Abstract
Vaccination strategies that can block or limit heterosexual human immunodeficiency virus (HIV) transmissions to local and systemic tissues are the goal of much research effort. Herein, in a mouse model, we aimed to determine whether the enhancement of antibody responses through mucosal and systemic immunizations, previously observed with protein-based vaccines, applies to immunizations with DNA- or RNA-based vectors. Intranasal (i.n.) followed by intramuscular (i.m.) immunizations (i.n./i.m.) with polylactide-coglycolide (PLG)-DNA microparticles encoding HIV-gag (PLG-DNA-gag) significantly enhanced serum antibody responses, compared with i.m., i.n. or i.m. followed by i.n. (i.m./i.n.) immunizations. Moreover, while i.n./i.m., i.n. or i.m./i.n. immunizations with PLG-DNA-gag resulted in genital tract antibody responses, i.m. immunizations alone failed to do so. Importantly, β7-deficient mice developed local and systemic antibody responses following i.n./i.m. immunization, or immunization via any other route, similar to those of wild-type mice. To compare the DNA with an RNA delivery system, immunizations were performed with VEE/SIN-gag replicon particles, composed of Venezuelan equine encephalitis virus (VEE) replicon RNA and Sindbis surface structure (SIN). i.n./i.m., compared with any other immunizations, i.n./i.m. immunization with VEE/SIN-gag resulted in enhanced genital tract but not serum antibody responses. These data show for the first time that mucosal followed by systemic immunizations with gene delivery systems enhance B-cell responses independent of the mucosal homing receptors α4β7 and αEβ7.
Keywords: HIV, mucosal vaccine, DNA, migration, reproductive immunology
Introduction
There is an urgent need for the development of a safe, effective and inexpensive vaccine against human immunodeficiency virus (HIV) infection. The majority of HIV infections currently occur through heterosexual intercourse, by transmission through the vaginal mucosa.1,2 Importantly, if HIV virions or HIV-infected cells that enter through the vaginal mucosa are not eliminated in situ, they may spread systemically through the draining lymphatics.3–6 Therefore, a successful HIV vaccine will probably need to elicit long-term immunity at sites of viral entry at the vaginal mucosa and the draining lymph nodes (LN), as well as systemically.
It is well established that mucosal immunization is one of the most effective means to induce long-term immunity at local and distant mucosal as well as systemic lymphoid tissues.7–9 Therefore, the development of vaccines for mucosal administration is an important objective. The intranasal (i.n.) route of administration offers a practical and effective means to induce local and distant mucosal immunity in the nasal and upper respiratory mucosa and the draining LN, as well as the vaginal mucosa.10–12
In recent years, a new strategy has been described to enhance the immunogenicity of antigens, involving the combination of a mucosal with a systemic route of immunization. Several studies have investigated the systemic followed by mucosal,13–18 and mucosal followed by systemic, routes of immunization.15,19–24 However, these studies used a combination of proteins, vaccinia virus or DNA for priming or boosting of animals, and whether a DNA or RNA delivery system alone can enhance mucosal and systemic antibody responses following combinations of mucosal and systemic immunizations remains unknown.
Although traditional vaccines have comprised subunit proteins, live attenuated viruses, or killed bacteria, much attention has recently focused on non-replicating DNA or RNA vaccine delivery systems. Immunization with DNA has several advantages over immunization with proteins, including improved vaccine stability, and reduced costs for vaccine production. Moreover, compared with attenuated viruses as delivery vehicles for HIV genes, plasmid DNA offers a safe alternative. However, although immunization with DNA-based vaccines, compared with protein-based vaccines, has resulted in stronger T-cell responses in small-animal models, induction of immune responses in primates, including humans, has not been as effective. Consequently, there is a need to improve the potency of DNA vaccines for humans. Polylactide-coglycolide (PLG) microparticles are a biodegradable and biocompatible delivery system, and have been safely used in humans for a wide range of biomedical purposes, including the controlled release of peptide and protein drugs.25,26 In addition, PLG microparticles have been used as vaccine delivery systems for both systemic and mucosal vaccines.27 We previously reported that i.n. immunizations with PLG-DNA, as opposed to naked DNA, encoding HIV-gag prolonged gene expression, and enhanced local and systemic B- and T-cell responses.28
RNA, as opposed to DNA, delivery systems offer the advantage of expressing the gene of interest in the cytoplasm, thus precluding the possibility of chromosomal integration of the gene of interest and other genes in the nucleus. Alphaviruses, including the Sindbis virus, Semliki Forest virus and Venezuelan equine encephalitis virus (VEE), are enveloped RNA viruses that have been developed into replication-defective RNA delivery systems.29,30 Alphavirus replicon RNA vectors maintain the non-structural protein gene and cis replication sequences required to drive abundant expression of heterologous antigens from the viral subgenomic 26s promoter, but are devoid of any alphaviral structural protein genes required for propagation and spread. These vectors also offer the prospect of natural adjuvanticity and stimulation of the innate immune response in addition to the antigen-specific adaptive response, arising from the cytoplasmic amplification of these vectors through double-stranded RNA intermediates.31
Given the advantages of both DNA and RNA delivery systems, it is important to determine their immunogenicity following mucosal and/or systemic immunizations against HIV infection. We recently reported that mucosal followed by systemic immunizations with Helicobacter pylori-derived recombinant proteins induced significantly higher antibody responses in serum than systemic alone, mucosal alone or systemic followed by mucosal immunizations.32 However, it is not known whether the combinations of mucosal and systemic immunizations with a DNA or an RNA delivery system also result in enhanced systemic and/or mucosal B-cell responses. Because we have previously reported on the systemic and mucosal immunogenicity of our DNA (PLG-DNA)28 and RNA (chimeric alphavirus-based replicon particles) delivery systems,6 we have focused in this study on induction of mucosal and systemic B- and T-cell responses following combinations of mucosal and systemic immunizations with PLG-DNA or VEE/SIN replicon particles expressing HIV-gag.
Several studies have shown a role for the β7 integrins α4β7 and αEβ7 in homing of lymphocytes to the gastrointestinal33–37 and genitourinary6 tracts. While the role of α4β7 expression by antigen-specific B cells in the gastrointestinal tract following oral immunizations or challenge is well established,38–41 its role on antigen-specific B cells in the respiratory or genitourinary tract following i.n. or combinations of i.n. and systemic immunizations is less clear, as roles for both α4β7 and l-selectin have been suggested.42,43 Because we observed significantly enhanced antibody responses following i.n./intramuscular (i.m.) immunizations with PLG-DNA, we determined, in mice genetically deficient in expression of the β7 integrin, whether this molecule played a role in the enhancement of local and systemic antibody responses observed in wild-type mice.
