S. citri were able to prime spleen cells reciprocally for proliferation and to modify cytokine production of cells primed with the other peptide. Our results suggest that the phenotype of Th2-primed splenic T cells can be modulated by modified allergen peptides.
Materials and methods
Immunization of mice
Female BALB/c, C57BL/6, and CBA mice (8 weeks old) were obtained from Taconic M & B A/S (Ry, Denmark). The mice were maintained under pathogen-free conditions throughout the study. Three to five mice in each group were injected once subcutaneously (s.c.) at the base of the tail with peptides (0·005 µmol) in complete Freund's adjuvant (CFA; Sigma, St Louis, MO; F-5881). Control mice were treated with phosphate-buffered saline (PBS) or CFA. Experiments were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg 18 March 1986, adopted in Finland 31 May 1990). The study was approved by the Animal Care and Use Committee of the University of Kuopio.
Synthetic peptides
The Bos d 2 peptide p127–142 (ELEKYQQLNSERGVPN), its 16-mer analogues, truncated derivatives, and other peptides described below were synthesized using a PerSeptive 900 Plus automated peptide synthesizer (Millipore, Bedford, MA) with a Fmoc (N-[9-fluorenyl]methoxycarbonyl) strategy. The peptides were purified by high-performance liquid chromatography (HPLC) and confirmed by mass spectrometry. The peptides were dissolved in sterile PBS, filtered through a 0·2-μm filter, and handled aseptically throughout. Single amino acid substitutions (conservative and non-conservative, including 16 alanine and 18 other amino acid substitutions) in the peptide analogues were introduced in all positions. The sequences for the peptides SP1–SP9 homologous to p127–142 (SP; database search peptide) were obtained from the Swiss-Prot protein sequence database (http://www.expasy.ch/sprot or http://www.expasy.ch/cgi-bin/list?allergen.txt) using the program FindPatterns (Accelrys Ltd, San Diego, CA), whereas the sequences for the peptides SP10–SP20 corresponding to p127–142 were obtained by sequence homology alignments with lipocalin allergens (from dog Can f 1 and Can f 2, horse Equ c 1, cow Bos d 5, cockroach Bla g 4, mouse Mus m 1, and rat Rat n 1), mouse non-allergen lipocalins and human lipocalins from the same database using the program BestFit (Accelrys Ltd), as described previously.11
Proliferation assays
The spleens were removed from the mice 10 days after immunization and prepared as a single-cell suspension. The culture medium was Dulbecco's modified Eagle's minimal essential medium (DMEM; BioWhittaker, Walkersville, MD) supplemented with 2 mm l-glutamine (Gibco, Grand Island, NY), 50 µm 2-mercaptoethanol (2-ME), 1 mm sodium pyruvate (BioWhittaker), 10 mm HEPES (BioWhittaker), 100 IU/ml penicillin (Orion, Espoo, Finland), 100 µg/ml streptomycin (Sigma), and 10% inactivated fetal calf serum (Biological Industries, Beit Haemek, Israel). Proliferation tests were performed as described previously.4 In brief, 2 × 105 cells/well were incubated in triplicate in 96-well round-bottomed microtitre plates (Corning, Acton, MA) with antigens at 0·5–50 µm for 3 days in a humidified 5% CO2 incubator at 37° and pulsed for 16 hr with 0·5 µCi/well [3H]thymidine (Amersham, Little Chalfont, UK). The radionuclide uptake was measured by scintillation counting and the results expressed as a stimulation index [SI; the ratio of mean counts per minute (c.p.m.) in cultures with stimulant to mean c.p.m. in cultures without stimulant].
Determination of restriction by major histocompatibility complex (MHC)
CD4-positive T cells were separated from the spleen cell suspension by the SpinSep™ Cell Enrichment Method (StemCell Technologies, Vancouver, Canada) according to the protocol of the manufacturer. The purity of CD4+ T cells determined by flow cytometry was > 95% after two purification cycles (data not shown). The CD4+ cells (1 × 105 cells/well) were cultured in triplicate in 96-well round-bottomed microtitre plates with γ-irradiated (6000 rad) I-Ad- or I-Ed-transfected fibroblasts [LA(d) and LE(d)]20 as feeder cells (0·5 × 105 cells/well). The p127–142 concentration ranged from 0·5 to 50 µm. The proliferative response was measured as described above.
Binding of peptides to I-Ador I-Ed
The competitive MHC II–peptide binding assay was performed as previously described.21 Briefly, the purified I-Ad molecules were incubated with serial dilutions of competitor peptides and the biotinylated MYO 106–118 peptide while the I-Ed molecules were incubated with serial dilutions of competitor peptides and the biotinylated HA 306–318 peptide. The samples were incubated for 24 or 72 hr for I-Ad or I-Ed, respectively. After neutralization the samples were incubated for 2 hr at room temperature on plates coated with 10 µg/ml MKD6 [monoclonal antibody (mAb) for I-Ad] and 14.4.4S (for I-Ed). The bound biotinylated peptide was detected using a streptavidin-alkaline-phosphatase conjugate (Amersham) and 4-methylumbelliferyl phosphate substrate (Sigma). Emitted fluorescence was measured at 450 nm upon excitation at 365 nm. Maximal binding was determined by incubating the biotinylated peptide with the MHC II molecule in the absence of a competitor. The results were expressed as the peptide concentration that prevented binding of 50% of the labelled peptide (IC50).
