Abstract
There is currently great interest in the idea of using helminth-derived molecules for therapeutic purposes and indeed we have shown that ES-62, a filarial nematode-derived phosphorylcholine-containing glycoprotein, significantly reduces the severity of arthritis in a murine model. Clearly, knowledge of mechanism of action is important when considering molecules for use in treating disease and although much is known regarding how ES-62 interacts with the immune system, gaps in our understanding remain. A feature of filarial nematode infection is a defective, T helper 2 (Th2)-polarized antigen-specific T-cell response and in relation to this we have recently shown that ES-62 inhibits clonal expansion and modulates effector function towards a Th2 phenotype, of antigen-specific T cells in vivo. ES-62 is also known to directly modulate B-cell behaviour and hence to determine whether it was mediating these effects on T cells by disrupting B–T-cell co-operation, we have investigated antigen-specific responses using an adoptive transfer system in which traceable numbers of tg ovalbumin (OVA)-specific T cells and hen egg lysozyme (HEL)-specific B cells respond to a chemically coupled form of OVA–HEL that contains linked epitopes that promote cognate T- and B-cell interactions. Surprisingly, these studies indicate that activated B cells restore T-cell expansion and prevent Th2-like polarization. However, ES-62-treated double cell transfer mice demonstrate a more generalized immunosuppression with reduced levels of Th1 and -2 type cytokines and antibody subclasses. Collectively, these results suggest that whilst ES-62 can target B–T-cell co-operation, this does not promote polarizing of T-cell responses towards a Th2-type phenotype.
Keywords: B cells, ES-62, helminth, immunomodulation, T cells
Introduction
Parasitic filarial nematodes represent highly significant causes of ill health for humans in the Third World.1 Infection with these worms persists for many years2 and it is now accepted that this is caused by the parasites interfering with the host immune system.3,4 Thus, filarial nematode infections are associated with altered T-cell effector responses5 not only in terms of inhibition of parasite-specific immune responses6 but also with respect to non-specific stimuli7 and bystander antigens, such as routine vaccinations.8,9 There is agreement that the immunomodulation incorporates not only impairment of lymphocyte proliferation but also a bias in production of both cytokines and antibodies. With respect to cytokines there is reduced release of interferon-γ (IFN-γ) and increased synthesis of interleukin (IL)-10. For antibodies, there are imbalances in immunoglobulin G (IgG) subclasses – greatly elevated IgG4 (often considered a ‘blocking’ antibody) and decreases in other IgG subclasses. Overall, the picture is of an immune response demonstrating a somewhat suppressed, anti-inflammatory, T helper 2 (Th2)-like, phenotype. It has been speculated that such a phenotype is conducive to both parasite survival and, by limiting pathology resulting from aggressive immune responses, host health.
The basis of this parasite-driven immunomodulation has been studied extensively and it is now clear that excretory–secretory (ES) products released by filarial nematodes subvert the host immune system to help maintain infection and parasite survival.10 We have previously characterized the immunomodulatory activities of one such molecule from the rodent filarial nematode Acanthocheilonema viteae, ES-62, which is also produced by human filarial nematodes.11–18 Specifically, these studies showed that ES-62 inhibits the ability of B and T lymphocytes to respond to ligation of their antigen receptors by rendering cells hypo-responsive to stimulation in vitro (reviewed in 19). It can also bias the immune response towards a Th2 phenotype, thereby preventing the induction of Th1-mediated-pathology, which would be deleterious to both host and parasite.15–17 More recently we have shown that ES-62 modifies clonal expansion and effector function of antigen-specific T cells in vivo. Demonstrating this has involved exploiting a model in which T cells bearing a transgenic (tg) T-cell receptor (TCR) specific for the major immunodominant epitope of the model antigen, ovalbumin (OVA) are transferred into normal BALB/c mice in numbers large enough to trace in vivo with an anti-TCR-specific antibody, but small enough to reflect, and indeed not interfere with, the normal physiological response to antigen.20–22 Using this model, we showed that exposure to ES-62 in vivo modulates responses to heterologous antigen by biasing T-cell effector function towards a modified Th2-like cytokine and antibody phenotype.23 Thus, ES-62-treated mice exhibited a Th2-biased IgG antibody response as evidenced by stable enhancement of anti-OVA IgG1 production and a profound inhibition of anti-OVA IgG2a. Consistent with this, T cells from ES-62-treated mice produced a Th2-like cytokine response when re-challenged with antigen ex vivo. Moreover, such T cells produced lower levels of IL-2 and proliferated less upon antigen re-challenge ex vivo. Finally, pre-exposure to ES-62 inhibited the clonal expansion of the transferred antigen-specific CD4+ T cells and altered the functional response of such T cells in vivo, by modulating the kinetics and reducing the extent of their migration into B-cell follicles.23
The precise target of ES-62 was not revealed by these studies, but it is generally accepted that dendrtitic cells (DC) are the primary cell type responsible for priming naïve CD4+ T cells in vivo. Indeed, we have previously shown that ES-62 can functionally modulate bone marrow derived (bm)-DC maturation in vitro and in vivo.16,24 Thus, ES-62 may be modulating effector CD4 T-cell function in vivo via modulation of DC maturation and hence, T-cell priming. However, B cells which are also a direct target of ES-62, similarly have the capacity to present antigen to T cells, although it is has been proposed that this process take place after T cells have initially been activated by DC in the paracortex. These B-cell–T-cell interactions are important for promoting the production of early IgM antibody and the induction of signals required for germinal centre formation and high affinity class-switched antibody responses. Furthermore, it has recently been proposed that interactions between antigen-specific T and B cells are required for CD4+ T cells to undergo maximal clonal expansion, terminal differentiation and to acquire effector and memory function.25 Consequently, the observed reduction in antigen-specific T-cell expansion, altered anti-OVA IgG antibody production and differential cytokine production following ex vivo re-challenge in ES-62 treated transfer recipients, could also be the result of lesions in T- and B-cell interactions. Indeed, examination of the migratory properties of antigen-specific T cells revealed that ES-62 reduced their migration into B cell follicles.23 Therefore to determine whether ES-62 was mediating its effects on effector T-cell function by disrupting B–T-cell co-operation, we have investigated OVA responses using the adoptive transfer system in which traceable numbers of OVA-specific tg T cells and hen egg lysozyme (HEL)-specific tg B cells respond to a chemically coupled form of OVA–HEL that contains linked epitopes that promote cognate T- and B-cell interactions. Surprisingly, these studies have demonstrated that the presence of activated B cells restored T-cell expansion and prevented Th2-like polarization of effector function. At the same time, these ES-62-treated mice demonstrated a more generalized functional immunosuppression with reduced levels of both Th1 and -2 type cytokines and antibody subclasses. Collectively, these findings suggest that whilst ES-62 can target B–T-cell co-operation, it does not utilize this mechanism for polarizing T-cell responses towards an anti-inflammatory/Th2-type phenotype.
Materials and methods
Animals
Eight-week-old, male BALB/c mice (H-2d/d, IgMa) were purchased from Harlan-Olac (Oxford, UK) and used as recipients. Mice homozygous for the transgenic TCR that is specific for chicken (c) OVA peptide323−339 in the context of I-Ad were used as T-cell donors. The tg TCR (which is expressed on 70–80% of the CD4+ T cells from DO.11.10 BALB/c mice and recognizes the major immunodominant epitope of chicken ovalbumin) was detected by flow cytometry using the clonotypic monoclonal antibody KJ1.26.20,26 Similarly, mice heterozygous for the anti-HEL IgMa and IgDa transgenes on a BALB/c background (MD4) were screened for the expression of the MD4 transgenes by flow cytometry and positive mice were used as B-cell donors. All animals were specified pathogen-free and were maintained under standard animal house conditions at the University of Glasgow Central Research Facilities in accordance with local and home office regulations.