Materials and methods
Preparation of PLG-DNA
Cationic PLG-cetyltrimethylammonium bromide (CTAB) microparticles were prepared using a modified solvent evaporation process as described previously.28 The microparticles were prepared using an homogenizer (IKA, Wilmington, NC) at high speed to emulsify 10 ml of a 5%[weight/volume (w/v)] PLG polymer solution in methylene chloride with 1 ml of phosphate-buffered saline (PBS). The primary emulsion was then added to 50 ml of distilled water containing CTAB [0·5% (w/v)]. This resulted in the formation of a water-in-oil-in-water emulsion that was stirred at 6000 r.p.m. for 12 hr at room temperature, allowing the methylene chloride to evaporate. The resulting microparticles were washed four times in distilled water by centrifugation at 10 000 g and lyophilized. Plasmid DNA was adsorbed to PLG-CTAB microparticles by incubating 1 mg of DNA in 1 ml of 1 × Tris-ethylenediaminetetraacetic acid (EDTA) (TE) buffer with 100 mg of microparticles overnight at 4° with gentle rocking. The microparticles were then pelleted by centrifugation at 11 424 g for 10 min, washed with 1 × TE buffer, re-centrifuged, and suspended in 5 ml of deionized water and lyophilized. The size distribution of the microparticles was determined using a particle size analyser (Mastersizer; Malvern Instruments, Malvern, UK).
Preparation of alphavirus replicon particles
The VEE/SIN-gag replicon particles were prepared as described previously.44 Briefly, replicon particles were generated by cotransfection of in vitro-transcribed RNA species corresponding to a replicon and two defective helpers, with one helper expressing capsid protein and the other expressing envelope glycoproteins E2 and E1.45 Titres of replicon particles on BHK-21 cells were determined as previously described.46 Replicon particles expressing HIV-1 p55gag were harvested as culture supernatants at 20 and 30 hr post transfection, clarified by filtration, and purified by cation exchange chromatography. Replicon particle titres as infectious units (IU) per ml were determined by intracellular staining of expressed Gag, following overnight infection of BHK-21 cells with serial dilutions of particles. Infected cells were permeabilized and fixed using a Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) and then stained with fluorescein isothiocyanate-conjugated antibodies to HIV-1 core antigen (Beckman Coulter, Fullerton, CA). Using flow cytometry analysis, the percentage of Gag-positive cells was determined and used to calculate titres. The absence of contaminating replication-competent virus was determined by five consecutive infections of naïve BHK-21 cell monolayers and confirmation of the absence of plaque-forming units. Endotoxin levels were measured for all replicon particle samples and shown to be < 0·5 endotoxin units/ml.
Mice
Female C57BL/6 or BALB/c mice were purchased from Charles River Breeding Laboratories (Wilmington, MA) at the age of 6–8 weeks at the beginning of the studies. Female C57BL/6 ITGB7 < tm 1 Cgn > /J strain mice with a homozygous deletion of the β7 gene were purchased from Jackson Laboratories (Bar Harbor, ME) (originally provided by the University of Cologne, Cologne, Germany and used under licence from Ascenion GmbH, München, Germany) at 6–8 weeks of age. Groups of three or six mice were used for each immunization. Sera were collected and assayed from individual animals, whereas tissues [spleen (SP), vaginal/uterine mucosa (VUM) and iliac lymph nodes (ILN)] were collected and assayed in pools as stated in the Results section.
Immunizations
Groups of three or six female BALB/c, β7-deficient or wild-type C57BL/6 mice that were 6–8 weeks old at the start of immunization were used for each vaccine or immunization route, and the tissues were pooled when the mice were killed. Immunizations were performed four times at 2-week intervals. To this end, groups of three or six mice were immunized with PLG-DNA encoding HIV-gag, four times i.m. (4 i.m.), four times i.n. (4 i.n.), two times i.m. then two times i.n. (2 i.m./2 i.n.), or two times i.n. then two times i.m. (2 i.n./2 i.m.). For simultaneous i.n./i.m. immunizations, the animals were immunized four times at 2-week intervals and the doses for each route were reduced by 50% so that the total dose was equal to that of the single-route immunizations. They were bled at 1–2 weeks after each immunization to measure anti-gag immunoglobulin G1 (IgG1) and IgG2a responses. The dose for i.m. and i.n. immunizations with PLG-DNA-gag was 10 μg and 85 μg respectively. Immunizations with VEE/SIN-gag were performed with similar immunization routes and intervals with PLG-DNA-gag and with a dose of 106 international units for both i.m. and i.n. immunizations.
Tissue collection and preparation of single-cell suspensions
Spleens, ILN and VUM were harvested and pooled from five mice per group. Single-cell suspensions were used for fluorescence-activated cell sorting (FACS) analysis. One week following the final immunization or 5 days following the intravaginal (i.vag.) challenge, SP, ILN, and VUM from groups of five immunized mice each were harvested and pooled. SP and ILN tissues were teased through a nylon mesh with a pore diameter of 250 μm, washed three times in medium [enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) medium, composed of RPMI containing 10% fetal calf serum (FCS), antibiotics, HEPES and l-glutamine (complete RPMI)], counted, and seeded into wells. Single-cell suspensions were prepared from VUM by removing the entire vagina, uterus, and uterine horns from five mice per group as previously described.47 Uterine horns were cut longitudinally, and, together with vaginal and uterine tissues, were diced into 5-mm pieces. The tissue pieces were washed three times in Hanks' balanced salt solution (HBSS) without calcium (Ca2+) and magnesium (Mg2+) containing 10% fetal calf serum and 5 mm HEPES (complete HBSS) and treated enzymatically with agitation at 37° once with 1 mg/ml collagenase/dispase (Sigma, St Louis, MO) plus 0·5 mg/ml DNase (Roche Molecular Biochemicals, Indianapolis, IN) in 20 ml of complete HBSS medium for 30 min and twice with 800 U/ml collagenase (type XI; Sigma) plus 0·5 mg/ml DNase in 20 ml of complete RPMI for 45 min. Following each enzymatic treatment, the released cells were recovered and washed twice with complete RPMI. The cell suspensions from each enzymatic treatment were pooled and counted. This method routinely resulted in the recovery of a minimum of 107 mononuclear cells per five mice at a viability level of > 90%.
Magnetic bead enrichment
Cell suspensions were prepared at 107 cells/ml, and biotinylated anti-lymphocyte Peyer’s patch adhesion molecule (LPAM)-1 (α4β7) antibody (Southern Biotechnology Associates, Birmingham, AL) was added at a 1 : 50 dilution in PBS and incubated at 4° for 10 min. Unbound antibody was washed with PBS, the cells were resuspended in staining buffer [provided with the magnetic activated cell sorting (MACS) kit; Miltenyi, Auburn, CA] and streptavidin-magnetic microbeads were added at a 1 : 16 dilution. The cells were incubated at 4° for 15 min and washed with the staining buffer. The cells were then run over columns provided with the kit and placed on the magnet, and non-adherent cells were collected. A volume of 0·5 ml of staining buffer was run over the column twice and the non-adherent cells were collected and pooled each time with the original non-adherent cells. The adherent cells were recovered by removing the column from the magnet and running 1 ml of staining buffer with a plunger. The cells were counted using an automatic cell counter (Vi-cell XR; Beckman Coulter).