Enzyme-linked immunosorbent spot-forming cell assay (ELISPOT)
The ELISPOT analyses of cytokine production by spleen cells were performed as described previously.5 Briefly, ELISPOT plates (Cellular Technology Ltd, Cleveland, OH) were coated with anti-mouse IL-4 or interferon (IFN)-γ monoclonal antibodies (Pharmingen, San Diego, CA) at 4 µg/ml in PBS overnight at 4°. The medium used was HL-1 (BioWhittaker) supplemented with 2 mm l-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin. Spleen cells at a density of 106 cells/well (0·2 ml) were then added in duplicate in plates with peptides at concentrations ranging from 0·5 to 50 µm. After incubation of the cells for 24 hr (IFN-γ) or 48 hr (IL-4) in a humidified 5% CO2 incubator at 37°, biotinylated monoclonal antibodies to IL-4 or IFN-γ (Pharmingen) were added to the plates at 4 µg/ml. Incubation was overnight at 4°. Next, a streptavidin-horseradish peroxidase (HRP) conjugate (diluted 1 : 2000; Dako A/S, Glostrup, Denmark) was added and the plates were incubated for 2 hr at room temperature. The colour reaction was allowed to develop at room temperature for 1 hr using a solution containing 800 µl of 3-amino-9-ethyl carbazole [Sigma; 100 mg dissolved in 10 ml of N-N-dimethylformamide (Merck, Darmstadt, Germany)], 24 ml of 0·1 m sodium acetate, pH 5·0, and 12 µl of 30% H2O2 (Riedel-de Haën, Seelze, Germany). The spots were counted under a stereomicroscope.
Results
The anatomy of the immunodominant epitope of Bos d 2
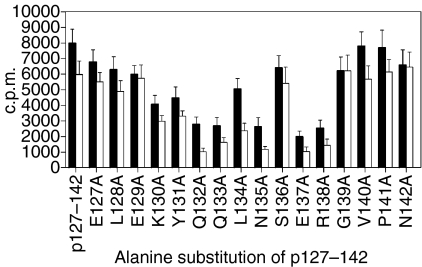
To examine the recognition of the immunodominant T-cell epitope of Bos d 2, the BALB/c mice were immunized with peptide p127–142 containing the epitope. The proliferative responses of spleen cells from primed mice upon in vitro stimulation with the alanine-substituted analogues of p127–142 are shown in Fig. 1. The central part of p127–142 was observed to be important for the recognition of the peptide. The residues Q132, Q133, N135, E137 and R138 tolerated notably fewer substitutions by alanine (Fig. 1) or other amino acids, classified as conservative or semiconservative22 (data not shown), suggesting their importance for T-cell recognition. The probable MHC anchor amino acids, Y131, L134, S136 and G139 (see below), were less sensitive to substitution as their replacement with conservative amino acids did not abrogate the response.
Figure 1.
Proliferative responses of spleen cells from BALB/c mice primed with p127–142 of Bos d 2 upon stimulation with its alanine-substituted analogues. The results are presented as mean counts per minute (c.p.m.) (± standard error of the mean) for four independent experiments. The peptide concentrations were 15 µm (solid bars) and 1·5 µm (open bars). The medium background has been subtracted (< 1400 c.p.m. in all tests).
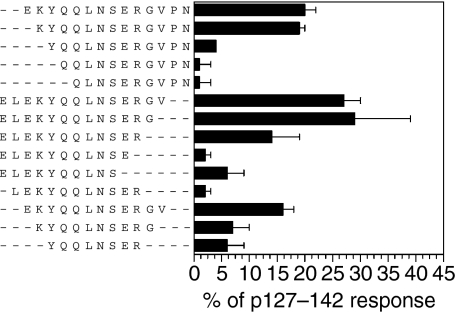
To determine more precisely the epitope core of the peptide p127–142, the spleen cell responses of p127–142-primed BALB/c mice were examined with truncated analogues of the peptide (Fig. 2). It was observed that the responsiveness to the analogues was very sensitive to the deletion of the terminal amino acids of the peptide, as removing four amino acids from the N-terminus (including K130) or four from the C-terminus (including G139) strongly reduced the response. When an analogue truncated at both ends by three amino acids (K130–G139) was used for stimulation, the response was at the background level. These results, together with the MHC binding analyses (see below), suggest that the critical amino acids for recognition by T cells from BALB/c mice are between K130 and G139.