Preparation of ES-62
ES-62 is a major secreted glycoprotein of the rodent filarial nematode Acanthoceilonema viteae and homologue of molecules found in filarial nematodes that parasitize humans. The molecule consists of a tetramer of identical monomers of 62 000 MW that contain phosphorylcholine (PC) attached to N-type glycans.27 ES-62 was purified to homogeneity from spent culture medium of adult A. viteae using endotoxin-free reagents essentially as described previously.11 Purity and identity of each batch was confirmed by a combination of sodium dodecyl sulphate–polyacrylamide gel electrophoresis and Western blotting. That ES-62 preparations were truly endotoxin-free was confirmed using an Endosafe kit (Charles River Laboratories, Margate, UK).
Flow cytometry
Analysis of cell surface marker expression was performed as described previously.22,28 Briefly for detection of CD4+ DO11.10 tg T cells, the cell suspensions were incubated with phycoerythrin (PE)-conjugated anti-CD4 and biotinylated clonotypic anti-TCR antibody KJ1.26 with fluoroscein isothiocyanate (FITC)-conjugated streptavidin (all BD Pharmingen, Oxford, UK) and for detection of B220+ MD4 B cells, cell suspensions were detected as above using PE-conjugated anti-B220 (BD Pharmingen) and biotinylated HEL or anti-IgMa (BD Pharmingen) with FITC-conjugated streptavidin at concentrations previously determined by titration of optimum binding. Immediately prior to data acquisition 50 µg/ml propidium iodide (Calbiochem, Nottingham, UK) was added to each sample to enable exclusion of dead cells from the analyses. Cellular fluorescence data were acquired using a Becton Dickinson FACSCaliber™ flow cytometer and analysed using FlowJo software (Tree Star Inc, OR).
Adoptive transfer and immunization
Peripheral lymph nodes (axillary, brachial, inguinal, cervical; PLN), mesenteric lymph nodes and spleens from MD4 BALB/c and DO.11.10 BALB/c mice were pooled and forced through Nitex (Cadisch Precision Meshes, London, UK). The suspensions were washed in sterile RPMI-1640 (Life Technologies, Paisley, UK). Cells were washed by adding 1 ml of washing medium before the suspensions were centrifuged at 400 g for 5 min The percentage of IgMa+ B220+ MD4 B cells or KJ1.26+ CD4+ DO.11.10 T cells in these preparations was determined by flow cytometric analysis as described above. Cell suspensions containing either 2·5 × 106 transgenic (tg) T cells (single transfer) or 2·5 × 106 both tg T and B cells (double transfer) each in 100 µl were mixed and 200 µl was injected intravenously (i.v.) into age matched, male BALB/c recipients as described previously.22 Where indicated, adoptive transfer recipient mice were injected subcutaneously (s.c.) with 2 µg ES-62 in phosphate-buffered saline (PBS) three times in total, 2 days before transfer, on the day of transfer and the following day when mice were also immunized. Such quantities of ES-62 are similar to those used in ameliorating collagen-induced arthritis (CIA)29 and will give serum concentrations within the range found for PC-containing molecules in filarial nematode infection (e.g. see refs 18,30). In indicated experiments, cells (5 × 107 cells/ml) were labelled with CFSE (5 µm 5-(and-6-) carboxyfluorescein diacetate, succinimidyl ester (5(6)-CFDA, SE; Molecular Probes Inc., Eugene, OR) prior to transfer as described above.22
Following adoptive transfer, recipient mice were exposed to antigen by immunizing s.c. in the scruff of the neck with either 130 µg of OVA–HEL conjugates or molar equivalents of OVA and HEL conjugates in 100 µl PBS/50% complete Freund's Adjuvant (CFA) (Sigma, Poole, UK). OVA–HEL and OVA–OVA or HEL–HEL conjugates were prepared by gluteraldehyde treatment as described previously.28
Measurement of antigen-specific proliferation ex vivo
Proliferation of antigen-specific T cells was assessed by the [3H]-thymidine uptake assay to evaluate DNA synthesis. Briefly, on day 10-post immunization, PLN were removed and single cell suspensions prepared as described above. Cells (2 × 105 cells/well) were cultured in triplicate in flat bottomed 96-well plates in RPMI-1640 medium supplemented with 2 mm glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin and 10% fetal calf serum (FCS; Sigma). Cells were stimulated with 10 µg/ml OVA323−339 peptide at a final well volume of 200 µl for 48 hr at 37° in a 5% (v/v) CO2 atmosphere at 95% humidity. DNA synthesis was assessed by pulsing with 0·5 µCi/well [6-3H]-thymidine (Amersham Pharmacia Biotech, Amersham, UK) for the last 4 hr of culture to allow incorporation into cellular DNA. Cells were harvested onto glass fibre filter mats (Wallac, Warrington, UK) using a Betaplate 96-well harvester (Amersham Pharmacia Biotech). Incorporated label was assessed by liquid scintillation counting and results are expressed as counts per minute (c.p.m.) incorporated ± SEM (of pooled triplicate values of three individual mice).
Measurement of antigen-specific cytokine production ex vivo
PLN were removed and single cell suspensions prepared as described above. Cells (2 × 106 cells/well) were cultured in triplicate in flat bottomed 24-well plates (Cellstar) in RPMI-1640 medium supplemented with 2 mm glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin and 10% FCS (Sigma). Cells were stimulated with 10 µg/ml OVA323−339 peptide at a final well volume of 1 ml. Cells were cultured for 72 hr at 37° in a 5% (v/v) CO2 atmosphere at 95% humidity. Cell culture supernatant was then removed and frozen at −20° until analysis.
Cytokine enzyme-linked immunosorbent assays (ELISA) were performed according to the antibody supplier's recommendations. IL-2, IL-4, IL-5, IL-10 and IFN-γ were analysed using OPTEIA Mouse ELISA kits (BD Pharmingen) and transforming growth factor-β (TGF-β) was analysed by Immunoassay ELISA kit system (Promega, Cheshire, UK). Briefly, Nunc-immuno Maxisorp plates were coated overnight at 4° in 0·1 m NaHCO3 buffer, pH 7·4 (or 0·2 m sodium phosphate buffer, pH 6·5 for IL-10 ELISA) before blocking for 2 hr with 10% FCS in PBS at 37° and samples and standard were diluted in culture medium and incubated for 1 hr at 37° or overnight at 4°. Detection antibodies and ExtrAvidin (Sigma) were diluted in blocking buffer and incubated for 1 hr each at 37°. Plates were washed at least five times between stages. Finally, plates were developed using tetramethylbenzidine (TMB) substrate and absorbances at 630 nm were determined. In some experiments, the production of cytokines from cell cultures supernatants was also analysed using Cytometric Bead Array assay (CBA; BD Pharmingen) according to the manufacturer's instructions. Briefly, five bead populations with distinct fluorescent intensities were coated with capture antibodies specific for IL-2, IL-4, IL-5 and IFN-γ. These cytokine capture beads were mixed with PE-conjugated detection antibodies and then incubated with recombinant cytokine standards or 50 µl test culture supernatant. Following acquisition of sample data using a Becton Dickinson FACSCalibar™ flow cytometer, data were analysed using the BD CBA Analysis Software (BD Biosciences).
Detection of serum antibodies from adoptive transfer recipients
To detect B cell-derived anti-HEL IgMa in serum, Nunc-Immuno plates (Nalge Nunc International, Denmark) were coated with 100 µl of 20 µg/ml HEL in PBS at 4° overnight. Plates were then washed at least three times with PBS−0·05% Tween 20 (Sigma) before being blocked with PBS−10% FCS for 1 hr at 37°. Plates were washed and incubated with serially diluted serum samples at 4° overnight before further washing. IgMa levels in serum were determined by incubation with biotinylated anti-IgMa (2 µg/ml; BD Pharmingen) for 1 hr at 37°. Plates were then washed and incubated with Extravidin (1/1000; Sigma) for 1 hr at 37°. Plates were washed again and TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added. All ELISAs were read on a plate reader at 630 nm. The above protocol was similarly used to detect anti-OVA IgG1, IgG2a, total IgG and IgE, except the plates were coated with 100 µl of 20 µg/ml OVA (biotinylated anti-IgMa was substituted with either 2 µg/ml biotinylated anti-IgG, IgG1, IgG2a or IgE (BD Pharmingen)).