ELISPOT
Single-cell suspensions from pooled VUM, SP and ILN from groups of three or five mice were added to nitrocellulose or polyvinylidene fluoride (pvdf) plates (Milipore, Billerica, MA) precoated with HIV-gag p55 antigen (5 µg/ml PBS) and blocked with complete RPMI medium at pH 7·2, containing 10% fetal calf serum, 5 mm HEPES and antibiotics. Following overnight incubation of cells, the plates were washed and biotinylated goat-anti IgA or IgG (Southern Biotechnology Associates) was added in PBS/0·1% bovine serum albumin (BSA)/0·02% Tween, and the plates were incubated at room temperature for 2 hr. The plates were then washed with PBS/0·02% Tween (P/T) and incubated for 1 hr at 37° with avidin-peroxidase (Pharmingen) at 1 : 1000 dilution. The plates were washed with P/T and the spots were visualized by adding 3′3′-diaminobenzidine (DAB) substrate (Bio-Rad, Concord, CA) in Tris-HCl (pH 7·5) buffer for 30 min. The plates were washed with deionized H2O and air-dried. The spots were counted with a Zeiss automatic ELISPOT reader (Oberkochen, DE). The data are presented as mean ± standard deviation of two or three independent experiments expressed as the number of gag-specific antibody-secreting cells (ASCs) per 106 mononuclear cells (MNCs).
Enzyme-linked immunosorbent assay (ELISA)
HIV-gag-specific serum IgG1 and IgG2a titres were quantified by a standard ELISA. Briefly, ELISA plates (96-well U-bottomed plates; Nunc Maxisorp, Nunc, Denmark) were coated with HIV-gag protein at 1 µg/well in PBS overnight at 4°. After washing with 1 × PBS + 0·03% Tween 20 (Sigma), the wells were blocked with PBS/2% FCS for 30 min at 37° and serum samples were added at an initial dilution of 1 : 5000 and 1 : 2 serial dilutions performed in the blocking reagent. A standard serum was included in each assay as positive control. The samples and standard sera were incubated at 4° overnight and washed with PBS/0·03% Tween. The plates were washed and, after the addition of biotinylated anti-mouse IgA or IgG (Southern Biotechnology Associates) at 1 : 8000 in PBS/2% FCS, incubated for 2 hr at room temperature. The plates were washed again and, after the addition of avidin-HRP (Pharmingen) at 1 : 1000, incubated at 37° for 30 min. The plates were then washed and developed with tetramethylbenzidine (TMB; Kirkegaard and Perry, Laboratories Inc., Gaithersberg, MD) for 15 min, the reaction being stopped with 2 N HCl. The optical density of each well was measured using a Titertek optical density reader at 450 nm (Huntsville, AL).
Statistical analysis
Student's t-test on log-transformed values was performed for two means assuming equal variances (F-test, P > 0·05), using the statistical analysis software program in Microsoft Excel.
Results
Intranasal/intramuscular immunizations with PLG-DNA-gag enhanced serum antibody responses compared with other single or combined routes
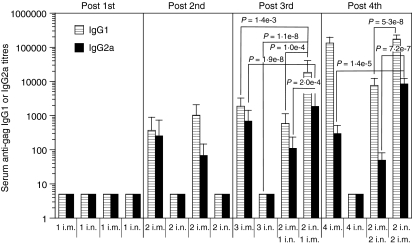
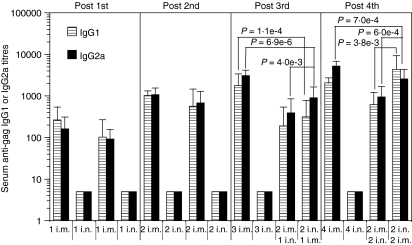
To determine the optimal routes of immunization with a DNA delivery system for induction of systemic antibody responses, mice were immunized with PLG-DNA-gag i.n., i.m. or with a combination of i.n. and i.m. routes and their serum antibody responses were monitored following each immunization. The first i.m. or i.n. immunization did not induce any IgG1 or IgG2a antibody responses (Fig. 1). Following 2 i.m. immunizations, anti-gag IgG1 and IgG2a responses were measurable, with IgG2a several-fold lower than IgG1 responses (Fig. 1). Interestingly, however, after the third immunization, 2 i.n./1 i.m. immunizations induced significantly higher serum anti-gag IgG1 and IgG2a responses than 3 i.n. (P = 1·1 × 10−8 for both IgG1 and IgG2a), 2 i.m./1 i.n. (P = 1 × 10−4 for IgG1 and P = 2 × 10−4 for IgG2a) and 3 i.m. (P = 1·4 × 10−3 for IgG1 and P = 1·9 × 10−8 for IgG2a) immunizations (Fig. 1). Following the fourth immunization, however, the 2 i.n./2 i.m. immunizations induced significantly higher IgG2a responses compared with any other immunizations (for 4 i.n., P = 1·1 × 10−8; for 2 i.m./2 i.n., P = 7·2 × 10−7; for 4 i.m., P = 1·4 × 10−5). Importantly, only the 2 i.n./2 i.m. immunizations induced splenic interferon (IFN)-γ responses, indicating a correlation between the enhanced serum IgG2a and a systemic T helper type 1 (Th1) response (data not shown). Of note, even 4 i.n. immunizations induced background-level IgG1 and IgG2a antibody responses. The anti-gag IgG1 response in the 2 i.n./2 i.m. immunizations, although higher than in the 2 i.m./2 i.n. or 4 i.n. immunizations, did not reach statistical significance when compared with the 4 i.m. immunizations. Of note, no serum IgA responses were detectable in any groups at any time-points (data not shown). These data show that two i.n. immunizations followed by one or two i.m. immunizations with PLG-DNA-gag significantly enhanced serum antibody responses compared with other single or combined routes of immunization.
Figure 1.
Intranasal (i.n.) followed by intramuscular (i.m.) immunizations with polylactide-coglycolide DNA microparticles encoding human immunodeficiency virus (HIV)-gag (PLG-DNA-gag) induced significantly higher serum antibody responses compared with i.n. alone, i.m. alone, or i.m. followed by i.n. immunization. Groups of female BALB/c mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes at 2-week intervals. Sera were collected at 2 weeks after each of the first three immunizations (Post 1st, 2nd and 3rd, respectively) and 7 days after the final immunization (Post 4th) and anti-gag antibody responses were measured by enzyme-linked immunosorbent assay (ELISA). The data are presented as mean serum anti-gag immunoglobulin G1 (IgG1) or IgG2a titres for individual mice from two independent experiments, with six or 10 mice in each experiment, plus the standard error of the mean; each group of mice is shown at the same relative position in the histograms at the different time-points. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization experienced by the group of mice at that time-point. The P-values between groups with statistically significant differences are shown between the compared groups.
Intranasal immunizations, alone or in combination with intramuscular immunizations, with PLG-DNA-gag were required for induction of vaginal immune responses
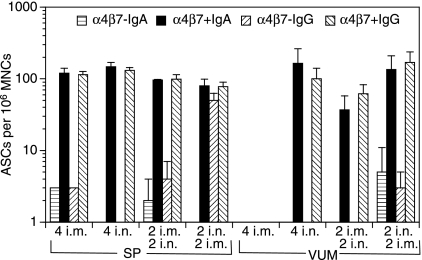
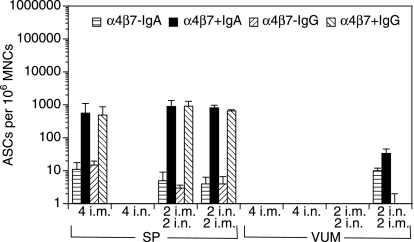
We next addressed the question of whether i.n./i.m. immunizations, which enhanced serum antibody responses, also resulted in increased antibody responses in the vaginal/uterine mucosa (VUM) and spleen. To this end, the mice were killed 7 days after the final immunization. To determine whether the expression of anti-gag IgG- or IgA-secreting cells could distinguish the systemic (splenic) from the mucosal (VUM) responses, we enriched for cells expressing α4β7 by MACS, and plated the α4β7 positively and negatively enriched cells on ELISPOT plates precoated with the gag antigen. Intranasal immunizations, either alone or in combination with i.m. immunizations, were required for the induction of IgG and IgA ASCs in VUM (Fig. 2). Most of the IgG and IgA ASCs appeared to be within the α4β7 positively enriched cells (Fig. 2). Importantly, while i.m. immunizations alone induced IgA- and IgG-secreting cells in SP, i.m. immunizations alone did not induce any ASCs in the VUM. Conversely, although i.n. immunizations alone did not induce any detectable serum antibody responses (Fig. 1), they did induce splenic and VUM ELISPOT responses in the α4β7 positively enriched population (Fig. 2).
