Abstract
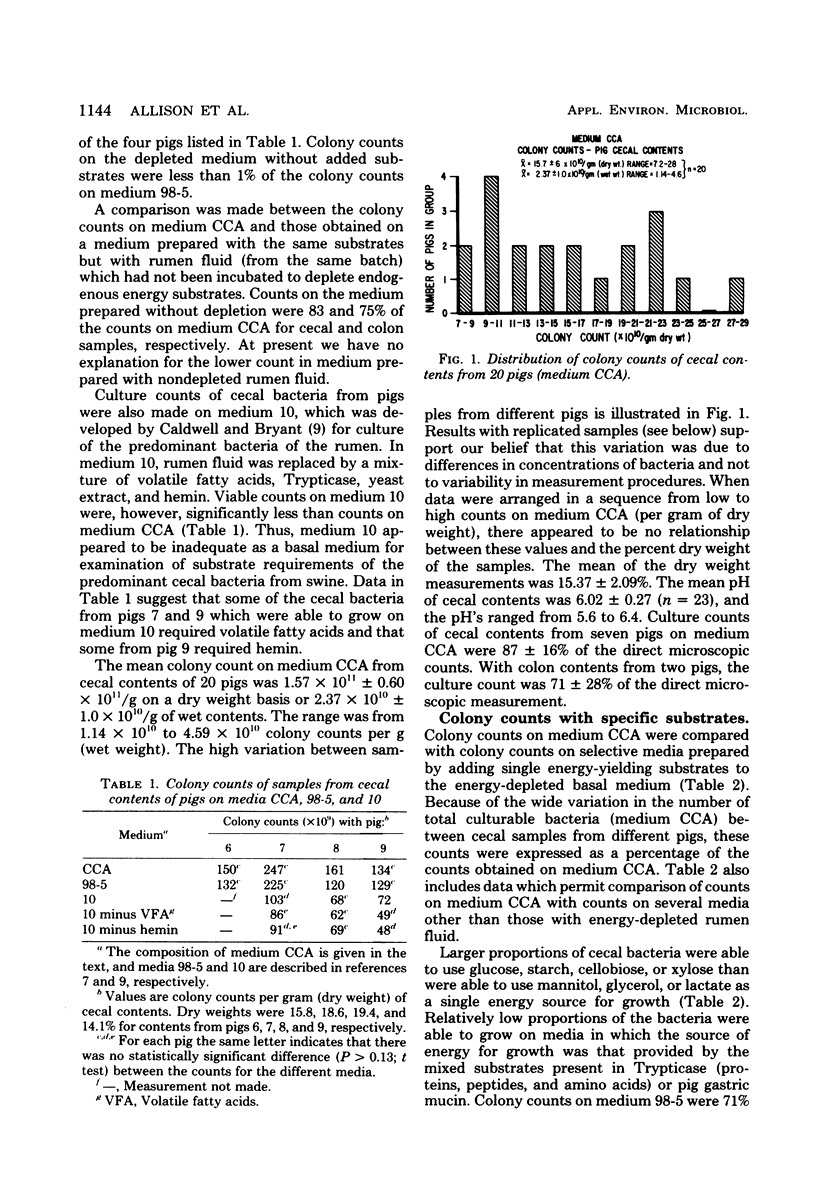
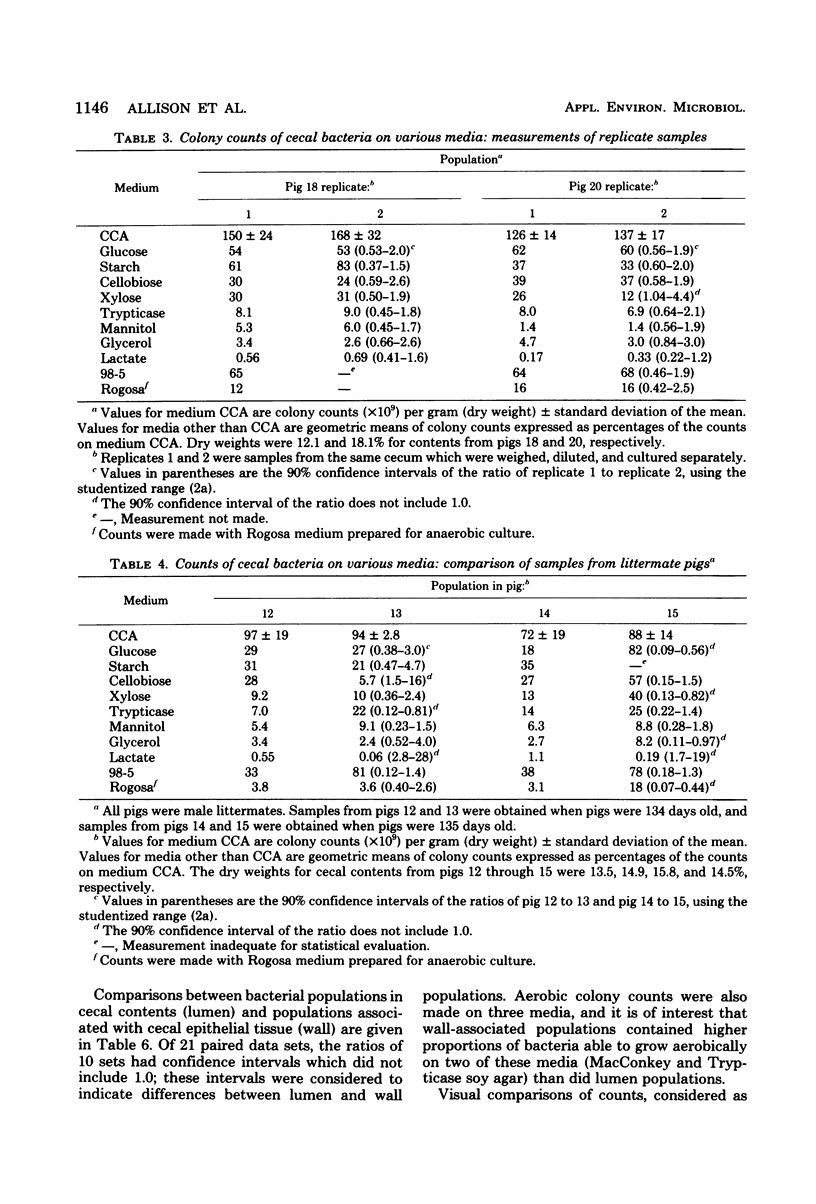
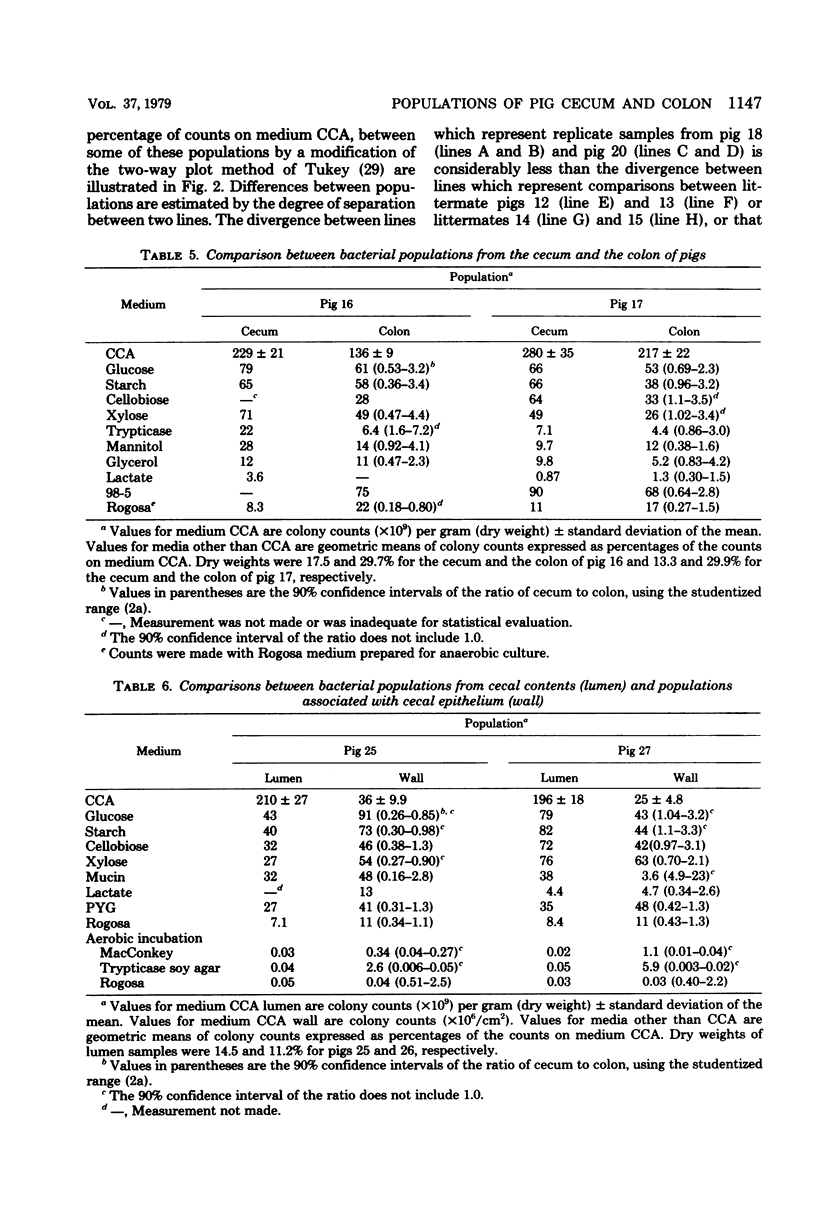
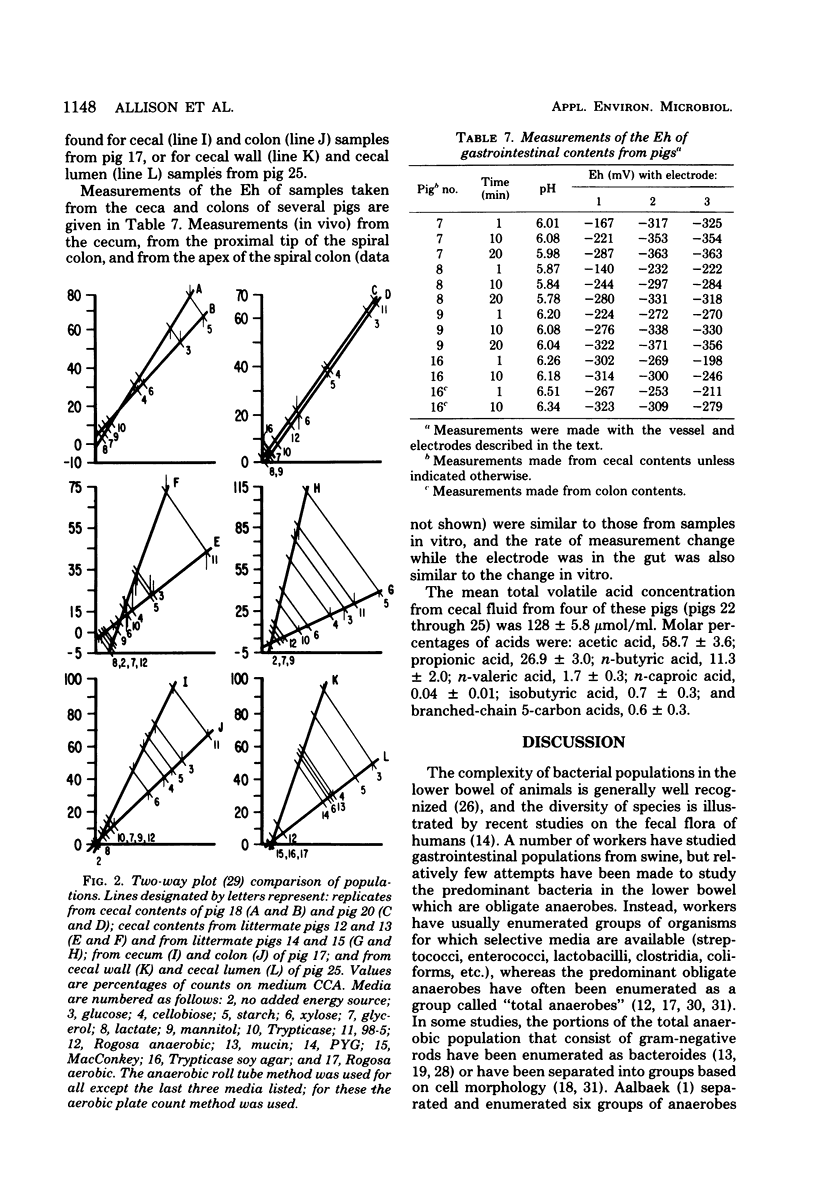
Concentrations of bacteria in the ceca and colons of pigs were measured by determinations of colony counts on rumen fluid-based media in anaerobic roll tubes. With our most complete medium (medium CCA), the mean colony count of cecal samples from 20 pigs was 2.37 X 10(10) +/- 1.0 X 10(10) (+/- standard deviation)/g (wet weight). The mean number of bacteria attached to or associated with cecal epithelial tissues from three pigs on medium CCA was 2.67 X 10(7) +/- 0.81 X 10(7)/cm2 of tissue. The proportions of gut bacterial populations able to use various energy substrates were estimated on the basis of relative colony counts. The following substrates are listed in descending order of their capacity to support growth of cecal bacteria: glucose, starch, cellobiose, xylose, Trypticase, gastric mucin from