Laser scanning cytometry (LSC)
Sections were stained as described previously.22 Briefly, PLN were frozen in liquid nitrogen in OCT embedding medium (Miles Diagnostic Division, Elkart, IN) and stored at −70°. Sections (8 µm) were cut, mounted on microscope slides (Shandon Co, Runcorn, UK) and stained immediately with biotinylated anti-KJ1.26 and FITC-anti-B220 (BD Pharmingen), for 30 min, washed and then incubated with streptavidin–horseradish peroxidase (HRP) for 30 min After washing, the cells were treated with biotinylated-tyramide (TSA™ Biotin system, Perkin Elmer Life Sciences, Boston, MA) for 10 min, washed and then incubated with Streptavidin Alexa Fluor® 647 (Molecular Probes) for 30 min. Finally, slides were washed three times and mounted in Vectashield (Vector, Peterborough, UK) for analysis by LSC. Tissue maps were generated from this data. Upon these tissue maps, equally sized regions were randomly placed within follicular and paracortical areas. This allowed statistical data on the number and percentage of KJ1.26+ located within B-cell follicles to be determined using Wincyte software (Compucyte Corp, Essex, UK). Using the relocation feature of the LSC, areas surrounding follicular regions were relocated to and high quality digital images of the fluorescently stained tissue sections were obtained using a Hammamatsu camera and Openlab software (Improvision, Coventry, UK).
Statistical analysis
Statistical significance was determined by Student's t-test and P < 0·05 was considered to be significant.
Results
Recipients of double cell transfers are protected from the inhibitory effects of ES-62 on T-cell expansion
To investigate whether the polarizing effects of ES-62 on antigen-specific T-cell responses in vivo23,31 were mediated via modulation of T-cell and B-cell interactions, mice were either transferred with 2·5 × 106 OVA-specific TCR tg CD4+ T cells from donor DO.11.10 mice (single transfer) as before, or cotransferred with 2·5 × 106 OVA-specific TCR tg CD4+ T cells from donor DO.11.10 mice and 2·5 × 106 HEL-specific B-cell receptor (BCR) tg B220+ B cells from donor MD4 mice (double transfer). All groups were subsequently immunized with OVA chemically coupled to HEL (OVA–HEL), which contains linked epitopes that can be recognized by both tg T and B cells. Importantly, this promotes cognate interactions between OVA-specific Tcells and HEL-specific B cells, which have been shown to be required for optimum HEL-specific B-cell responses.21
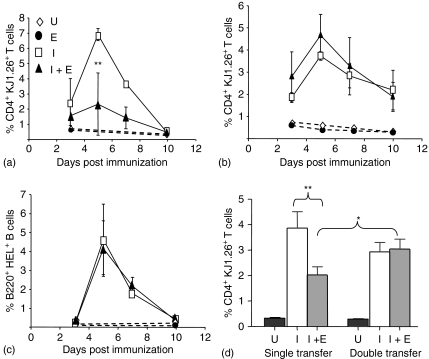
Unlike our previous adoptive transfer experiments where mice were only transferred with OVA-specific T cells (single transfer) (Fig. 1a), treatment of mice with ES-62 did not inhibit antigen-specific T-cell clonal expansion when both antigen-specific T and B cells were transferred together (double transfer) (Fig. 1b). The transfers were performed simultaneously and under identical experimental conditions, using the same donor pool of T cells and same batch of recipient mice. Indeed, in the double transfer, both ES-62 treated and control groups had very similar kinetics and profiles of expansion. Similarly, both control and ES-62 treated groups, demonstrated analogous levels of B-cell expansion, which peaked at day 5 post immunization and subsequently declined towards day 10 (Fig. 1c). Importantly, because B cells require T-cell help to undergo optimal clonal expansion21,32 the analogous levels of HEL-specific B-cell expansion between ES-62 and control groups, strongly suggests that ES-62 was not preventing interactions between OVA-specific T cells and HEL-specific B cells. Similar results were obtained when mice were transferred with a variety of T : B cell ratios ranging from 0·25 : 1 to 10 : 1, with ES-62 treated groups showing comparable levels of T-cell expansion levels to those in control groups (results not shown). Although the peak T-cell clonal expansion obtained in response to control mice appeared to be higher in single relative to double transfers, analysis of the day 5 T-cell clonal expansion data pooled from four independent experiments, indicated that these differences were not significant. Nevertheless, this analysis confirmed that exposure to ES-62 in vivo significantly inhibited T-cell expansion in single but not double transfers (Fig. 1d).
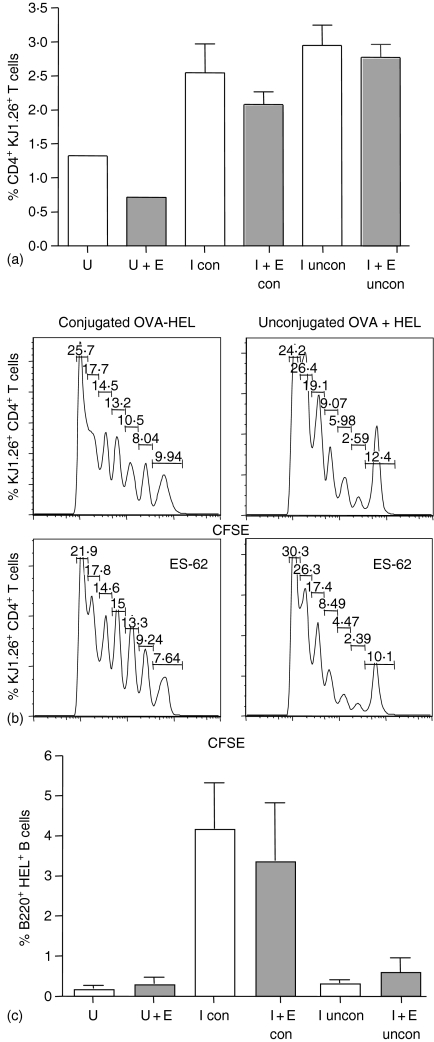
Figure 1.
Effects of ES-62 on antigen-specific CD4+ T cell and B220+ B-cell clonal expansion in vivo. Non-transgenic BALB/c recipient control and ES-62 treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells (a) or 2·5 × 106 CD4+ KJ1.26+ T cells and B220+ HEL+ B cells (b and c). Twenty-four hr later, these mice were immunized s.c with 130 µg OVA–HEL in CFA. The percentage of CD4+ KJ1.26+ T cells (a and b) and B220+ HEL+ B cells (c) in the draining lymph nodes of adoptively transferred recipients was assessed by flow cytometry on days 3, 5, 7 and 10 after immunization. Each time point represents the mean ± SEM for three mice per group, **P < 0·01 where U represents unimmunized, control mice; E represents unimmunized, ES-62-treated mice; I represents immunized control mice and I + E represents immunized, ES-62-treated mice. Data are representative of at least four independent experiments and the day 5 T-cell clonal expansion data from four of these experiments have been pooled and presented in (d) **P < 0·01 for I (immunized, control mice) versus I + E (immunized, ES-62-treated mice) from single transfer experiments and *P < 0·05 for immunized, ES-62-treated single transfer versus double transfer mice.
