Abstract
Phosphorylation of the regulatory light chain (RLC) activates the actin-dependent ATPase activity of Dictyostelium myosin II. To elucidate this regulatory mechanism, we characterized two mutant myosins, MyΔC1225 and MyΔC1528, which are truncated at Ala-1224 and Ser-1527, respectively. These mutant myosins do not contain the C-terminal assembly domain and thus are unable to form filaments. Their activities were only weakly regulated by RLC phosphorylation, suggesting that, unlike smooth muscle myosin, efficient regulation of Dictyostelium myosin II requires filament assembly. Consistent with this hypothesis, wild-type myosin progressively lost the regulation as its concentration in the assay mixture was decreased. Dephosphorylated RLC did not inhibit the activity when the concentration of myosin in the reaction mixture was very low. Furthermore, 3xAsp myosin, which does not assemble efficiently due to point mutations in the tail, also was less well regulated than the wild-type. We conclude that the activity in the monomer state is exempt from inhibition by the dephosphorylated RLC and that the complete regulatory switch is formed only in the filament structure. Interestingly, a chimeric myosin composed of Dictyostelium heavy meromyosin fused to chicken skeletal light meromyosin was not well regulated by RLC phosphorylation. This suggests that, in addition to filament assembly, some specific feature of the filament structure is required for efficient regulation.
The ATPase activities of smooth muscle and several nonmuscle myosin IIs (simply myosin hereafter) are inhibited by dephosphorylated regulatory light chain (RLC). Phosphorylation of RLC at analogous Ser residues relieves such inhibition (1). Much has been learned about the molecular mechanism of regulation of smooth muscle myosin by RLC phosphorylation. Classic proteolysis analysis demonstrated that subfragment 1 (S1) is not regulated (2), whereas heavy meromyosin (HMM) is well regulated by RLC phosphorylation (3). Requirement of the HMM structure indicated that association between the head and the tail is involved in the regulatory mechanism (4, 5). Recent studies by using single-headed HMM further suggested that an interaction between two heads in one molecule also is crucial for regulation (6, 7). Structurally, the activation by RLC phosphorylation is accompanied by significant, salt-sensitive conformational changes; smooth muscle myosin exists in either the folded 10S or extended 6S conformation depending on the phosphorylation state of RLC (8, 9). In the 6S conformation, two heads fold over along the amino-terminal portion of subfragment 2 (S2) of the tail (9). Taken together, it is now believed that physical, probably ionic, interactions among two heads and the amino-terminal portion of S2 is primarily responsible for inhibition of the enzymatic activities of dephosphorylated smooth muscle myosin (5, 10).
Several types of myosins from various sources of nonmuscle cells have been characterized in terms of regulation. Among them Acanthamoeba myosin is most extensively studied (11, 12). Its activity is regulated by phosphorylation of the heavy chain. Recently, Pato et al. (13) reported that recombinant chicken nonmuscle HMM IIB also is weakly regulated by heavy chain phosphorylation. Our knowledge about the regulatory mechanism of nonmuscle myosins by RLC phosphorylation is relatively limited, with only two nonmuscle myosins, thymus and platelet myosins, being characterized thus far. It is reported that RLC phosphorylation activates these two myosins only by decreasing Kapp for actin without effect on Vmax (14, 15). Myosin of the cellular slime mold Dictyostelium discoideum is composed of two identical 243-kDa heavy chains, a pair of 18-kDa regulatory light chains and a pair of 16-kDa essential light chains. Phosphorylation of Ser-18 in the RLC activates its actin-dependent ATPase activity ≈6-fold (16–19) and also activates its motor activity (16, 20). However, unlike in smooth muscle myosin, RLC phosphorylation is reported not to affect filament assembly (16). In Dictyostelium myosin, filament assembly is regulated by phosphorylation of three Thr residues in the distal tail portion of the heavy chain (21–23). Of the two regulatory switches, the mechanism of regulation by RLC phosphorylation still is only poorly understood.
In this study, we attempted to identify elements involved in the regulation of Dictyostelium myosin by RLC phosphorylation through analyses of a set of truncation, point, and chimeric mutations. The results suggest that, unlike the case of smooth muscle myosin, filament formation is required for efficient regulation of Dictyostelium myosin by RLC phosphorylation.
MATERIALS AND METHODS
Generation of Mutant Cells.