swine, mannitol, glycerol, and lactate. The effect of diet upon this distribution was not examined. The relative proportions of bacteria from a given population that were able to grow on various selective media were used as population profiles. Comparisons of populations in this way indicated that differences could be detected between (i) populations from the cecum of littermate pigs, (ii) populations from the cecum and colon of the same pig, and (iii) populations in the lumen of the cecum as compared with populations associated with cecal mucosa.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalbaek B. Gram-negative anaerobes in the intestinal flora of pigs. Acta Vet Scand. 1972;13(2):228–237. doi: 10.1186/BF03548576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranwell P. D. Microbial fermentation in the alimentary tract of the pig. Nutr Abstr Rev. 1968 Jul;38(3):721–730. [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Basal medium for the selective enumeration of rumen bacteria utilizing specific energy sources. Appl Environ Microbiol. 1976 Nov;32(5):703–710. doi: 10.1128/aem.32.5.703-710.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazhary M. A., Tremblay A., Lagacé A., Roy R. S. A preliminary study on the intestinal flora of cecum and colon of eight, ten and 12 week old swine. Can J Comp Med. 1973 Oct;37(4):369–374. [PMC free article] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolacz J. W., Wescott R. B., Dommert A. R. Microflora of Hormel miniature swine: fecal flora of adult sows. Am J Vet Res. 1970 Jul;31(7):1173–1178. [PubMed] [Google Scholar]
- Kovács F., Nagy B., Sinkovics G. The gut bacterial flora of healthy early weaned piglets, with special regard to factors influencing its composition. Acta Vet Acad Sci Hung. 1972;22(4):327–338. [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951 Jul;62(1):132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall G. D., Wood A. J., Wescott R. B., Dommert A. R. Distribution of bacteria in feces of swine. Appl Microbiol. 1970 Nov;20(5):789–792. doi: 10.1128/am.20.5.789-792.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977 Jan;33(1):79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Slyter L. L., Oltjen R. R., Kern D. L., Weaver J. M. Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr. 1968 Feb;94(2):185–192. doi: 10.1093/jn/94.2.185. [DOI] [PubMed] [Google Scholar]
- Vervaeke I. J., Van Nevel C. J. Comparison of three techniques for the total count of anaerobes from intestinal contents of pigs. Appl Microbiol. 1972 Sep;24(3):513–515. doi: 10.1128/am.24.3.513-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke I. J., Van Nevel C. J., Decuypere J. A., Van Assche P. F. A comparison of two methods for obtaining anaerobic counts in different segments of the gastro-intestinal tract of piglets. J Appl Bacteriol. 1973 Sep;36(3):397–405. doi: 10.1111/j.1365-2672.1973.tb04121.x. [DOI] [PubMed] [Google Scholar]