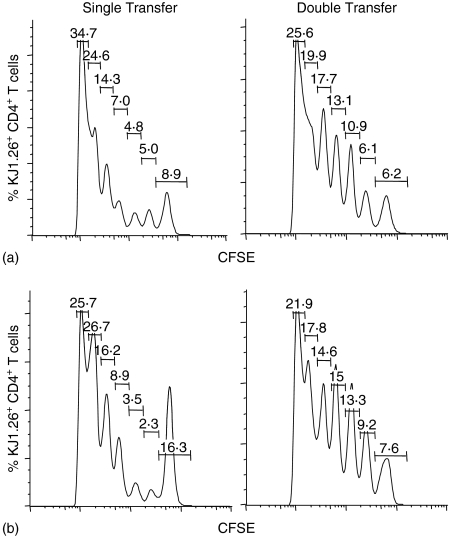
Our previous experiments examining the number of cell divisions undergone by adoptively transferred CFSE-labelled OVA-specific T cells in vivo23 provided corroborative evidence of ES-62-mediated inhibition of antigen-specific T-cell expansion (as indicated by flow cytometry analysis of the tg TCR expression) rather than migration. Therefore, the profile of CFSE staining of OVA-specific T cells in control and ES-62 treated single and double transfer mice was examined (Fig. 2). In the single transfer system it can be seen as established previously that ES-62 induces a population of non-dividing cells – 16·3% versus 8·9% for the control (Fig. 2 and ref 23). The mean value obtained from four independent experiments for this population was 14·7 ± 2·4% of cells versus 8·6 ± 1·5% in the absence of ES-62. Although this difference may appear on the small side when considering the ∼60% reduction in clonal expansion induced by ES-62, it is possible that the helminth product targets the most actively dividing cells and/or that it may after all additionally affect T-cell migration/homeostasis. These points were addressed by the quantitative, statistical analysis afforded by FloJo software which indicated that whilst exposure to ES-62 reduced the percentage of cells that had divided from 32·3 ± 2·1% to 20·5 ± 3·5% (numbers consistent with the increase in the non-dividing population cited above), it did not significantly affect the proliferation index (i.e. the average number of divisions of the cells that divided underwent; 2·95 ± 0·09 versus 2·27 ± 0·62). However, it did substantially reduce the division index (i.e. average number of divisions that a cell in the starting population has undergone) from 0·95 ± 0·02 to 0·56 ± 0·06, P < 0·01 suggesting that ES-62 may have indeed converted a small population of highly dividing cells into a non-responsive population. By contrast, tg T cells from the double transfer from both control and ES-62 groups exhibited very similar CFSE staining of tg T cells indicating essentially identical populations of non-dividing cells at day 5 post immunization (8·4 ± 1·2% versus 8·1 ± 0·7%, n = 4 independent experiments) results consistent with the similar levels of clonal expansion observed. Moreover, exposure to ES-62 had no significant effect on any of the division (0·97 ± 0·10 versus 0·90 ± 0·05), proliferation (2·96 ± 0·34 versus 2·94 ± 0·20) or percentage cells divided, indices (33·0 ± 1·7% versus 32·3 ± 1·8%). Taken together, these results suggest that the presence of a large population of activated antigen-specific B cells restores the proliferative capacity of OVA-specific T cells in ES-62-treated mice.
Figure 2.
Effects of antigen-specific B cells on the ability of ES-62 to modulate cell division of antigen-specific CD4+ T cells in vivo following immunization. Non-transgenic BALB/c recipient control and ES-62-treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells (single transfer) or 2·5 × 106 CD4+ KJ1.26+ T cells and B220+ HEL+ B cells (double transfer). Prior to transfer, T cells were labelled with CFSE. Twenty-four hr later, these mice were immunized s.c. with 130 µg OVA–HEL in CFA. Five days after immunization, draining lymph nodes from immunized control (a) and immunized and ES-62-treated (b) adoptive transfer recipients were removed and CFSE fluorescence, a measure of cell division, was assessed in CD4+ KJ1.26+ T cells by flow cytometry. Data are shown as histograms of CFSE fluorescence intensity (increasing from left to right) versus number of CD4+ KJ1.26+ T cells and are from one mouse, which is representative of three mice per group and a total of two independent experiments. Each peak represents a cell division event with the number of cell divisions increasing from right to left. The percentage of cells present in each peak is shown within the figure.
Exposure to ES-62 is associated with inhibition of both Th1 and Th2 antibodies in double cell transfer mice
The nature of the double transfer system allows the helper ability of the OVA-specific T cells for anti-HEL IgM production by the transferred HEL-specific B cells to be examined. This is because immunization with the linked OVA–HEL antigen permits HEL-specific B cells to endocytose the antigen via their BCR and subsequently process and present OVA epitopes to the transferred OVA-specific T cells. Therefore, because B cells require T-cell-mediated help for IgM production21,32 the effects of ES-62 on the ability of OVA-specific T cells to provide help to HEL-specific B cells can be investigated.
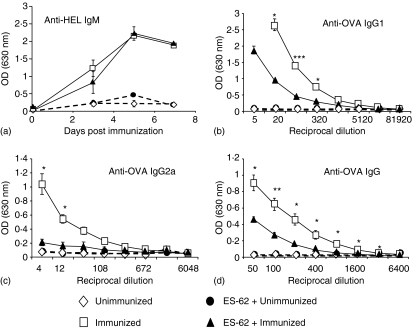
Thus to examine the effects of ES-62 on the functional response of the transferred HEL-specific B cells, the production of anti-HEL IgM in serum prepared from mice at 3, 5 and 7 days post immunization was analysed. Both control and ES-62-treated groups demonstrated comparable kinetics and levels of antibody production, indicating that ES-62 was not altering the ability of OVA-specific T cells to provide help for IgM production (Fig. 3a). Furthermore, this finding, together with the earlier data demonstrating that ES-62 did not affect HEL-specific B-cell clonal expansion, strongly argued that ES-62 was not preventing OVA-specific T-cell and HEL-specific B-cell interactions. The production of anti-OVA IgM by endogenous B cells was also examined, but no significant production could be detected in immunized groups (results not shown).
Figure 3.
Effects of ES-62 on the anti-HEL IgM response and of antigen-specific B cells on the ability of ES-62 to modulate the production of OVA-specific IgG1 and IgG2a in vivo. Non-transgenic BALB/c recipient control and ES-62-treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells and B220+ HEL+ B cells. Twenty-four hr later, these mice were immunized s. c with 130 µg OVA–HEL in CFA. In (a), serum (1/200 dilution) was prepared at 3, 5 and 7 days after immunization and HEL-specific IgMa was assessed by ELISA. Each time point represents the mean OD at 1/200 dilution for three mice per group ± SEM. Data are representative of two independent experiments. In (b–d), serum was collected at 10 days after immunization and OVA-specific IgG1 (b), IgG2a (c) and IgG (d) were assessed by ELISA. Data are presented as the mean of three mice per group ± SEM and are representative of two independent experiments. ***P < 0·005, **P < 0·01, *P < 0·05.
One of the most striking effects of ES-62 treatment in the previously presented single transfer experiments was that ES-62-treated mice exhibited a Th2-biased anti-OVA IgG response, evidenced by increased anti-OVA IgG1 production and inhibition of anti-OVA IgG2a (ref. 23 and results not shown). In contrast, in double transfers, ES-62 treatment resulted in suppression of both Th1 and Th2 associated antibodies at day 10 post immunization (Figs 3b, c). Not only did ES-62 treated mice have significantly lower levels of anti-OVA IgG1 than control animals (Fig. 3b), but the production of anti-OVA IgG2a in ES-62 treated mice was completely abrogated, exhibiting levels comparable to those in unimmunized animals (Fig. 3c). Consistent with this the production of total anti-OVA IgG was also lower in ES-62-treated mice (Fig. 3d), also indicating that the decrease in IgG1 and IgG2a production in ES-62-treated animals was not likely to be associated with increased production of another IgG isotype, such as IgG3. Furthermore, in agreement with single transfer data, ES-62 did not induce the production of anti-OVA IgE as no anti-OVA IgE could be detected in either ES-62-treated or control mice (data not shown). Taken together, these results suggest that although the interactions between OVA-specific T cells and HEL-specific B cells may not be prevented by ES-62, the signals being delivered to endogenous OVA-specific B cells by such T cells are modulated following ES-62 treatment.