Figure 2.
Intranasal (i.n.) immunizations alone, or in combination with intramuscular (i.m.) immunization, with polylactide-coglycolide DNA microparticles encoding human immunodeficiency virus (HIV)-gag (PLG-DNA-gag) induced gag-specific antibody-secreting cells (ASCs) in the cervicovaginal mucosa. Groups of female BALB/c mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes with PLG-DNA-gag at 2-week intervals. The mice were killed 7 days after the final immunization. Single-cell suspensions were prepared from spleen (SP) and vaginal/uterine mucosa (VUM) from pools of three to five mice, and the cells were enriched by magnetic antibody cell sorting (MACS) for the populations expressing or not expressing the mucosal homing receptor α4β7. The α4β7 negatively and positively enriched populations were then used in an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) to detect anti-gag ASCs. The data are presented as average number of anti-gag immunoglobulin A (IgA)- or IgG-secreting cells per million α4β7 negatively (α4β7–) or positively (α4β7+) enriched mononuclear cells (MNCs) from two subgroups of three to five mice per experiment and two independent experiments, plus standard deviation. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization.
In SP, the various routes of immunization, alone or in combination, resulted in similar numbers of IgA or IgG ASCs, which were mostly within the α4β7 positively enriched population. Moreover, in SP, only 2 i.n./2 i.m. immunizations induced IgG-secreting cells, with similar numbers in the α4β7 positively and negatively enriched populations (Fig. 2). Of note, anti-gag IgG- and IgA-secreting cells were detectable in ILN only after 2 i.n./2 i.m., and not after any other single or combined routes of immunization (data not shown). These data show that the i.n. route of immunization, alone or in combination with i.m. immunization, with PLG-DNA-gag was required for induction of mucosal antibody responses in the genital tract. Moreover, most of the anti-gag IgA- and IgG-secreting cells were found in the α4β7 positively enriched populations.
Enhanced antibody responses following intranasal/intramuscular immunizations with PLG-DNA independent of the mucosal homing receptors α4β7 and αEβ7
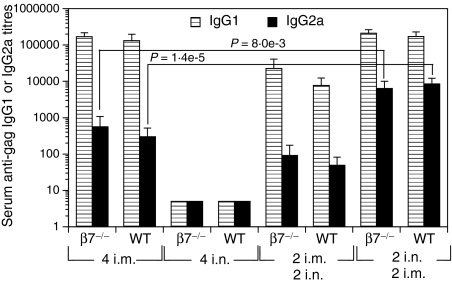
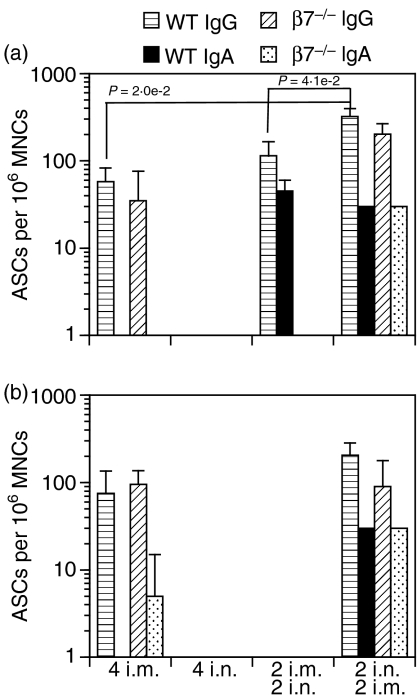
Expression of the mucosal homing receptors α4β7 and αEβ7 by antigen-specific B and T cells has been shown to be important for the induction of mucosal immune responses in the gastrointestinal and respiratory tracts. Because we had observed enhanced antibody responses following i.n./i.m. as opposed to i.m./i.n., i.m. alone or i.n. alone immunizations, we sought to establish the role of β7 integrins in the enhancement of the responses. To this end, β7 gene knockout (β7–/–) and wild-type C57BL/6 (WT) mice were immunized with PLG-DNA-gag 2 i.n./2 i.m., 2 i.m./2 i.n., 4 i.n. or 4 i.m. and 7 days after the final immunization serum anti-gag IgG1 and IgG2a responses were measured. No significant differences in serum anti-gag IgG1 or IgG2a responses were observed between β7–/– and WT mice (Fig. 3). Moreover, compared with 4 i.m. immunizations, 2 i.n./2 i.m. immunizations induced enhanced serum IgG2a antibody responses in β7–/– (P = 8 × 10−3) and WT mice (P = 1·4 × 10−5) (Fig. 3). Because only i.n./i.m. immunizations induced antibody responses in ILN of WT mice, we next compared antibody responses in ILN and SP of WT and β7–/– mice. We found increased numbers of local anti-gag IgG-secreting cells in the SP (Fig. 4a), but not ILN (Fig. 4b), of both β7–/– and WT mice following 2 i.n./2 i.m. immunizations compared with all other immunization routes (Figs 4a and b). Of note, 2 i.m./2 i.n. immunizations induced both IgG- and IgA-secreting cells in SP of WT but not β7–/– mice (Fig. 4a). These data suggest that β7-integrin does not play a significant role in the enhancement of local and systemic antibody responses following immunizations through the i.n./i.m. routes.
Figure 3.
The enhancement of serum antibody responses measured after intranasal (i.n.) followed by intramuscular (i.m.) immunizations with polylactide-coglycolide DNA microparticles encoding human immunodeficiency virus (HIV)-gag (PLG-DNA-gag) is independent of the expression of the mucosal homing receptor α4β7 or αEβ7. Groups of female mice genetically deficient in β7 expression (β7–/–) and wild-type C57BL/6 (WT) mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes at 2-week intervals. Sera were collected at 7 days after the final immunization and anti-gag antibody responses were measured by enzyme-linked immunosorbent assay (ELISA). The data are presented as mean serum anti-gag immunoglobulin G1 (IgG1) or IgG2a titres for six individual mice, plus the standard error of the mean. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization. The P-values between groups with statistically significant differences are shown between the compared groups.
Figure 4.