Figure 2.
Proliferative responses of spleen cells from BALB/c mice primed with p127–142 upon stimulation with the truncated analogues of the peptide. The results are expressed as mean percentages (± standard error of the mean) of the response to p127–142 for three independent experiments. The peptide concentration was 15 µm. The proliferative response to p127–142 was > 2100 counts per minute (c.p.m.) and the background was < 885 c.p.m. in all tests.
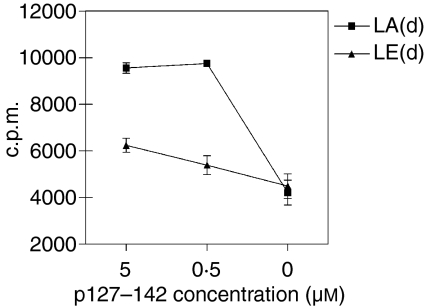
The MHC molecule restricting the response to p127–142 was determined with purified spleen T cells and I-Ad- or I-Ed-transfected fibroblasts as antigen-presenting cells. As shown in Fig. 3, I-Ad-transfected fibroblasts had a superior capacity to present the peptide to T cells. To confirm this finding, the binding of p127–142 and its analogues to I-Ad and I-Ed was measured. I-Ed had no capacity to bind p127–142 at all (data not shown), while I-Ad bound the peptide (Table 1). The results obtained with the peptide analogues of p127–142 indicate that Y131 is the MHC anchor amino acid at position 1 (Table 1). The amino acids of p127–142 at positions 1 and 4 are in accordance with the motif reported for I-Ad (Database Syfpeithi; http://www.syfpeithi.de/).
Figure 3.
Proliferative responses of purified spleen T cells (1 × 105 cells/well) from BALB/c mice with I-Ad or I-Ed-transfected fibroblasts as feeder cells [LA(d) and LE(d), respectively; 0·5 × 105 cells/well] upon stimulation with p127–142. The results are presented as mean counts per minute (c.p.m.) ± standard error of the mean for three independent experiments.
Table 1.
Binding of p127–142 and its analogues with notably reduced binding capacity to I-Ad
| Peptide | IC50 ratio | IC50 |
|---|---|---|
| P127-142 | 1·0 | 3·5 ± 7·07 × 10−6 |
| 1·0 | 8·5 ± 2·12 × 10−6a | |
| Y131F | 2·6 | 9·0 ± 0·0 × 10−6 |
| L134A | 5·3 | 4·5 ± 0·71 × 10−5 |
| L134D | > 28 | > 1·0 ± 0·0 × 10−4 |
| S136Y | > 28 | > 1·0 ± 0·0 × 10−4 |
| G139Y | 11·4 | 4·0 ± 0·0 × 10−5 |
The binding of 34 analogues was measured. Analogues other than those shown in the table did not exhibit a notable reduction (IC50 < 2·5) in their binding (data not shown). IC50 values are expressed in nm (mean ± standard error of the mean) for two independent experiments. IC50 ratios are presented in comparison with p127–142.
The control IC50 value for alanine mutants.
IC50, the peptide concentration that prevented binding of 50% of the labelled peptide.
Crossreactivity of p127–142-recognizing spleen cells
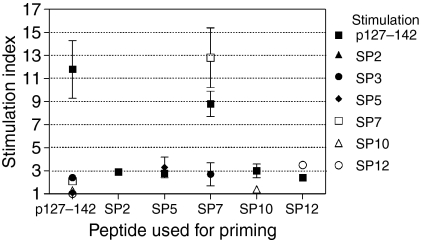
To examine the potential cross-reactivity of p127–142-recognizing T cells, the spleen cells of p127–142-primed BALB/c mice were stimulated in vitro with p127–142, database search peptides SP1–SP9 or lipocalin peptides SP10–SP20 at 50 µm. In repeated experiments, only SP3 (Bacillus subtilis; VLELIQQLNRERGITF) and SP7 (S. citri; HVIEVQQINSERSWFF) were able to give rise to a proliferative response, but at a low level (SI 2·4 ± 0·3 and 2·1 ± 0·2, respectively) in comparison with p127–142 (11·8 ± 2·5).
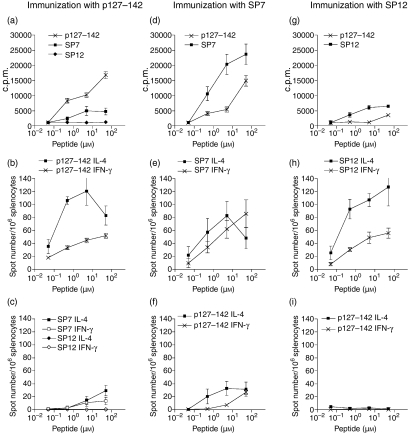
When the mice were primed with SP peptides, it was observed that most of them were not immunogenic: only SP4 (human 5-HT-2C; PRGTMQAINNERKASK), SP5 (human nestin; VRLELQQLQAERGGLL), and SP7 (HVIEVQQINSERSWFF) from non-lipocalin peptides and lipocalin peptide SP12 (horse Equ c 1; IKEEFVKIVQKRGIVK) elicited a response upon in vitro stimulation at 50 µm (SI > 2; at least three independent experiments; Figs 4, 5d and 5g, and data not shown). Of these peptides, only SP7 induced a response comparable with that obtained with p127–142 (Figs 4 and 5d).