ES-62 has no effect on the localization of antigen-specific T cells within draining lymph nodes in double cell transfer recipients
In order for antigen-specific B cells to undergo affinity maturation and class switching of immunoglobulin transcripts for IgG antibody production, germinal centres must be formed within B-cell follicle regions.33 This process of germinal centre formation and production of high affinity class-switched IgG antibodies requires interactions between antigen-specific T and B cells34–36 and thus, T-cell migration into B-cell follicles has been proposed to play an essential role in the generation of an IgG-mediated immune response. Therefore, using laser-scanning cytometry, it was investigated whether the decreased production of both IgG1 and IgG2a in ES-62-treated double transfer mice was caused by inhibition of antigen-specific T-cell migration into B-cell follicles. Indeed, in single transfers we have already shown that ES-62 had the capacity to inhibit T-cell migration within lymph nodes following immunization.23
Surprisingly, and unlike the single transfer data, ES-62 treatment of double transfer recipient mice had effectively no impact on the migration of antigen-specific T cells into B-cell follicles. Thus, when the average number and percentage of KJ1.26+ T cells contained within the follicle was calculated it was found that ES-62 was having little effect. Both groups demonstrated similar number of KJ1.26+ T cells within follicles (data not shown) and these were equivalent to comparable percentages of the total numbers contained within the whole lymph node under study (at day 5, control: 1·5 ± 0·1% and ES-62: 1·3 ± 0·2%).
ES-62 inhibits Th1/Th2 cytokine production and cell proliferation following ex vivo rechallenge of lymph node cells from double transfer recipients
In contrast to single transfer mice23 ES-62 treatment of double transfer mice resulted in suppression of anti-OVA IgG1 and IgG2a production, rather than promoting an IgG1-biased response. Because the suppressed IgG antibody production could not be explained by reduced antigen-specific T-cell entry into B-cell follicles, it was hypothesized that perhaps T cells activated in the presence of ES-62 in double transfer recipients were non-functional and did not produce the cytokines necessary to support anti-OVA IgG1 or IgG2a production. Alternatively, the reduced production of anti-OVA IgG1 and IgG2a antibodies could reflect the possibility that T cells activated in ES-62 treated double transfer mice were induced to develop into regulatory T cells that suppressed the generation of anti-OVA IgG responses. Therefore, similarly to before, lymph node cells were removed at 10 days post immunization and re-challenged ex vivo with OVA to examine the cytokine profile of the culture supernatant.
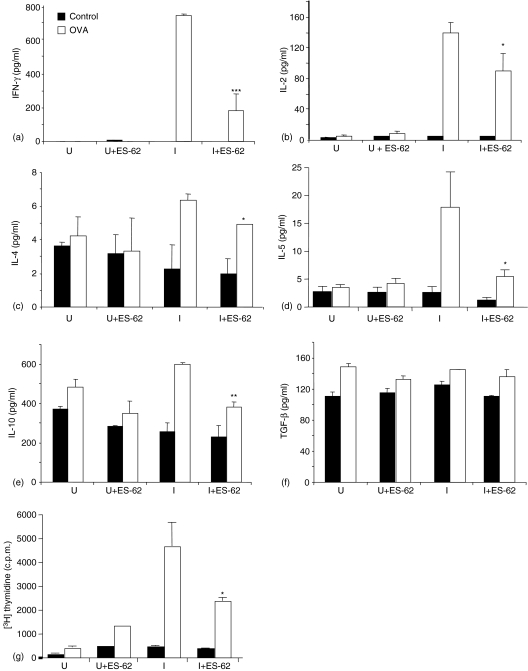
Consistent with the idea that suppression of anti-OVA IgG1 and IgG2a production may reflect ES-62-mediated modulation of cytokine production, ES-62 treated mice exhibited inhibition of both Th1 and Th2 associated cytokines upon ex vivo re-challenge (Fig. 4a–d). Analysis of cell culture supernatants demonstrated that compared to control groups, ES-62 treated groups produced less IL-2 and IFN-γ as well as IL-4 and IL-5. The inhibition of IL-5 production in ES-62-treated mice was unique in being in contrast to that observed with single transfer data in which IFN-γ, IL-2, IL-6, IL-17, IL-4 and IL-13 were all reduced and IL-5 production was elevated in cells that had been exposed to ES-62 in vivo.23
Figure 4.
Effects of antigen-specific B cells on ES-62-mediated modulation of antigen-specific CD4+ T cell cytokine production and proliferation following antigen re-challenge ex vivo. Non-transgenic BALB/c recipient control and ES-62 treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells and 2·5 × 106 B220+ HEL+ B cells. Twenty-four hr later, these mice were immunized s. c with 130 µg OVA–HEL in CFA. Draining lymph nodes were removed 10 days post immunization and cells were stimulated in vitro with 10 µ g /ml Ova323−339 peptide for 72 hr. Cell culture supernatants from unimmunized control (U) and ES-62-treated (U + ES-62) and immunized control (I) and ES-62-treated (I + ES-62) groups, were then removed and assessed for production of IFN-γ (a), IL-2 (b), IL-4 (c) and IL-5 (d) by CBA assay and IL-10 (e) and TGF-β (f) by ELISA. Data are presented as the mean from three mice per group ± SEM. Data presented for TGF-β production is from a single experiment, whereas IL-2, IFN-γ, IL-4, and IL-5 are representative of two other independent experiments using conventional cytokine analysis by ELISA (data not shown). ***P < 0·005, **P < 0·01, *P < 0·05 compared to relative immunized group. In (g), cells from unimmunized control (U), unimmunized ES-62-treated (U + ES-62), immunized control (I) and immunized, ES-62-treated (I + ES-62) groups were cultured for 48 hr and pulsed with 0·5 µCi/well [6-3H]-thymidine for the last 4 hr of culture. Incorporated, [3H]-thymidine was assessed by liquid scintillation counting. Data are presented as the mean from three mice per group ± SEM and are representative of two independent experiments. *P < 0·05, OVA recall responses, control/immunized versus ES-62/immunized groups.
The overall suppression in IgG production and both pro-inflammatory/Th1 and Th2 cytokines could have reflected the preferential production of IL-10 and TGF-β by T cells from ES-62 treated mice. These cytokines have been shown to mediate suppression of both Th1 and Th2 immune responses and previous in vitro data had demonstrated that T cell activation by ES-62 matured DC in vitro was associated with increased production of IL-1016. However, surprisingly, IL-10 production was also inhibited in ES-62-treated mice and there was no difference in total TGF-β (and activated TGF-β, results not shown) production (Fig. 4e, f). This suggests that ES-62 may not be inducing the development of regulatory T cells that produce these ‘suppressive’ cytokines to inhibit antibody production, but instead may mediate inhibition of IgG1 and IgG2a production via decreased IFN-γ and IL-4 production.
Although T cells from ES-62-treated double transfer recipients did not exhibit increased production of regulatory T-cell associated IL-10 and TGF-β production, they exhibited decreased proliferation compared to control groups (Fig. 4g). Of interest, this phenotype may only be witnessed when cells receive a secondary stimulation because as shown in Fig. 1, the presence of B cells in the transfer system protects primary T-cell expansion. Such a phenotype is commonly associated not only with the development of regulatory T cells but also anergic T cells. However, it is unlikely that the cells in ES-62 treated double transfer recipients develop a state of anergy, as we have previously shown that anergic T cells do not have the capacity to ‘help’ B cells nor do they home to follicles in vivo.37
Unconjugated OVA–HEL mirrors conjugated OVA–HEL with respect to clonal expansion but not cytokine inhibition in ES-62-treated mice
The presence of antigen-specific B cells in the adoptive transfer system was found to dramatically alter ES-62 modulation of the immune response observed in animals adoptively transferred with T cells alone. Therefore, to address whether antigen-presentation by B cells plays a role in modulating the effects of ES-62 treatment, in particular restoring T-cell clonal expansion and suppressing the production of both IgG1 and IgG2a production, the immunization strategy was modified. In the conventional double transfer system, immunization with conjugated OVA–HEL permits the transferred HEL-specific B cells to preferentially take up OVA–HEL via their BCR and present OVA-derived epitopes to the transferred OVA-specific T cells. Although, it is somewhat controversial as to whether B cells have the capacity to activate naive CD4+ T cells, it has been reported that following uptake of antigen via the BCR, B cells up-regulate the necessary costimulatory molecules required for T-cell activation.38 Therefore, to reduce such potential B-cell presentation, mice were immunized with unconjugated OVA and HEL, which would result in the transferred HEL-specific B cells being unable to present OVA to the OVA-specific T cells. Consequently, in this situation, DC would then presumably carry out the majority of OVA presentation, although, albeit at a very low level, there may be some endocytosis/pinocytosis-driven presentation by endogenous and transferred B cells.