Local and systemic antibody responses in α4β7–/– and wild-type (WT) mice following combinations of intranasal (i.n.) and intramuscular (i.m.) or i.m. alone or i.n. alone immunizations with polylactide-coglycolide DNA microparticles encoding human immunodeficiency virus (HIV)-gag (PLG-DNA-gag). Groups of female mice genetically deficient in α4β7 expression (α4β7–/–) and wild-type C57BL/6 (WT) mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes at 2-week intervals. Spleens (a) and iliac lymph nodes (b) were collected at 7 days after the final immunization. Single-cell suspensions were prepared from spleen and iliac lymph nodes, which drain the vaginal mucosa, from pools of three mice, and used in an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) to detect anti-gag immunoglobulin G (IgG)- or IgA-secreting cells. The data are presented as the average number of anti-gag IgA- or IgG-secreting cells (ASCs) per million mononuclear cells (MNCs) from two subgroups of three mice per experiment, plus standard deviation. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization. The P-values between groups with statistically significant differences are shown between the compared groups.
Intranasal/intramuscular immunizations with VEE/SIN-gag chimeric replicon particles resulted in similar serum antibody responses compared with intramuscular alone immunizations
Because we found that mucosal followed by systemic immunizations with PLG-DNA resulted in enhanced serum antibody responses, we tested whether the same regimen using an RNA delivery system resulted in enhanced serum antibody responses. We found that, in contrast to PLG-DNA, one i.m., but not one i.n., immunization with VEE/SIN-gag induced measurable IgG1 and IgG2a serum anti-gag responses (Fig. 5). Following 2 i.m., but not 2 i.n., immunizations, anti-gag IgG1 and IgG2a responses were enhanced compared with the 1 i.m. immunization, with similar levels of IgG2a and IgG1 responses (Fig. 5). After the third immunization, i.m. immunizations alone still induced the highest anti-gag IgG1 and IgG2a titres. However, the 2 i.n./1 i.m. immunizations induced significantly higher serum anti-gag IgG2a compared with IgG1 responses, albeit at several-fold lower levels than 3 i.m. immunizations. The group that received 2 i.m./1 i.n. immunizations mimicked the 2 i.n./1 i.m. group, although the responses were generally lower (Fig. 5). Following 2 i.n./2 i.m. immunizations, similar serum anti-gag IgG1 and lower IgG2a (P = 1·1 × 10−4) responses were induced compared with 4 i.m. immunizations. The 2 i.m./2 i.n. immunization group had several-fold lower responses than the 2 i.n/2 i.m. (P = 3·8 × 10−3 for IgG1 and P = 6 × 10−4 for IgG2a) or 4 i.m. (P = 7 × 10−4 for IG2a) immunization group, while the 4 i.n. group did not show any serum anti-gag antibody responses (Fig. 5). These data show that, using the VEE/SIN-gag RNA delivery system, 2 i.n./2 i.m. immunizations induced similar or lower serum antibody responses compared with 4 i.m. immunizations.
Figure 5.
Intranasal (i.n.) followed by intramuscular (i.m.) immunizations with Venezuelan equine encephalitis virus (VEE)/Sindbis surface structure (SIN)-gag alphavirus-based replicon particles induced similar serum antibody responses compared with i.m. alone immunizations. Groups of female BALB/c mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes at 2-week intervals. Sera were collected at 2 weeks after each of the first three immunizations (Post 1st, 2nd and 3rd, respectively) and 7 days after the final immunization (Post 4th) and anti-gag antibody responses were measured by enzyme-linked immunosorbent assay (ELISA). The limit of detection for this assay was a titre of 5. The data are presented as mean serum anti-gag immunoglobulin G1 (IgG1) or IgG2a titres for individual mice from two independent experiments, with five mice in each experiment, plus the standard error of the mean; each group of mice is shown at the same relative position in the histograms at the different time-points. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization experienced by the group of mice at that time-point. The P-values between groups with statistically significant differences are shown between the compared groups.
Only intranasal/intramuscular immunizations with VEE/SIN-gag chimeric replicon particles induced antibody-secreting cells in the vaginal/uterine mucosa
To determine whether 2 i.n./2 i.m. immunizations with VEE/SIN-gag induced enhanced mucosal responses, the mice were killed 7 days after the fourth immunization, and anti-gag ASCs were enumerated in single-cell suspensions prepared from the VUM and SP by ELISPOT. To determine whether the expression of α4β7 anti-gag IgG- or IgA-secreting cells could distinguish the systemic (SP) from the mucosal (VUM) responses, we enriched for cells expressing α4β7 by MACS, and plated the α4β7 positively and negatively enriched cells on ELISPOT plates precoated with the gag antigen. Only 2 i.n./2 i.m. immunizations induced anti-gag IgA, but not IgG, ASCs in VUM. While most of the ASCs appeared to reside within the α4β7-enriched group, there were no significant differences between α4β7 positively and negatively enriched cells (Fig. 6). All other single or combinational routes of immunizations, except the i.n. alone route, induced similar numbers of anti-gag IgG and IgA ASCs in spleens, mostly within the α4β7-enriched population (Fig. 6). These data show that only the 2 i.n./2 i.m. regimen, and not 4 i.m., 4 i.n. or 2 i.m./2 i.n. immunizations, with the VEE/SIN-gag RNA delivery system induced ASCs in mucosal (VUM) as well as in systemic (SP) lymphoid tissues.
Figure 6.
Only intranasal (i.n.) followed by intramuscular (i.m.) immunizations with Venezuelan equine encephalitis virus (VEE)/Sindbis surface structure (SIN)-gag alphavirus-based replicon particles induced gag-specific antibody-secreting cells (ASCs) in the cervicovaginal mucosa. Groups of female BALB/c mice were immunized i.n., i.m. or through combinations of i.n. and i.m. routes with VEE/SIN-gag at 2-week intervals. The mice were killed 7 days after the final immunization. Single cell suspensions were prepared from spleen (SP) and vaginal/uterine mucosa (VUM) from pools of three to five mice, and the cells were enriched by magnetic antibody cell sorting (MACS) for the populations expressing or not expressing the mucosal homing receptor α4β7. The α4β7 negatively and positively enriched populations were then used in an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) to detect anti-gag ASCs. The data are presented as average number of anti-gag immunoglobulin A (IgA)- or IgG-secreting cells per million α4β7 negatively (α4β7–) or positively (α4β7+) enriched mononuclear cells (MNCs) from groups of five mice per experiment and two independent experiments, plus standard deviation. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization.
Simultaneous intranasal and intramuscular immunizations with PLG-DNA, but not with VEE/SIN-gag replicon particles, enhanced serum anti-gag antibody responses compared with intranasal or intramuscular alone immunizations
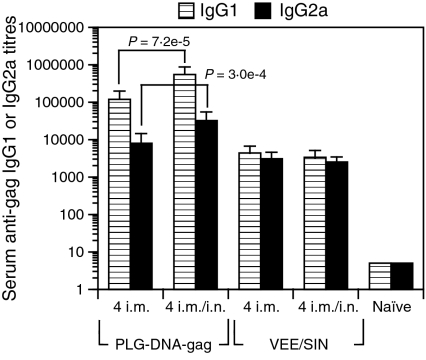
It would arguably be more practical in the clinic to perform simultaneous i.n./i.m. immunizations, as opposed to separated i.n. and i.m. immunizations, in order to avoid human error regarding the order of each route. To determine whether PLG-DNA-gag given as simultaneous mucosal/systemic immunizations induced similar enhanced serum antibody responses to single routes of immunization separated over time, we immunized mice by i.m. or simultaneous i.m./i.n. immunizations four times at 2-week intervals and measured serum anti-gag IgG1 and IgG2a responses in sera 2 weeks after the final immunization. We found that the simultaneous i.m./i.n. immunizations given four times with PLG-DNA induced enhanced serum IgG1 (P = 7·2 × 10−5) and IgG2a (3·1 × 10−4) anti-gag responses compared with 4 i.m. immunizations, with IgG1 predominating over IgG2a responses in both groups (Fig. 7). In contrast, simultaneous i.m./i.n. immunizations given four times with the chimeric VEE/SIN-gag RNA delivery system induced similar serum antibody responses to i.m. alone immunizations, with similar levels of IgG1 and IgG2a (Fig. 7). Of note, while measurement of local ASCs in VUM would have been valuable, they were not measured, as it was anticipated that the simultaneous i.n./i.m. immunizations would induce responses similar to those induced by separate i.n./i.m. immunizations, and that ILN, which drain VUM, would show similar responses. Thus, regardless of the use of a DNA or RNA vaccine delivery system, simultaneous i.m./i.n. immunizations induced similar responses to separate i.n. followed by i.m. immunizations.