Figure 4.
Proliferative responses of spleen cells from BALB/c mice primed with p127–142 or peptides obtained through database searches upon in vitro stimulation. The peptide concentration was 50 µm. The results are expressed as mean stimulation index (± standard error of the mean) for three independent experiments. The background proliferation in wells without a stimulant was < 950 counts per minute in all experiments.
Figure 5.
Proliferative responses and cytokine production of spleen cells from BALB/c mice primed with p127–142, SP7 or SP12 upon in vitro stimulation. The cytokine production analysed using the enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) is expressed as mean spot number/106 spleen cells [± standard error of the mean (SEM)] and the proliferation as mean counts per minute (c.p.m.) (± SEM) for at least four independent experiments. In each experiment, three to five mice per peptide group were primed. The spot numbers in the unstimulated cultures remained < 4 spots/106 spleen cells in all experiments (data not shown).
Stimulation of the cells of SP7-primed mice in vitro with p127–142 induced a strong cross-reactive response (Figs 4 and 5d). In general, however, priming with the immunogenic SP peptides (SP4, SP5, SP7 and SP12) did not generate distinct cross-reactive T-cell responses (SI > 4; Fig. 4 and data not shown) upon stimulation with the other SP peptides or p127–142. Furthermore, p127–142 was able to induce low-level proliferation of spleen cells of unprimed (Fig. 6) and SP-primed mice (Figs 4 and 5g, and data not shown) (excluding SP9-primed mice) (SI range 2·2–3·2) at the highest stimulation dose used (50 µm). Importantly, the phenomenon was observed with two independently synthesized lots of p127–142 (data not shown) and was not seen with other peptides (e.g. SP12 in Fig. 5a, and data not shown).
Figure 6.
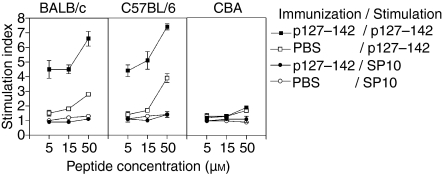
Proliferative responses of spleen cells from three mouse strains (BALB/c, C57BL/6 and CBA) primed with p127–142 in Freund's adjuvant (CFA) or treated with phosphate-buffered saline (PBS) upon stimulation with p127–142 (or SP10). Results are presented as mean stimulation index (± standard error of the mean) for two independent experiments. The background levels were < 2900 counts per minute (c.p.m.) for BALB/c mice, < 1000 c.p.m. for C57BL/6 mice and < 3300 c.p.m. for CBA mice in all experiments.
Considering that the capacity of p127–142 to induce the low-level proliferation of spleen cells from unprimed BALB/c mice (at a high peptide concentration) was related to the mouse strain, three strains were examined more in detail. As previously observed, the spleen cells from PBS-treated BALB/c mice proliferated dose-dependently upon stimulation with the peptide in vitro (Fig. 6). Interestingly, the cells of C57BL/6 mice immunized with p127–142 specifically recognized p127–142, although this strain was previously observed to be a non-responder to recombinant Bos d 2.4 Moreover, the spleen cells of PBS-treated C57BL/6 mice reacted to the peptide. One of the strains, CBA, previously found to be a non-responder to Bos d 2,4 did not mount a spleen cell response to p127–142. Furthermore, the response of PBS-treated CBA mice was also at the background level. A control peptide, SP10, found to be non-immunogenic in BALB/c mice (data not shown), did not elicit responses in any of the three mouse strains upon in vitro stimulation (Fig. 6).
Peptide-induced production of IFN-γ and IL-4 by spleen cells
To analyse the capacity of the peptides to modulate the Th1–Th2 balance of the immune response, the IFN-γ and IL-4 secretion induced by SP7 and SP12 was compared with that induced by p127–142 (Fig. 5). SP7 induced the distinctive cross-reactive proliferative response of spleen cells of BALB/c mice primed with p127–142, whereas SP12 had weak immunogenicity (Figs 4 and 5g).
ELISPOT analyses revealed that the responses induced by p127–142 and SP12 resembled each other in that they were IL-4-dominant (Figs 5b and h). This is of interest as there was a distinct difference in their capacity to induce lymphocyte proliferation (Figs 5a and g). In contrast, SP7, which was able to elicit the strongest proliferative response of spleen cells (Fig. 5d), gave rise to a Th0-type response (Fig. 5e). The ratio of IL-4/IFN-γ, at a concentration of 5 µm, for example, was 3·1 ± 0·4 with p127–142, while it was 1·9 ± 0·6 with SP7 (Figs 5b and e).