Following immunization of double transfer recipients with conjugated OVA–HEL or OVA plus HEL, the clonal expansion of CD4+ KJ1.26+ T cells was examined at day 7 post immunization (Fig. 5a). In mice that were immunized with conjugated OVA–HEL, the levels of expansion between ES-62-treated and control groups were comparable, in agreement with previous double transfer data (Fig. 1). Similarly, mice that were immunized with unconjugated OVA and HEL, demonstrated lack of inhibition of T-cell clonal expansion in the ES-62-treated group compared to the control group (Fig. 5a). These data were corroborated by analysis of CFSE levels in the transferred T cells which showed essentially identical proliferation profiles in cells from control and ES-62-treated mice, irrespective of the type of antigen used (Fig. 5b), although a slightly higher percentage of the control tg T cells did not respond to unconjugated OVA plus HEL relative to the equivalent population in mice that were immunized with OVA–HEL and this was confirmed by analysis of the division (OVA–HEL: 0·97 ± 0·10 versus 0·90 ± 0·05; OVA + HEL: 0·78 ± 0·05 versus 1·08 ± 1·0), proliferation (OVA–HEL: 2·96 ± 0·34 versus 2·94 ± 0·20%; OVA + HEL: 2·88 ± 0·04 versus 3·07 ± 0·09) and percentage cells divided (OVA–HEL: 33·0 ± 1·7% versus 32·3 ± 1·8%; OVA + HEL: 27·0 ± 2% versus 35·7 ± 4·2%) indices. Analysis of antigen-specific B-cell expansion at day 7 post immunization also supported the necessity of T-cell help for optimal B-cell clonal expansion. Thus, clonal expansion of the transferred B cells was only seen in mice immunized with OVA–HEL, which allows HEL-specific B cells to present OVA and receive help from OVA-specific T cells (Fig. 5c). Collectively, these results therefore suggest that the presence of an increased frequency of antigen-stimulated (but not necessarily proliferating) B cells rather than presentation of linked antigen by B cells may be responsible for restoring T-cell clonal expansion in the double transfer model.
Figure 5.
Effects of ES-62 on antigen-specific CD4+ T-cell and B220+ B-cell clonal expansion in vivo following immunization with unconjugated OVA and HEL. Non-transgenic BALB/c recipient control and ES-62-treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells and B220+ HEL+ B cells. Twenty-four hr later, these mice were either immunized s.c. with 130 µg of conjugated OVA–HEL (con) or unconjugated OVA and HEL (uncon) in CFA. The percentage of CD4+ KJ1.26+ T cells (a) and B220+ HEL+ B cells (c) in the draining lymph nodes of unimmunized control (U) and unimmunized ES-62-treated (U + E) and immunized control (I) and immunized ES-62-treated (I + E) adoptively transferred recipients was assessed by flow cytometry on day 7 after immunization. Data are presented as a bar chart of the mean ± SEM for three mice per group. Prior to transfer, T cells were labelled with CFSE and five days after immunization, draining lymph nodes from immunized control and ES-62-treated adoptive transfer recipients were removed and CFSE fluorescence, a measure of cell division, was assessed in CD4+ KJ1.26+ T cells by flow cytometry (b) Data are shown as histograms of CFSE fluorescence versus number of CD4+ KJ1.26+ T cells and are from one mouse, which is representative of three mice per group and a total of two independent experiments.
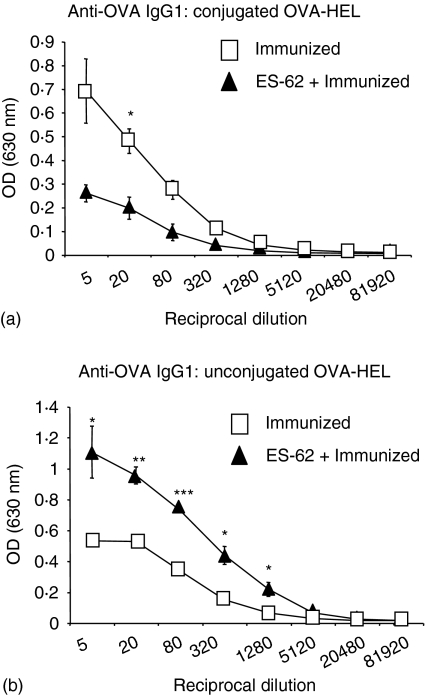
These results demonstrated that ES-62 had little effect on antigen-specific T-cell clonal expansion in double transfer recipients that were immunized with either conjugated or unconjugated OVA and HEL. Therefore, taking this into account it was hypothesized that ES-62 would induce a similar suppression in IgG1 and IgG2a production in double transfer mice that were immunized with unconjugated OVA and HEL. However, our data revealed that the production of anti-OVA IgG1 by ES-62-treated mice that were immunized with unconjugated OVA and HEL, more closely resembled that observed in single transfer mice, that is, an increase in OVA-specific IgG1 (Fig. 6) and, although control levels were very low, a decrease in OVA-specific IgG2a (results not shown). Thus, collectively these data suggest that B cells may restore antigen-specific T-cell clonal expansion in ES-62-treated mice in an antigen-independent manner. By contrast, however, the suppression of OVA-specific IgG1 production observed in double transfer mice appears to be the result of ES-62-mediated modulation of B- and T-cell cognate interactions.
Figure 6.
Effects of ES-62 on OVA-specific IgG1 production in vivo following immunization with unconjugated OVA and HEL. Non-transgenic BALB/c recipient control and ES-62-treated mice were injected with 2·5 × 106 CD4+ KJ1.26+ T cells and B220+ HEL+ B cells. Twenty-four hr later, these mice were either immunized s.c. with 130 µg of conjugated OVA–HEL (a) or unconjugated OVA and HEL (b) in CFA. Serum was collected at 7 days after immunization and OVA-specific IgG1 assessed by ELISA. There was little or no OVA-specific IgG1 detected in unimmunized groups. Data are presented as the mean of three mice per group ± SEM and are from a single experiment. ***P < 0·005, **P < 0·01, *P < 0·05.
Discussion
In this study we have further explored the immunomodulatory properties of ES-62 in vivo in order to more fully understand and dissect the mechanisms by which it alters the immune response. In order to specifically examine the effects of ES-62 on T- and B-cell interactions a double (tg BCR and tg TCR) transfer procedure was used.21 ES-62 treated mice were recipients for cotransfer with OVA-specific tg TCR CD4+ T cells and HEL-specific tg BCR B cells followed by immunization with conjugated OVA–HEL/CFA to promote cognate interactions between these cells. Importantly, this experimental set-up has shown that the clonal expansion and production of HEL-specific IgM by the transferred B cells depends critically on help from the transferred OVA-specific T cells.21
Rather surprisingly, ES-62 appeared to have different effects on T-cell effector function in the double transfer system compared to the single transfer system. For example, we have previously demonstrated using the single transfer system that ES-62 was able to inhibit antigen-specific T-cell expansion in vivo. In contrast, when ES-62-treated mice were cotransferred with antigen-specific B cells prior to immunization, the capacity of T cells to undergo clonal expansion and division was restored. Moreover, these antigen-specific T cells displayed normal B-cell follicle homing potential. However, the observed lack of anti-OVA IgG Abs and also production of both Th1 and Th2 cytokines indicated that the helper and effector functions of these T cells were severely attenuated. Further investigation using non-conjugated antigens into the role of these antigen-specific B cells in vivo suggested that whereas the restoration of T-cell expansion in ES-62-treated mice may only require the presence of an increased frequency of partially activated B cells regardless of specificity, the reduced production of anti-OVA IgG antibody may have been a consequence of dysfunctional B-cell presentation during B–T-cell co-operation.