Figure 7.
Simultaneous intranasal (i.n.) and intramuscular (i.m.) immunizations with polylactide-coglycolide DNA microparticles encoding human immunodeficiency virus (HIV)-gag (PLG-DNA-gag) or Venezuelan equine encephalitis virus (VEE)/Sindbis surface structure (SIN)-gag alphavirus-based replicon particles. Groups of female BALB/c mice were immunized i.m. or through simultaneous combinations of i.n. and i.m. routes four times at 2-week intervals. Sera were collected at 7 days after the final immunization and anti-gag antibody responses were measured by enzyme-linked immunosorbent assay (ELISA). The data are presented as mean serum anti-gag immunoglobulin G1 (IgG1) or IgG2a titres for individual mice from two independent experiments, with five mice in each experiment, plus the standard error of the mean. Numbers followed by ‘i.n.’ or ‘i.m.’ indicate the numbers of each type of immunization. The P-values between groups with statistically significant differences are shown between the compared groups.
Discussion
In this study, we showed that mucosal followed by systemic (i.n./i.m.) immunizations with PLG-DNA-gag induced enhanced mucosal as well as systemic antibody responses, being superior to i.n. alone, i.m. alone or i.m./i.n. routes of immunization. Although i.n./i.m. immunizations with VEE/SIN-gag did not enhance the serum antibody responses, they did enhance the mucosal antibody responses in VUM. Thus, using two different delivery systems for in vivo delivery of DNA (PLG-DNA) or RNA (VEE/SIN), we showed that i.n./i.m. immunizations enhanced systemic and/or mucosal antibody responses. It is interesting to note that our results from a previous study using H. pylori-derived protein antigens showed that splenic IFN-γ or interleukin (IL)-4 responses were not increased following i.n./i.m. immunizations.32
Several studies have investigated the systemic followed by mucosal,13–18 or mucosal followed by systemic, routes of immunization.15,19–24 Most of these studies used a combination of proteins, vaccinia virus or DNA for priming or boosting of animals. Using hepatitis B surface antigen as a model protein, it was found that, although mucosal followed by systemic or systemic followed by mucosal immunizations induced higher serum antibody titres than mucosal or systemic immunizations alone, there was no significant difference between priming mucosally or systemically.15 However, we and others19 found that the systemic followed by mucosal route was inferior to the mucosal followed by systemic route of immunization for induction of immunity. These differences may be explained in terms of the differences in the immunogenicities of the various immunogens and adjuvants used in these studies.
The finding that mucosal followed by systemic immunizations with PLG-DNA, VEE/SIN (current study) or H. pylori-derived protein antigens32 enhanced local and/or systemic antibody responses suggests that the route of immunization rather than the adjuvant or the delivery system used in mucosal immunizations was important for the enhancement of the response. Although the correlates of protection against HIV are not well established, it is generally agreed that induction of both neutralizing antibodies against surface HIV-env and cytotoxic T-cell responses against HIV-gag or -pol would be ideal features of an anti-HIV vaccine. The question then arises as to whether the i.n./i.m. strategy would induce enhanced antibody responses against HIV-env. Our preliminary data show that i.n./i.m. immunizations with various doses of HIV-env-derived oligomeric glycoprotein 140 (o-gp140) (containing the envelope gp120 plus a portion of the trans-membrane domain) induced strong serum IgG and cervicovaginal IgA responses in mice in a dose-sparing manner (data not shown). Moreover, in a rhesus macaque model, i.n./i.m. immunizations with HIV-env-derived o-gp140 induced strong anti-HIV-env serum and cervicovaginal antibody responses and neutralized various strains of HIV-1.48 Collectively, these data suggest that i.n. followed by i.m. immunizations with protein-, DNA- and RNA-based delivery systems enhance serum and/or mucosal antibody responses.
We made interesting observations regarding the antibody responses in the various immune compartments following various routes of immunization with the two gene delivery systems. For instance, although i.n. immunizations with PLG-DNA-gag alone did not induce any detectable serum antibody responses, they did induce SP and VUM ELISPOT responses in the α4β7 positively enriched population. This may be attributable to the fact that, while serum antibody responses reflect antibody production in many immune compartments, including the bone marrow as a major site of antibody production, the antibody response in SP is limited only to this site. Another noteworthy observation was the lack of induction of vaginal responses after i.m. immunizations alone. This was expected, as i.m. immunizations alone are well known to induce strong systemic but poor mucosal antibody responses.
α4β7 is important for homing of lymphocytes to the intestinal tract through binding to the mucosal vascular addressin mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1).39,40,49 However, a peripheral homing receptor, l-selectin, has also been found to be important for intestinal immune responses, as it too can bind to MAdCAM-1.50 While in many studies α4β7 has been found to be important for homing of antigen-specific cells to the intestinal tract after oral immunizations or challenge,33,39–41,49 the expression of this homing receptor has not been as well established for cells that traffic to the respiratory or the genitourinary tracts after i.n. immunizations. Csencsits and Pascual showed a delayed, but not over time diminished, nasal passage and reproductive tract mucosal B-cell response, which was not evident in the local draining lymph nodes early or late, after i.n. immunizations of l-selectin-deficient mice with cholera toxin.51 While this study did not examine the same immunization strategy in β7-deficient mice, it did show that nasal passage and reproductive tract mucosal B cells contained similar percentages of α4β7-expressing cells as intestinal lamina propria B cells.51 In human volunteers, i.n. immunizations induced the expression of both α4β7 and l-selectin on antigen-specific ASCs in peripheral blood.42 It was also shown in sheep that lymphocytes emigrating from lung expressed both α4β7 and l-selectin.38 Moreover, in humans, while most lung memory/effector T cells expressed low levels of l-selectin and no α4β7, almost half expressed αEβ7.52 These studies mostly addressed the expression of α4β7 or l-selectin by B or T cells in lungs after i.n. immunizations, and relatively little is known about the expression of these homing receptors on B or T cells in the vaginal mucosa after i.n. immunizations. Our previous study showed that i.n. immunizations with VEE/SIN replicon particles encoding HIV-gag followed by intravaginal challenge with vaccinia virus (VV)-gag induced anti-gag responses in CD8+ T cells in VUM, which comprised mostly IFN-γ-expressing cells which coexpressed surface α4β7 and chemokine receptor 5 (CCR5), while the anti-gag CD8+ T cells expressed mostly l-selectin in SP.6 Importantly, l-selectin deficient mice have been shown to mount defective B- and T-cell responses following systemic immunizations.53,54
On the basis of the above findings, we aimed to determine the role of α4β7 in the enhancement of B-cell responses following i.n./i.m. immunizations. However, we found no significant differences in the systemic or mucosal responses in the presence or absence of α4β7, following immunizations with PLG-DNA-gag through i.n./i.m. or any other routes. This was unexpected in the light of our findings that the majority of antigen-specific plasma cells or plasmablasts from both mucosal (VUM) and systemic (SP) lymphoid tissues expressed α4β7. These data imply that compensatory mechanisms or coexpression of homing receptors other than α4β7 may be involved in the enhancement of antibody responses following i.n./i.m. immunizations. Moreover, these results demonstrate for the first time that the expression of the β7-associated integrins does not play a significant role in the induction of mucosal and systemic B-cells responses following immunizations with a DNA delivery system.