The peptides p127–142 and SP7 could prime spleen cells reciprocally for cytokine production (Figs 5c and f). Moreover, in vitro stimulation with the peptides appeared to be able to modify the cytokine responses of the primed spleen cells. For example, when the spleen cells from mice immunized with p127–142 were stimulated with SP7, the outcome of the response (Fig. 5c) resembled that generated when both priming and in vitro stimulation were performed with SP7 (Fig. 5e). Accordingly, the stimulation of spleen cells from SP7-primed mice with p127–142 resulted in a cytokine response (Fig. 5f) resembling the response generated when cells from mice were both primed and in vitro stimulated with p127–142 (Fig. 5b). The IL-4-deviating capacity of p127–142 was most obvious at lower peptide concentrations, independently of the priming peptide (Figs 5b and f).
Discussion
To explore the prospects for peptide-based allergen immunotherapy, we have characterized here in BALB/c mice the immunodominant epitope of Bos d 2 and the corresponding areas in other lipocalins. We have previously shown that BALB/c mice recognize one immunodominant epitope in the lipocalin allergen Bos d 2.4 The epitope is identical to that recognized by human Bos d 2-specific T cells in that it co-localizes in p127–142 of the allergen.3 Furthermore, the deletion of four amino acids from the ends of the peptide resulted in the abrogation or a strong reduction of the response with both a human T-cell clone11 and the spleen cells of BALB/c mice (Fig. 2). It is also noteworthy that the critical amino acids for the recognition by the T cells of BALB/c mice (Fig. 1 and data not shown) and the human T-cell clone11 co-localized closely.
We have observed previously with human T cells that Bos d 2 contains a limited number of epitopes.3 As human T cells recognize a determinant in the corresponding area in two other lipocalin allergens, rat Rat n 16 and dog Can f 1,23 we wished to examine whether BALB/c mice would recognize this area in lipocalins in general. This turned out not to be the case, as only one peptide, SP12 from horse Equ c 1, out of 11 lipocalin peptides was immunogenic in BALB/c mice. In contrast, four non-lipocalin peptides out of nine peptides homologous to p127–142 elicited a cellular response. While several factors, including antigen processing and the T-cell repertoire, can account for the weak immunogenicity of the lipocalin peptides, it seems that the phenomenon reflects the poor stimulatory capacity of lipocalins, as observed with Bos d 2,3,4 Rat n 1,6 Can f 124 and Equ c 1.7
Our previous study suggested that the allergenic capacity of Bos d 2 is associated with the response to its immunodominant epitope.4 The present results support this view, as the spleen cell response to p127–142 was IL-4-dominated while the response to SP7 was balanced (Figs 5b and e). Out of the three peptides studied in more detail, SP7 exhibited the strongest effect in terms of proliferation in vitro (Figs 5a, d and g). In contrast, SP12, a peptide from a lipocalin allergen, Equ c 1, induced the weakest proliferative response of the three peptides (Fig. 5g) and elicited an IL-4-biased response (Fig. 5h) similar to that induced by p127–142. These results are in accordance with findings that suggest that weak stimulation through the TCR,13,14 low antigen dose25–27 or low affinity of the TCR–MHC interaction,28 favours a Th2-biased outcome of the cellular immune response.
We did not consider the question of whether the lower stimulatory potential of p127–142 compared with SP7 is primarily dependent on T-cell recognition or MHC binding. However, if we assume that the core of the epitope in SP7 is in the middle of the peptide, as it is in p127–142 (Figs 1 and 2), and the cellular response against it is I-Ad-restricted, as it is with p127–142, it appears that MHC binding contributes to the weaker response to p127–142, because only the probable MHC anchor amino acids at positions P1 and P4 differ between the cores of the two peptides. We cannot rule out the possibility, however, that the response to SP7 is restricted by I-Ed, as the peptide contains two binding motifs for the allele (Database Syfpeithi; http://www.syfpeithi.de/). Similarly, the response against SP12 (Equ c 1) can be restricted by both alleles. Nevertheless, our previous results provide evidence that weak stimulation through the TCR can be one factor determining the allergenic capacity of a protein.10,11
BALB/c mice did not exhibit T-cell cross-reactivity between the lipocalin-derived peptides corresponding to p127–142 of Bos d 2. This is not surprising, as the peptide sequences differed considerably (Table 4 in Kinnunen et al.11). In contrast, when BALB/c mice were primed with p127–142 and the spleen cells were then stimulated in vitro with two non-lipocalin-derived peptides (SP3 and SP7) homologous to p127–142, low proliferative responses were produced repeatedly. This can probably be ascribed to the complete identity of the probable TCR contact amino acids in the core region of the peptides.