Although the unconjugated antigen studies suggested that the restoration of T-cell clonal expansion in ES-62 treated mice was not caused by antigen presentation by transferred tg B cells, a role for B–T-cell interactions in the priming of naïve CD4+ T-cell activation cannot be ruled out. Indeed, although it is widely accepted that DC are the primary cell type responsible for the generation of adaptive immune responses39 it has been shown that following antigen uptake via the BCR, B cells subsequently acquire a phenotype (increased major histocompatibility complex (MHC) class II and CD86) that supports naïve T-cell activation.38 Accordingly, in the double transfer system it is possible that tg B cells uptake OVA–HEL via their BCR and subsequently up-regulate costimulatory expression to a sufficient extent to allow effective presentation of OVA to antigen-specific T cells. Indeed, because naïve B cells are also found transiently in the paracortex as they migrate to the follicles40,41 it is possible that the relatively large population of tg B cells could also acquire antigen during this passage and induce or modulate T-cell activation.21,42 Of relevance, Brocker's group has recently shown43 by adoptively transferring tg TCR CD4+ T cells into transgenic mice expressing selectively the T-cell restricting MHC class II molecules on either DC, B cells or DC and B cells, that whilst DC were sufficient to induce clonal expansion, IL-2 production and follicular migration of antigen-specific T cells, B cells alone did not possess antigen-presenting capacities in vivo. Interestingly, however, in mice in which both DCs and B cells were capable of presenting antigen in vivo, there was increased clonal expansion and follicular migration by antigen-specific T cells in vivo, suggesting that whilst DC are the primary antigen-presenting cells for T-cell activation, B cells can further modulate CD4 T-cell responses.43 However, as it might be expected that most of the transferred HEL-specific B cells would be unable to present OVA to OVA-specific T cells in the case of immunization with unconjugated OVA and HEL antigens, our data may suggest that an increased frequency of partially activated B cells per se may be sufficient to provide ‘help’ for enhanced clonal expansion of T cells, presumably via antigen-independent cell–cell contact or cytokine interactions, rather than an absolute requirement for cognate B- and T-cell interactions. Alternatively, our findings may imply that, under conditions of simultaneous immunization/infection with multiple antigens (e.g. OVA and HEL), such increased frequencies of partially activated B cells in the lymph nodes are indeed capable of acquiring, processing and presenting heterologous antigen (in this case HEL-specific B cells presenting OVA-derived peptides) to modulate polyclonal T-cell activation.
Taking this into account, ES-62 could be modulating the initial stages of antigen presentation between DC and T cells as described previously for the single transfer system, but the outcome of this reaction is subsequently affected by the presence of a large population of antigen-specific B cells. For example, Ingulli et al. have demonstrated that as the immune response progresses from the initial stages of T-cell priming by DC, these DC soon lose the capacity to present antigen and leave the lymph node.44 As a result, this may create a situation where B-cell presentation may be favoured in the face of limiting numbers of DC, providing a means to restore the proliferative capacity of the T cells ‘activated’ by ES-62-matured DC. Indeed, a study by Linton et al. demonstrated that B cells could play an essential role in vivo to amplify the CD4 response, the size of the CD4 pool being determined by the degree of B-cell dependent T-cell expansion. Interestingly, it was shown that not only did the transfer of naïve B cells with the capacity for BCR-mediated antigen-uptake restore the frequency of IL-2-producing T cells, but also and pertinent to this study, transfer of activated B cells loaded with either relevant or irrelevant antigen to the CD4 response could also restore the frequencies of primary effector T cells.45 Therefore, the presence of activated B cells in ES-62-treated mice may be simply amplifying the effector T cells that were induced during initial activation by ES-62 matured DC.
Because activated B cells could support T-cell expansion, it is possible that costimulation, particularly reduced expression of CD80/CD86, is the primary deficiency that limits T-cell expansion in response to presentation by ES-62-DC. Not only are CD28/CD80/CD86 interactions necessary for IL-2-dependent CD4 cell expansion46–48 but through up-regulation of antiapoptotic Bcl-xL, they may maintain T-cell survival during the primary response.49 Consequently, the restoration of T-cell clonal expansion in ES-62-treated mice may reflect the pool of tg B cells up-regulating costimulatory molecule expression following antigen-uptake via their BCR38 providing supplementary proliferative/survival signals to the low frequency of T cells originally promoted by ES-62-DC. These supplementary signals may be delivered to T cells around the edges of B-cell follicles, consistent with evidence that following BCR engagement B cells migrate to the outer T-cell zone and following activation by DC, T cells migrate towards follicular regions.21,50 Indeed, unlike previous single transfers where suboptimal clonal expansion was associated with reduced follicular migration, in this system ES-62 had little effect on the movement of T cells to B cell follicles, supporting the report by Smith et al. proposes that passage through B-cell follicles is required for optimal T-cell expansion.25
Interestingly, although the helper ability of these T cells to promote tg B-cell expansion and anti-HEL IgM production appears to be unaltered by exposure to ES-62, the production of anti-OVA IgG antibody and production of cytokines upon ex vivo re-challenge are suppressed, indicating the restoration of clonal expansion has not fully revived the effector function of these T cells. It was surprising to observe inhibition of both anti-OVA IgG1 and IgG2a antibodies in this system, because previously it was demonstrated that ES-62, consistent with its Th2-like promoting ability, induced the development of CD4+ T cells, which favoured an IgG1 bias. Because the production of IgG1 and IgG2a isotypes has been shown to be dependent on the production of IL-4/IL-5 and IFN-γ, respectively,51 the reduced production of these cytokines by T cells upon ex vivo re-challenge may go some way to explaining why there appeared to be an overall suppression in IgG production. Alternatively, because of the nature of the linked OVA–HEL immunization strategy, the majority of transferred OVA-specific T cells are likely to be interacting with transferred HEL-specific B cells in double transfers. Consequently, those endogenous OVA-specific B cells, which have been selected for IgG1 production, may not receive the optimal levels of T cell help resulting in suppression of anti-OVA IgG1 production. Consistent with this, when double transfer mice were immunized with uncoupled OVA and HEL to reduce the levels of cognate B–T-cell interactions and promote T-cell help to the endogenous OVA-specific B cells, the IgG1 antibody response was restored.
Although the mechanisms underlying the functional differences observed in the single and double transfer models are unclear, it is plausible that ES-62 exhibits differential effects on B-cell (suppression-mediated), relative to DC (polarization-mediated), presentation of antigen and modulation of T-cell effector responses eliciting differential functional phenotypes depending on the immunological status of the host. Thus, ES-62, like other parasite immunomodulatory molecules52,53 may inhibit DC migration from the site of antigen-uptake to draining lymph nodes, thus reducing the frequency of antigen-presenting DC. If this occurs when a large population of B cells with the capacity to present antigen to T cells are available, then B-cell presentation may be favoured. Interestingly, we have previously shown that B-cell CD40 expression, which plays a key role in B–T-cell interactions, is down-regulated following exposure to ES-62 in vivo.18 Consequently, the observed suppressed phenotype in ES-62-treated double transfer mice may reflect ES-62 modulation of antigen-presentation by B cells with resulting induction of T-cell unresponsiveness, as demonstrated by studies indicating that naïve B-cell antigen presentation can promote T-cell tolerance as a result of lack of CD40/CD40L interactions.54 Perhaps consistent with this, there is evidence that as clinical disease progresses in murine models of experimentally induced rheumatoid arthritis there is a concomitant increase in the infiltration of B cells that have the capacity to present antigen and activate T cells,55,56 and we have previously demonstrated that ES-62 given therapeutically to such mice exhibiting clinical symptoms of arthritis results in amelioration of inflammation and disease in an IL-10- and Th2-independent manner, with a phenotype of suppression similar to the global suppression described in double transfers.29,57
Taken together, these results demonstrate that in a system where an expanded population of activated B cells has the capacity to present antigen to T cells and/or modulate DC–T-cell priming, ES-62 can interfere with the outcome of the immune response, such that there is an overall suppression of immunity. This proposal may have implications for filarial nematode-infected individuals who are often subject to infection with multiple pathogens at any given time, in the sense that it may go some way to explaining why filarial nematode infection gives rise to both specific and generalized immunosuppression.