There are inherent differences between the PLG-DNA and VEE/SIN delivery systems and hence we did not aim to directly compare their immunogenicities. However, in vitro, it appeared that 10 µg of DNA induced the same level of gag expression and secretion as a dose of between 2 × 104 and 2 × 105 international units of VEE/SIN (data not shown). It is interesting to note that i.n./i.m. immunizations with PLG-DNA induced higher serum antibody responses than i.n./i.m. immunizations with VEE/SIN. PLG-DNA is a DNA delivery system that releases DNA over a more sustained period of time compared with naked DNA. We have shown that i.n. immunizations with PLG-DNA encoding HIV-gag induced enhanced mucosal and systemic antibody responses compared with naked DNA. A possible mechanism for the increased immune responses following i.n. immunization with PLG-DNA versus naked DNA was sustained expression of gag by antigen-presenting cells in cervical lymph nodes over a 1-week period.28 In contrast, the alphavirus-based replicon particles, including the chimeric VEE/SIN particles used in this study, infect target cells that are lysed 48 hr later, and have a peak expression of the gene of interest at 24 hr after uptake by target cells.44,55 Moreover, at 24 hr after a single i.n. and i.vag., but not i.m., immunization with VEE/SIN-gag, many cells in ILN (which drain the vaginal mucosa) expressed gag.6 Thus, while the DNA (PLG-DNA) and RNA (VEE/SIN) delivery systems are inherently different in terms of transcribing the gene of interest in the nucleus or the cytoplasm of target cells and the number of particles taken up by target cells, they also differ in terms of sustained expression of the gene of interest, offering a possible explanation for their different immunogenicities. Ultimately, comparison of the VEE/SIN and the PLG-DNA systems will require careful in vivo analysis at the single-cell level by, for example, in situ hybridization.
Acknowledgments
This study was supported by NIH R21 grant 1 R21 AI50430-01, by NIH IPCAVD grant 1 U19 AI51596, by DDT contract N01-AI-05396 and by the Chiron Corporation.
Abbreviations
- ASC
antibody-secreting cell
- CTAB
cetyltrimethylammonium bromide
- DAB
3′3′-diaminobenzidine
- HIV
human immunodeficiency virus
- HIV-env
HIV envelope
- ILN
iliac lymph nodes
- i.m.
intramuscular
- i.n.
intranasal
- i.vag.
intravaginal
- MACS
magnetic activated cell sorting
- MNC
mononuclear cell
- o-gp140
oligomeric glycoprotein 140
- PFU
plaque-forming units
- PLG
polylactide-coglycolide
- PLG-DNA-gag
PLG-DNA microparticles encoding HIV-gag
- pvdf
polyvinylidene fluoride
- SIN
Sindbis surface structure
- SP
spleen
- TE
Tris-ethylenediaminetetraacetic acid (EDTA) buffer
- VEE
Venezuelan equine encephalitis virus
- VUM
vaginal/uterine mucosa.
References
- 1.Quinn TC. Global burden of the HIV pandemic. Lancet. 1996;348:99–106. doi: 10.1016/s0140-6736(96)01029-x. [DOI] [PubMed] [Google Scholar]
- 2.De Schryver A, Meheus A. Epidemiology of sexually transmitted diseases: the global picture. 68:639–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Parr MB, Parr EL. Vaginal immunity in the HSV-2 mouse model. Int Rev Immunol. 2003;22:43–63. doi: 10.1080/08830180305228. [DOI] [PubMed] [Google Scholar]
- 4.Miller CJ, Lu FX. Anti-HIV and -SIV immunity in the vagina. Int Rev Immunol. 2003;22:65–76. doi: 10.1080/08830180305230. [DOI] [PubMed] [Google Scholar]
- 5.Vajdy M. Induction of optimal immune responses against human immunodeficiency virus at mucosal portals of entry. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:222–33. doi: 10.2174/1568008033340225. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Janani R, Bin Q, et al. Characterization of HIV-gag-specific IFNγ-expressing cells following protective mucosal immunization with alphavirus replicon particles. J Virol. 2005;79:7135–45. doi: 10.1128/JVI.79.11.7135-7145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallichan W, Rosenthal K. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–90. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4-targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 9.McGhee J, Lamm M, Strober W. Mucosal immune responses. An overview. In: Ogra P, Lamm M, Bienenstock J, Mestecky J, Strober W, Mcghee J, editors. Mucosal Immunology. 2. San Diego, CA: Academic Press; 1999. pp. 485–506. [Google Scholar]
- 10.Imaoka K, Miller CJ, Kubota M, et al. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gag and cholera toxin adjuvant induces Th1/Th2 help for virus-specific immune responses in reproductive tissues. J Immunol. 1998;161:5952–8. [PubMed] [Google Scholar]
- 11.Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the Cholera toxin B subunit. Infect Immun. 1996;64:1272–83. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris MT. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–9. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantis NJ, Kozlowski PA, Mielcarz DW, Weissenhorn W, Neutra MR. Immunization of mice with recombinant gp41 in a systemic prime/mucosal boost protocol induces HIV-1-specific serum IgG and secretory IgA antibodies. Vaccine. 2001;19:3990–4001. doi: 10.1016/s0264-410x(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 14.Kowalczyk DW, Wlazlo AP, Shane S, Ertl HC. Vaccine regimen for prevention of sexually transmitted infections with human papillomavirus type 16. Vaccine. 2001;19:3583–90. doi: 10.1016/s0264-410x(01)00070-6. [DOI] [PubMed] [Google Scholar]
- 15.McCluskie MJ, Weeratna RD, Payette PJ, Davis HL. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol Med Microbiol. 2002;32:179–85. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 16.Marx PA, Compans RW, Gettie A, et al. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–7. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 17.Israel ZR, Gettie A, Ishizaka ST, et al. Combined systemic and mucosal immunization with microsphere-encapsulated inactivated simian immunodeficiency virus elicits serum, vaginal, and tracheal antibody responses in female rhesus macaques. AIDS Res Hum Retroviruses. 1999;15:1121–36. doi: 10.1089/088922299310412. [DOI] [PubMed] [Google Scholar]
- 18.Moldoveanu Z, Vzorov AN, Huang WQ, Mestecky J, Compans RW. Induction of immune responses to SIV antigens by mucosally administered vaccines. AIDS Res Hum Retroviruses. 1999;15:1469–76. doi: 10.1089/088922299309982. [DOI] [PubMed] [Google Scholar]
- 19.Eo SK, Gierynska M, Kamar AA, Rouse BT. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J Immunol. 2001;166:5473–9. doi: 10.4049/jimmunol.166.9.5473. [DOI] [PubMed] [Google Scholar]
- 20.Kanellos TS, Byarugaba DK, Russell PH, Howard CR, Partidos CD. Naked DNA when co-administered intranasally with heat-labile enterotoxin of Escherichia coli primes effectively for systemic B- and T-cell responses to the encoded antigen. Immunol Lett. 2000;74:215–20. doi: 10.1016/s0165-2478(00)00257-1. [DOI] [PubMed] [Google Scholar]
