Abstract
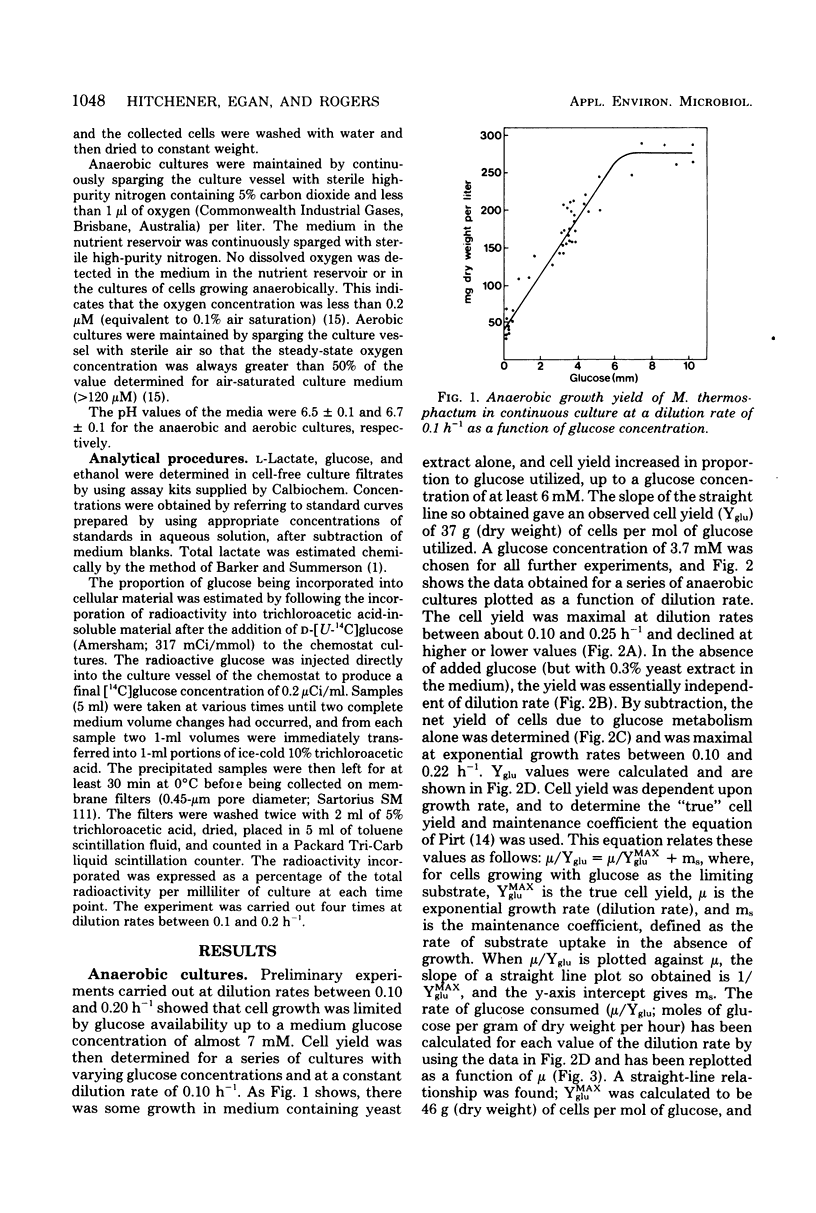
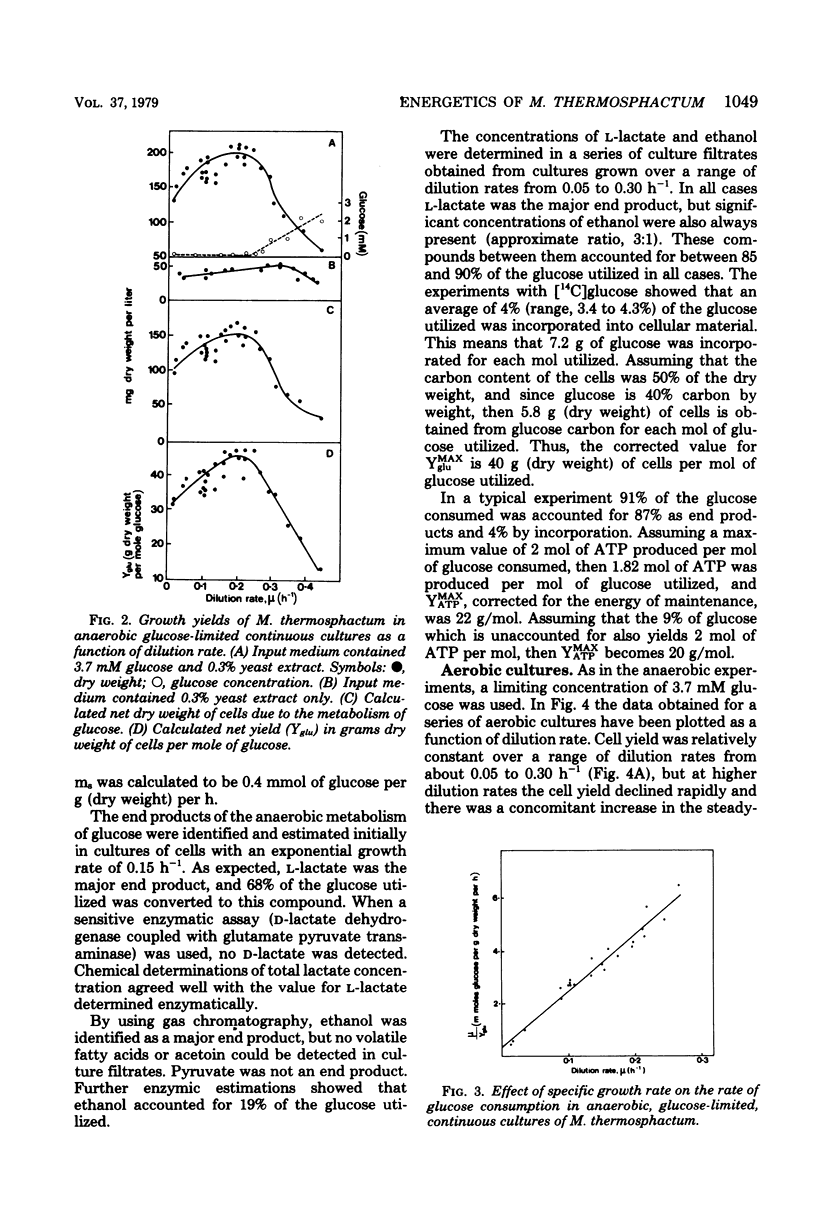
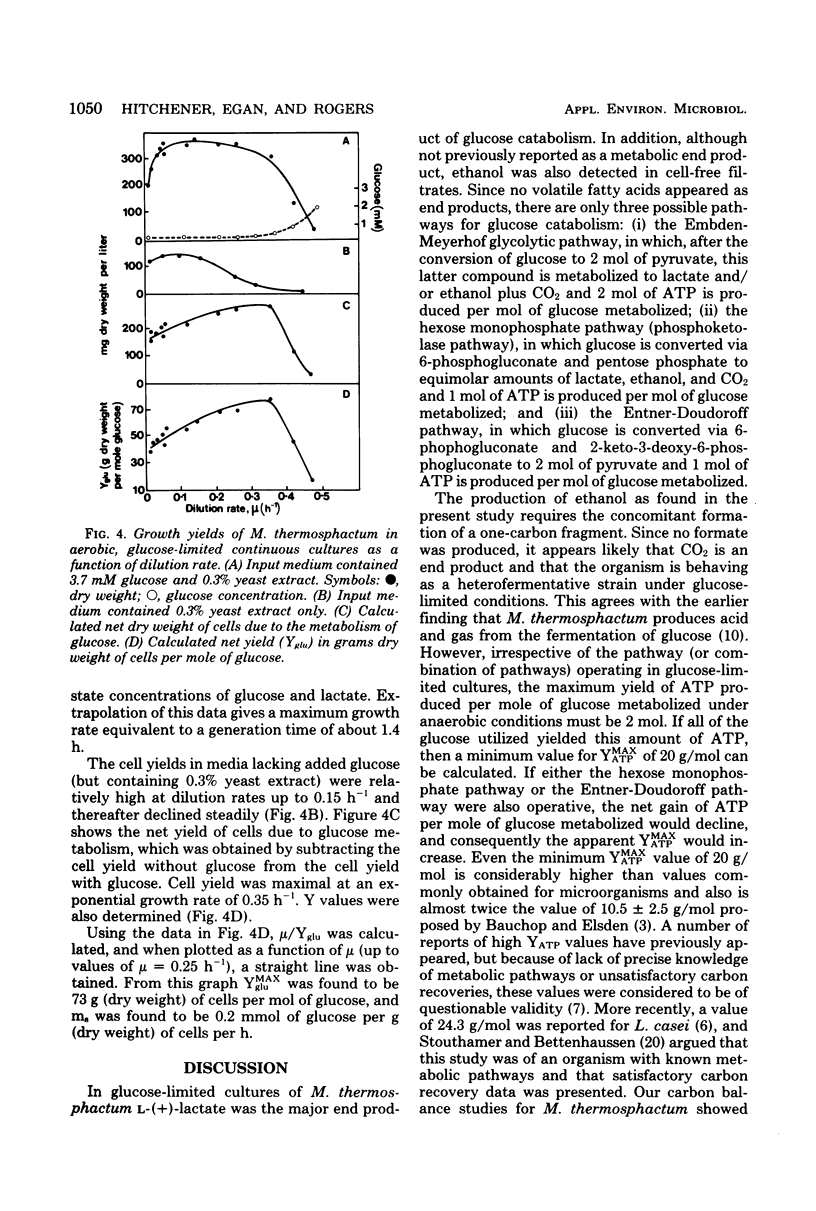
Microbacterium thermosphactum was grown at 25 degrees C in glucose-limited continuous culture under aerobic (greater than 120 microM oxygen) and anaerobic (less than 0.2 microM oxygen) conditions. The end products of the anaerobic metabolism of glucose were identified as L-lactate and ethanol. Together these compounds accounted for between 85 and 90% of the glucose utilized over the full range of growth rates studied. In addition, 4% of the glucose utilized was incorporated into cellular material. Under anaerobic conditions the molar growth yield was 40 g (dry weight) of cells per mol of glucose utilized, and the maintenance energy coefficient was 0.4 mmol of glucose utilized per g (dry weight) of cells per h. For cells grown under aerobic conditions in the corresponding values were 73 g/mol and 0.2 mmol/g per h, respectively. The molar growth yield with respect to adenosine 5'-triphosphate varied with the growth rate of the culture, and the true molar growth yield with