Acknowledgments
This work received the financial support of the MRC and the Wellcome Trust; F.A.M. held an MRC PhD training studentship.
References
- 1.WHO. Filariasis. Geneva: World Health Organization; 1987. [Google Scholar]
- 2.Vanamail P, Ramaiah KD, Pani SP, Das PK, Grenfell BT, Bundy DA. Estimation of the fecund life span of Wuchereria bancrofti in an endemic area. Trans R Soc Trop Med Hyg. 1996;90(2):119–21. doi: 10.1016/s0035-9203(96)90106-6. [DOI] [PubMed] [Google Scholar]
- 3.Kazura JW, Nutman TB, Greene B. Filariasis. In: Warren KS, editor. Immunology and Molecular Biology of Parasitic Infections. 3. Oxford: Blackwell Scientific Publications; 1993. pp. 473–95. [Google Scholar]
- 4.Maizels RM, Lawrence RA. Immunological tolerance: the key feature in human filariasis? Parasitol Today. 1991;7:271–6. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 5.Ottesen EA, Weller PF, Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33:413–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Piessens WF, Ratiwayanto S, Tuti S, Palmieri JH, Piessens PW, Koiman I, Dennis DT. Antigen-specific suppressor cells and suppressor factors in human filariasis with Brugia malayi. N Engl J Med. 1980;302:833–7. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- 7.Elkhalifa MY, Ghalib HW, Dafa'Alla T, Williams JF. Suppression of human lymphocyte responses to specific and non-specific stimuli in human onchocerciasis. Clin Exp Immunol. 1991;86:433–9. doi: 10.1111/j.1365-2249.1991.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–8. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 9.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72(5):2598–604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harnett W, Parkhouse RME. Structure and Function of Nematode Surface and Excretory-Secretory Products. New Delhi: M/S Narendra Publication House; 1995. [Google Scholar]
- 11.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151(9):4829–37. [PubMed] [Google Scholar]
- 12.Deehan M, Harnett M, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–11. [PubMed] [Google Scholar]
- 13.Deehan MR, Frame MJ, Parkhouse RM, Seatter SD, Reid SD, Harnett MM, Harnett W. A phosphorylcholine-containing filarial nematode-secreted product disrupts B lymphocyte activation by targeting key proliferative signaling pathways. J Immunol. 1998;160:2692–9. [PubMed] [Google Scholar]
- 14.Harnett MM, Deehan MR, Williams DM, Harnett W. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol. 1998;20:551–63. doi: 10.1046/j.1365-3024.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 15.Harnett W, Deehan MR, Houston KM, Harnett MM. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 1999;21:601–8. doi: 10.1046/j.1365-3024.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 16.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 17.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 18.Wilson EH, Deehan MR, Katz E, Brown KS, Houston KM, O'Grady J, Harnett MM, Harnett W. Hyporesponsiveness of murine B lymphocytes exposed to the filarial nematode secreted product ES-62 in vivo. Immunology. 2003;109:238–45. doi: 10.1046/j.1365-2567.2003.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harnett W, Harnett MM. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim Biophys Acta. 2001;1539:7–15. doi: 10.1016/s0167-4889(01)00101-x. [DOI] [PubMed] [Google Scholar]
- 20.Pape KA, Kearney ER, Khoruts A, et al. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 21.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 22.Smith KM, Brewer JM, Rush CM, Riley J, Garside P. In vivo generated Th1 cells can migrate to B cell follicles to support B cell responses. J Immunol. 2004;173:1640–6. doi: 10.4049/jimmunol.173.3.1640. [DOI] [PubMed] [Google Scholar]
- 23.Marshall FA, Grierson AM, Garside P, Harnett W, Harnett MM. ES-62, an immunomodulator secreted by filarial nematodes, suppresses clonal expansion and modifies effector function of heterologous antigen-specific T cells in vivo. J Immunol. 2005;175:5817–26. doi: 10.4049/jimmunol.175.9.5817. [DOI] [PubMed] [Google Scholar]
- 24.Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, Harnett W. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine (PC)-containing filarial neamtode glycoprotein ES-62 polarises their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113:491–8. doi: 10.1111/j.1365-2567.2004.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KM, Brewer JM, Mowat AM, Ron Y, Garside P. The influence of follicular migration on T-cell differentiation. Immunology. 2004;111:248–51. doi: 10.1111/j.1365-2567.2004.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 27.Harnett W, Harnett MM, Byron O. Structural/functional aspects of ES-62 – a secreted immunomodulatory phosphorylcholine-containing filarial nematode glycoprotein. Curr Protein Peptide Sci. 2003;4:59–72. doi: 10.2174/1389203033380368. [DOI] [PubMed] [Google Scholar]
- 28.Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–44. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 29.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 30.Lal RB, Paranjape RS, Briles DE, Nutman TB, Ottesen EA. Circulating parasite antigen (s) in lymphatic filariasis – use of monoclonal-antibodies to phosphocholine for immunodiagnosis. J Immunol. 1987;138:3454–60. [PubMed] [Google Scholar]
- 31.Houston KM, Wilson EH, Eyres L, Brombacher F, Harnett MM, Alexander J, Harnett W. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect Immun. 2000;68:5466–8. doi: 10.1128/iai.68.9.5466-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark A, Ledbetter JA. Review. How B and T cells talk to each other. Nature. 1994;367:425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 33.McHeyzer-Williams LJ, Driver DJ, McHeyzer-Williams MG. Germinal center reaction. Curr Opin Hematol. 2001;8:52–9. doi: 10.1097/00062752-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Gray D, Siepmann K, Wohlleben G. CD40 ligation in B cell activation, isotype switching and memory development. Semin Immunol. 1994;6:303–10. doi: 10.1006/smim.1994.1039. [DOI] [PubMed] [Google Scholar]
- 35.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–31. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 37.Smith KM, McAskill F, Garside P. Orally tolerized T cells are only able to enter B cell follicles following challenge with antigen in adjuvant, but they remain unable to provide B cell help. J Immunol. 2002;168:4318–25. doi: 10.4049/jimmunol.168.9.4318. [DOI] [PubMed] [Google Scholar]
- 38.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–703. [PubMed] [Google Scholar]
- 39.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 40.Lortan JE, Roobottom CA, Oldfield S, MacLennan IC. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987;17:1311–6. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 41.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 42.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–9. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 43.Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–64. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4 (+) T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 46.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–6. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–6. [PubMed] [Google Scholar]
- 48.Costello PS, Walters AE, Mee PJ, et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci U S A. 1999;96:3035–40. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 50.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 51.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 52.Angeli V, Faveeuw C, Roye O, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–47. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semnani RT, Law M, Kubofcik J, Nutman TB. Filaria-induced immune evasion. suppression by the infective stage of Brugia malayi at the earliest host–parasite interface. J Immunol. 2004;172:6229–38. doi: 10.4049/jimmunol.172.10.6229. [DOI] [PubMed] [Google Scholar]
- 54.Croft M, Joseph SB, Miner KT. Partial activation of naive CD4 T cells and tolerance induction in response to peptide presented by resting B cells. J Immunol. 1997;159:3257–65. [PubMed] [Google Scholar]
- 55.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl. 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 57.Harnett W, McInnes IB, Harnett MM. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol Lett. 2004;94:27–33. doi: 10.1016/j.imlet.2004.04.008. [DOI] [PubMed] [Google Scholar]