Our findings regarding T-cell cross-reactivity between p127–142 and SP7 (Figs 5a–f) are of special interest as it was obvious that the peptides could reciprocally prime for spleen cell proliferation and cytokine production. This suggests that a determinant from a protein not related to the allergen could prime cells for a Th2-biased response to the allergenic determinant (Fig. 5f). As discussed above, the requirement for the priming was probably the considerable homology between the peptides. It can be speculated that such homologies can be encountered randomly or they may be related to the common biological background of the proteins. It seems that in autoimmune diseases, such as in type 1 diabetes or multiple sclerosis, T cells cross-react with several homologous peptides of infectious pathogens.15,29,30
Our findings also suggest that the allergen-specific T-cell response may be modulated by a cross-reactive peptide that is more stimulatory than the natural peptide. We previously observed that with human T-cell clones the altered peptide ligands of the immunodominant epitope of Bos d 2 (p127–142) favoured Th1-biased cytokine responses.11 In a murine asthma model, the Th1-skewing peptide analogue of a dominant allergen epitope was observed to modulate favourably the polarized Th2 response in vitro and in vivo.13 Recently, we reported that an altered allergen peptide was able to induce novel Th1-biased T-cell populations from human peripheral blood mononuclear cells in vitro.31 As the T-cell populations also recognized the native peptide, it can be speculated that altered allergen peptides could modify the Th2-dominated response upon allergen encounter in vivo, for example, through bystander suppression.31 In autoimmune diseases, such as multiple sclerosis and type 1 diabetes, the altered peptide ligands have been shown to induce a new Th2-deviated cell population in vitro and in vivo, which dampens the pathogenic Th1 responses by modulating the cytokine environment.32,33 Therefore, if it is possible in general to find altered analogue peptides for allergen-specific T cells, it may prove feasible to use these peptides as an alternative to conventional allergen immunotherapy.31,34,35 In human autoimmune diseases, trials with Th2-skewing altered peptide ligands have already been performed.36–38
In addition to cross-reactivity with SP7, we found that p127–142 was able to induce proliferation of spleen cells of unprimed BALB/c mice in vitro (Fig. 6). The phenomenon was observed repeatedly with two independently synthesized lots of peptide but not with other peptides. No signs of contaminants, for example endotoxins, were detected previously in human T-cells studies or in mass spectrometric analysis of the peptides. As the phenomenon was also observed with the spleen cells of C57BL/6 mice, it is reasonable to assume that it is characteristic for p127–142 in these two mouse strains. It is possible that the property is related to the immunodominance of the epitope it contains. This view is supported by a previous observation that the T cells of unprimed SJL mice exhibited proliferation with the peptide 139–151 of myelin proteolipid protein (PLP),39 an immunodominant epitope of the autoantigen in experimental autoimmune encephalomyelitis.40–42 The immunodominance of PLP 139–151 appeared to be attributable to the presence of expanded numbers of T cells reactive to the epitope in the peripheral repertoire of the unimmunized SJL mice.39,43 Similarly, cells of healthy individuals have been observed to recognize an epitope (amino acids 61–79) of myelin basic protein (MBP) which is immunodominant for multiple sclerosis patients.44 Escape from central tolerance, combined with peripheral expansion by cross-reactive antigen(s), is thought to be responsible for the high frequency of PLP 139–151-reactive T cells in SJL mice.39 On the basis of the present data, however, it is not possible to conclude whether an analogous mechanism would account for the proliferation of naive spleen cells of BALB/c mice upon stimulation with p127–142.
To summarize, our results show that recognition of the immunodominant epitope of Bos d 2 by BALB/c mice exhibits similarities to recognition by a human Bos d 2-specific T-cell clone. Interestingly, the spleen cells primed with p127–142 cross-reacted with a bacterial (S. citri) peptide, and the peptides were reciprocally able to induce proliferation and cytokine production in vitro. On the one hand, this finding suggests the possibility that priming for a Th2 response against an allergen can be induced by an antigen unrelated to the allergen or by a homologue of an allergen. On the other hand, our results suggest that modified allergen peptides, which are more stimulatory than natural peptides, can skew the phenotype of primed T cells. The use of such peptides may open up new possibilities for allergen immunotherapy.
Acknowledgments
We thank Virpi Fisk and Kirsi Lehikoinen for skilful technical assistance. The work was financially supported by Kuopio University Hospital (Project 5021605), the Academy of Finland (Contracts 48657 and 205871), the Tuberculosis Foundation of Tampere and the Finnish-Norwegian Foundation of Medicine.