respect to adenosine 5'-triphosphate was found to be 20 g/mol of adenosine 5'-triphosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Pine L. Path of glucose breakdown and cell yields of a facultative anaerobe, Actinomyces naeslundii. J Gen Microbiol. 1967 Feb;46(2):225–236. doi: 10.1099/00221287-46-2-225. [DOI] [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- Gill C. O. Substrate limitation of bacterial growth at meat surfaces. J Appl Bacteriol. 1976 Dec;41(3):401–410. doi: 10.1111/j.1365-2672.1976.tb00652.x. [DOI] [PubMed] [Google Scholar]
- MCLEAN R. A., SULZBACHER W. L. Microbacterium thermosphactum, spec: nov.; a nonheat resistant bacterium from fresh pork sausage. J Bacteriol. 1953 Apr;65(4):428–433. doi: 10.1128/jb.65.4.428-433.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Oxidative phosphorylation in bacteria which contain different cytochrome oxidases. Eur J Biochem. 1973 Jul 2;36(1):144–151. doi: 10.1111/j.1432-1033.1973.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Newton K. G., Gill C. O. The development of the anaerobic spoilage flora of meat stored at chill temperatures. J Appl Bacteriol. 1978 Feb;44(1):91–95. doi: 10.1111/j.1365-2672.1978.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Energetic efficiency and maintenance. Energy characteristics of Saccharomyces cerevisiae (wild type and petite) and Candida parapsilosis grown aerobically and micro-aerobically in continuous culture. Arch Microbiol. 1974;99(1):25–46. doi: 10.1007/BF00696220. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Respiratory development in Saccharomyces cerevisiae grown at controlled oxygen tension. J Bacteriol. 1973 Jul;115(1):88–97. doi: 10.1128/jb.115.1.88-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L. A., Clark D. S. Effect of lactobacilli and carbon dioxide on the growth of Microbacterium thermosphactum on fresh beef. Can J Microbiol. 1975 May;21(5):629–632. doi: 10.1139/m75-090. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Sutherland J. P., Patterson J. T., Murray J. G. Changes in the microbiology of vacuum-packaged beef. J Appl Bacteriol. 1975 Dec;39(3):227–237. doi: 10.1111/j.1365-2672.1975.tb00568.x. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]