- 21.Bruhl P, Kerschbaum A, Eibl MM, Mannhalter JW. An experimental prime-boost regimen leading to HIV type 1-specific mucosal and systemic immunity in BALB/c mice. AIDS Res Hum Retroviruses. 1998;14:401–7. doi: 10.1089/aid.1998.14.401. [DOI] [PubMed] [Google Scholar]
- 22.Lee CK, Soike K, Giannasca P, Hill J, Weltzin R, Kleanthous H, Blanchard J, Monath TP. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–82. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]
- 23.Bergmeier LA, Mitchell EA, Hall G, Cranage MP, Cook N, Dennis M, Lehner T. Antibody-secreting cells specific for simian immunodeficiency virus antigens in lymphoid and mucosal tissues of immunized macaques. AIDS. 1998;12:1139–47. doi: 10.1097/00002030-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lehner T, Tao L, Panagiotidi C, et al. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J Virol. 1994;68:1624–32. doi: 10.1128/jvi.68.3.1624-1632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada H. One- and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv Drug Deliv Rev. 1997;28:43–70. doi: 10.1016/s0169-409x(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 26.Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16:153–7. doi: 10.1038/nbt0298-153. [erratum in Nat Biotechnol 1998; 16:478] [DOI] [PubMed] [Google Scholar]
- 27.O'Hagan DT. Prospects for the development of new and improved vaccines through the use of microencapsulation technology. In: Levine MM, Woodrow GC, Kaper JB, Cobon GS, editors. New Generation Vaccines. Vol. 2. New York: Marcel Dekker, Inc.; 1997. pp. 215–28. [Google Scholar]
- 28.Singh M, Vajdy M, Gardner J, Briones M, O'Hagan D. Mucosal immunization with HIV-1 gag DNA on cationic microparticles prolongs gene expression and enhances local and systemic immunity. Vaccine. 2001;20:594–602. doi: 10.1016/s0264-410x(01)00321-8. [DOI] [PubMed] [Google Scholar]
- 29.Schlesinger S. Alphavirus vectors: development and potential therapeutic applications. Expert Opin Biol Ther. 2001;1:177–91. doi: 10.1517/14712598.1.2.177. [DOI] [PubMed] [Google Scholar]
- 30.Rayner JO, Dryga SA, Kamrud KI. Alphavirus vectors and vaccination. Rev Med Virol. 2002;12:279–96. doi: 10.1002/rmv.360. [DOI] [PubMed] [Google Scholar]
- 31.Leitner WW, Hwang LN, deVeer MJ, et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med. 2003;9:33–9. doi: 10.1038/nmxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajdy M, Singh M, Ugozzoli M, et al. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology. 2003;110:86–94. doi: 10.1046/j.1365-2567.2003.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. 1-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 34.Csencsits KL, Walters N, Pascual DW. Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: alpha (E) versus 1-selectin. J Immunol. 2001;167:2441–5. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- 35.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 36.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 37.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 38.Abitorabi MA, Mackay CR, Jerome EH, Osorio O, Butcher EC, Erle DJ. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol. 1996;156:3111–7. [PubMed] [Google Scholar]
- 39.Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Muller W, Greenberg HB. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- 40.Kantele A, Westerholm M, Kantele JM, Makela PH, Savilahti E. Homing potentials of circulating antibody-secreting cells after administration of oral or parenteral protein or polysaccharide vaccine in humans. Vaccine. 1999;17:229–36. doi: 10.1016/s0264-410x(98)00193-5. [DOI] [PubMed] [Google Scholar]
- 41.Youngman KR, Franco MA, Kuklin NA, Rott LS, Butcher EC, Greenberg HB. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J Immunol. 2002;168:2173–81. doi: 10.4049/jimmunol.168.5.2173. [DOI] [PubMed] [Google Scholar]
- 42.Quiding-Jarbrink M, Nordstrom I, Granstrom G, et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–6. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csencsits KL, Jutila MA, Pascual DW. Mucosal addressin expression and binding interactions with naive lymphocytes vary among the cranial, oral, and nasal-associated lymphoid tissues. Eur J Immunol. 2002;32:3029–39. doi: 10.1002/1521-4141(200211)32:11<3029::AID-IMMU3029>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Perri S, Greer CE, Thudium K, et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J Virol. 2003;77:10394–403. doi: 10.1128/JVI.77.19.10394-10403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polo JM, Belli BA, Driver DA, et al. Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors. Proc Natl Acad Sci USA. 1999;96:4598–603. doi: 10.1073/pnas.96.8.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perri S, Driver DA, Gardner JP, Sherrill S, Belli BA, Dubensky TW, Jr, Polo JM. Replicon vectors derived from Sindbis virus and Semliki forest virus that establish persistent replication in host cells. J Virol. 2000;74:9802–7. doi: 10.1128/jvi.74.20.9802-9807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vajdy M, Gardner J, Neidleman J, Cuadra L, Greer C, Perri S, O'Hagan D, Polo JM. Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles. J Infect Dis. 2001;184:1613–6. doi: 10.1086/324581. [DOI] [PubMed] [Google Scholar]
- 48.Vajdy M, Singh M, Kazzaz J, et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intra-nasal and parenteral immunizations. AIDS Res Hum Retroviruses. 2004;20:1269–81. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]
- 49.McKenzie BS, Corbett AJ, Brady JL, Boyle JS, Rockman SP, Lew AM. Targeting lymphocyte Peyer's patch adhesion molecule-1: a relay approach to gut immunization. Vaccine. 2005;23:3668–78. doi: 10.1016/j.vaccine.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Pascual DW, White MD, Larson T, Walters N. Impaired mucosal immunity in 1-selectin-deficient mice orally immunized with a Salmonella vaccine vector. J Immunol. 2001;167:407–15. doi: 10.4049/jimmunol.167.1.407. [DOI] [PubMed] [Google Scholar]
- 51.Csencsits KL, Pascual DW. Absence of 1-selectin delays mucosal B cell responses in nonintestinal effector tissues. J Immunol. 2002;169:5649–59. doi: 10.4049/jimmunol.169.10.5649. [DOI] [PubMed] [Google Scholar]
- 52.Picker LJ, Martin RJ, Trumble A, Newman LS, Collins PA, Bergstresser PR, Leung DY. Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol. 1994;24:1269–77. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in 1-selectin-deficient mice. J Exp Med. 1996;183:589–98. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for 1-selectin during immune responses. J Exp Med. 1996;184:2341–51. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vajdy M, Srivastava I, Polo J, Donnelly J, O'Hagan D, Singh M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol Cell Biol. 2004;82:617–27. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]