References
- 1.Åkerstrom B, Flower DR, Salier JP. Lipocalins. Unity in diversity. Biochim Biophys Acta. 2000;1482:1–8. doi: 10.1016/s0167-4838(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen T, Zeiler T, Mäntyjärvi R. Important animal allergens are lipocalin proteins: Why are they allergenic? Int Arch Allergy Immunol. 1999;120:247–58. doi: 10.1159/000024277. [DOI] [PubMed] [Google Scholar]
- 3.Zeiler T, Mäntyjärvi R, Rautiainen J, Rytkönen-Nissinen M, Vilja P, Taivainen A, Kauppinen J, Virtanen T. T cell epitopes of a lipocalin allergen colocalize with the conserved regions of the molecule. J Immunol. 1999;162:1415–22. [PubMed] [Google Scholar]
- 4.Saarelainen S, Zeiler T, Rautiainen J, Närvänen A, Rytkönen-Nissinen M, Mäntyjärvi R, Vilja P, Virtanen T. Lipocalin allergen Bos d 2 is a weak immunogen. Int Immunol. 2002;14:401–9. doi: 10.1093/intimm/14.4.401. [DOI] [PubMed] [Google Scholar]
- 5.Immonen A, Saarelainen S, Rautiainen J, Rytkönen-Nissinen M, Kinnunen T, Mäntyjärvi R, Virtanen T. Probing the mechanisms of low immunogenicity of a lipocalin allergen, Bos d 2, in a mouse model. Clin Exp Allergy. 2003;33:834–41. [PubMed] [Google Scholar]
- 6.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Determination of the T cell epitopes of the lipocalin allergen, Rat n 1. Clin Exp Allergy. 2004;34:1919–25. doi: 10.1111/j.1365-2222.2004.02126.x. [DOI] [PubMed] [Google Scholar]
- 7.Immonen AK, Kinnunen TT, Sirven P, et al. The major horse allergen Equ c 1 contains one immunodominant region of T cell epitopes. Clin Exp Allergy. 2007;37:939–47. doi: 10.1111/j.1365-2222.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 8.Saarelainen S, Taivainen A, Rytkönen-Nissinen M, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004;34:1576–82. doi: 10.1111/j.1365-2222.2004.02071.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith W, Butler AJ, Hazell LA, Chapman MD, Pomes A, Nickels DG, Thomas WR. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–8. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen T, Zeiler T, Rautiainen J, Mäntyjärvi R. Allergy to lipocalins. A consequence of misguided T-cell recognition of self and nonself? Immunol Today. 1999;20:398–400. doi: 10.1016/s0167-5699(99)01515-7. [DOI] [PubMed] [Google Scholar]
- 11.Kinnunen T, Buhot C, Närvänen A, et al. The immunodominant epitope of lipocalin allergen Bos d 2 is suboptimal for human T cells. Eur J Immunol. 2003;33:1717–26. doi: 10.1002/eji.200322952. [DOI] [PubMed] [Google Scholar]
- 12.Badou A, Savignac M, Moreau M, et al. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur J Immunol. 2001;31:2487–96. doi: 10.1002/1521-4141(200108)31:8<2487::aid-immu2487>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Janssen EM, van Oosterhout AJ, van Rensen AJ, van Eden W, Nijkamp FP, Wauben MH. Modulation of Th2 responses by peptide analogues in a murine model of allergic asthma: Amelioration or deterioration of the disease process depends on the Th1 or Th2 skewing characteristics of the therapeutic peptide. J Immunol. 2000;164:580–8. doi: 10.4049/jimmunol.164.2.580. [DOI] [PubMed] [Google Scholar]
- 14.Brogdon JL, Leitenberg D, Bottomly K. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J Immunol. 2002;168:3825–32. doi: 10.4049/jimmunol.168.8.3825. [DOI] [PubMed] [Google Scholar]
- 15.Ellis RJ, Varela-Calvino R, Tree TI, Peakman M. HLA class II molecules on haplotypes associated with type 1 diabetes exhibit similar patterns of binding affinities for coxsackievirus P2C peptides. Immunology. 2005;116:337–46. doi: 10.1111/j.1365-2567.2005.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannini M, Braccioni F, Sella G, Contoli M, Perri G, Frati F, Incorvaia C. Comparison of allergen immunotherapy and drug treatment in seasonal rhinoconjunctivitis: a 3-years study. Allerg Immunol (Paris) 2005;37:69–71. [PubMed] [Google Scholar]
- 17.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 18.Westritschnig K, Focke M, Verdino P, et al. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, phl p 7. J Immunol. 2004;172:5684–92. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- 19.Alexander C, Tarzi M, Larche M, Kay AB. The effect of fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005;60:1269–74. doi: 10.1111/j.1398-9995.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 20.Tarzi M, Klunker S, Texier C, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–74. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 21.Texier C, Herve M, Pouvelle S, Menez A, Maillere B. On the diversity and heterogeneity of H-2(d)-restricted determinants and T cell epitopes from the major bee venom allergen. Int Immunol. 1999;11:1313–26. doi: 10.1093/intimm/11.8.1313. [DOI] [PubMed] [Google Scholar]
- 22.Tangri S, Ishioka GY, Huang X, Sidney J, Southwood S, Fikes J, Sette A. Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide. J Exp Med. 2001;194:833–46. doi: 10.1084/jem.194.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Immonen A, Farci S, Taivainen A, et al. T cell epitope-containing peptides of the major dog allergen Can f 1 as candidates for allergen immunotherapy. J Immunol. 2005;175:3614–20. doi: 10.4049/jimmunol.175.6.3614. [DOI] [PubMed] [Google Scholar]
- 24.Kinnunen T, Taivainen A, Partanen J, Immonen A, Saarelainen S, Rytkönen-Nissinen M, Rautiainen J, Virtanen T. The DR4-DQ8 haplotype and a specific T cell receptor vbeta T cell subset are associated with absence of allergy to Can f 1. Clin Exp Allergy. 2005;35:797–803. doi: 10.1111/j.1365-2222.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 25.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foucras G, Gallard A, Coureau C, Kanellopoulos JM, Guery JC. Chronic soluble antigen sensitization primes a unique memory/effector T cell repertoire associated with th2 phenotype acquisition in vivo. J Immunol. 2002;168:179–87. doi: 10.4049/jimmunol.168.1.179. [DOI] [PubMed] [Google Scholar]
- 27.Guery JC, Galbiati F, Smiroldo S, Adorini L. Selective development of T helper (th) 2 cells induced by continuous administration of low dose soluble proteins to normal and beta(2)-microglobulin-deficient BALB/c mice. J Exp Med. 1996;183:485–97. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995;181:1569–74. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croxford JL, Ercolini AM, Degutes M, Miller SD. Structural requirements for initiation of cross-reactivity and CNS autoimmunity with a PLP139-151 mimic peptide derived from murine hepatitis virus. Eur J Immunol. 2006;36:2671–80. doi: 10.1002/eji.200635876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ercolini AM, Ludovic Croxford J, Degutes M, Miller SD. Cross-reactivity between peptide mimics of the immunodominant myelin proteolipid protein epitope PLP139-151: Comparison of peptide priming in CFA vs. viral delivery. J Neuroimmunol. 2007;186:5–18. doi: 10.1016/j.jneuroim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Kinnunen T, Jutila K, Kwok WW, et al. Potential of an altered peptide ligand of lipocalin allergen Bos d 2 for peptide immunotherapy. J Allergy Clin Immunol. 2007;119:965–72. doi: 10.1016/j.jaci.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: result of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 33.Alleva DG, Gaur A, Jin L, et al. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immudominant type 1 diabetes autoantigen insulin B-chain (9–23) peptide. Diabetes. 2002;5:2126–34. doi: 10.2337/diabetes.51.7.2126. [DOI] [PubMed] [Google Scholar]
- 34.Ise W, Totsuka M, Takato R, et al. Primary response of naive CD4(+) T cells to amino acid-substituted analogs of an antigenic peptide can show distinct activation patterns: Th1- and Th2-type cytokine secretion, and helper activity for antibody production without apparent cytokine secretion. FEBS Lett. 2000;465:28–33. doi: 10.1016/s0014-5793(99)01716-0. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe S, Shibata R, Nishimura T. Hypoallergenic and T cell reactive analogue peptides of bovine serum albumin, the major beef allergen. Mol Immunol. 2004;41:885–90. doi: 10.1016/j.molimm.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Alleva DG, Maki RA, Putnam AL, et al. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol. 2006;63:59–69. doi: 10.1111/j.1365-3083.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Antel JP, Duquette P, Alleva DG, Conlon PJ, Bar-Or A. Persistence of immune responses to altered and native myelin antigens in patients with multiple sclerosis treated with altered peptide ligand. Clin Immunol. 2002;104:105–14. doi: 10.1006/clim.2002.5258. [DOI] [PubMed] [Google Scholar]
- 38.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 39.Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: Mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–70. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson JK, Croxford JL, Calenoff MA, Dal Canto MC, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. J Clin Invest. 2001;108:311–8. doi: 10.1172/JCI13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voskuhl RR, Farris RW 2nd, Nagasato K, McFarland HF, Dalcq MD. Epitope spreading occurs in active but not passive EAE induced by myelin basic protein. J Neuroimmunol. 1996;70:103–11. doi: 10.1016/s0165-5728(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 42.Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139–151. J Neurosci Res. 1996;45:680–9. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Anderson AC, Reddy J, Nazareno R, Sobel RA, Nicholson LB, Kuchroo VK. IL-10 plays an important role in the homeostatic regulation of the autoreactive repertoire in naive mice. J Immunol. 2004;173:828–34. doi: 10.4049/jimmunol.173.2.828. [DOI] [PubMed] [Google Scholar]
- 44.Tejada-Simon MV, Hong J, Rivera VM, Zhang JZ. Reactivity pattern and cytokine profile of T cells primed by myelin peptides in multiple sclerosis and healthy individuals. Eur J Immunol. 2001;31:907–17. doi: 10.1002/1521-4141(200103)31:3<907::aid-immu907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]