MyΔC1225 and 3xAsp mutant myosins have been described by De Lozanne and Spudich (24) and by Egelhoff et al. (23), respectively, except that MyΔC1225, originally called HMM-140, was newly tagged with six His residues at the C terminus. Two chimeric myosins, chimera A and chimera B, were constructed by using Dictyostelium and chicken fast skeletal myosin heavy chain genes, keeping the 28-aa repeat units in frame at the fusion point between the two sequences (S.M., A.H.-I., and T.Y., unpublished results). Briefly, there are totally 1,832 aa residues in chimera A, of which the N-terminal 940 residues were from Dictyostelium myosin and the C-terminal 892 residues of the tail were derived from the corresponding region of chicken skeletal myosin. Chimera B consisted of 1,224 N-terminal residues from Dictyostelium myosin and 650 C-terminal residues from chicken skeletal myosin. These chimeric myosins partially complements defects in vivo of Dictyostelium cells lacking the endogenous copy of myosin heavy chain gene (S. Shu, R. J. Lee, J. Leblanc-Straceski, and T.Q.P.U., unpublished observations), indicating that they correctly dimerize and assemble functional filaments. MyΔC1528 and S1 genes were constructed in the form of fusion with six His residues attached at the C terminus by using standard methods for DNA manipulations (25). Dictyostelium clones expressing one of these mutant myosin heavy chain genes were selected in the presence of the antibiotic G-418 (12 μg/ml) after electroporating each plasmid into cells devoid of the endogenous myosin heavy chain gene (19) as described previously (26).
Culture of Dictyostelium Cells.
Wild-type Ax2 and cells bearing chimeric myosins were grown in suspension culture in HL-5 containing 6 μg/ml each of penicillin and streptomycin at 22°C (27). Mutant cells expressing truncated myosins and 3xAsp were cultured in Petri dishes containing the same medium further supplemented with 10 μg/ml G-418.
Preparation of Proteins.
All procedures were carried out at 4°C.
Wild-type and chimeric myosins were prepared by the method of Ruppel et al. (19) with some modifications. In brief, cells were lysed by Triton X-100, and the insoluble cytoskeleton fraction was pelleted, washed with 10 mM Hepes buffer (pH 7.5) containing 150 mM NaCl, 3 mM MgCl2, and 1 mM DTT, and then extracted with 10 mM Hepes containing 125 mM NaCl, 1 mM DTT, and 3 mM ATP. The supernatant fraction after ultracentrifugation underwent two rounds of assembly–disassembly cycles to yield the purified fraction. Due to its poor filament assembly, several modifications were needed for the purification of 3xAsp myosin, as follows. The cytoskeleton fraction was extracted with the same buffer as above except that the concentration of NaCl was 25 mM, and the extract was concentrated to 3 ml after centrifugation. The resultant sample was dialyzed against 10 mM Pipes buffer (pH 6.9) containing 50 mM NaCl, 1 mM DTT, and 10 mM MgCl2. The pellet was recovered and dissolved in 10 mM Hepes buffer (pH 7.5) containing 250 mM NaCl, 3 mM ATP, and 1 mM DTT. It was further purified through two rounds of assembly and disassembly cycles.
The truncated myosins (S1, MyΔC1225 and MyΔC1528) were extracted from the cytoskeleton fraction as described above and purified through a nickel-agarose column chromatography (28). Concentration of purified Dictyostelium myosin was measured by the method of Bradford (29) by using rabbit skeletal muscle myosin as the standard (19).
Rabbit skeletal muscle actin was prepared by using the method of Spudich and Watt (30), and its concentration was spectrophotometrically determined by using the coefficient of E1%290 = 6.2. Actin was polymerized in the presence of 0.2 mM ATP, 4 mM MgCl2, 100 mM KCl, and 2 mM Tris (pH 8.0). The F-actin was pelleted and resuspended in a buffer of 4 mM MgCl2, 25 mM imidazole (pH 7.5), and 0.2 mM DTT for ATPase assays.
Phosphorylation of Myosin.
Phosphorylation of wild-type and chimeric myosins was performed after they were purified, using bacterially expressed myosin light chain kinase, which carried a T166E mutation (31), according to the method of Ruppel et al. (19). 3xAsp, S1, MyΔC1225, and MyΔC1528 were treated with the kinase during the purification procedure; after they were extracted from cytoskeleton with ATP, kinase was added and incubated overnight on ice before proceeding to the next step. The concentration of myosin light chain kinase in the reaction mixture was ≈10 μg/ml.
Electrophoretic Methods.
Urea-SDS-glycerol PAGE was performed according to the modified method of Perrie and Perry (32) as described previously (19).
ATPase Assays.
Steady–state ATPase activities were determined by measuring release of phosphate by using the method of Kodama et al. (33) under the conditions described by Ruppel et al. (19). The reaction mixtures contained 25 mM imidazole (pH 7.5), 25 mM KCl, 4 mM MgCl2, 1 mM DTT, 2 mM ATP, and F-actin. The reaction was started by the addition of myosin as described by Truong et al. (34). In each assay the activity was measured at four time points, showing a linear relationship between time and activities.
RESULTS
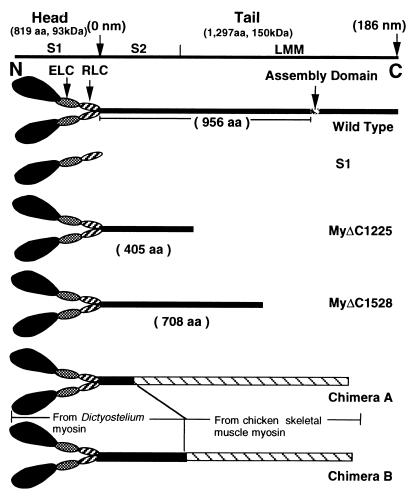
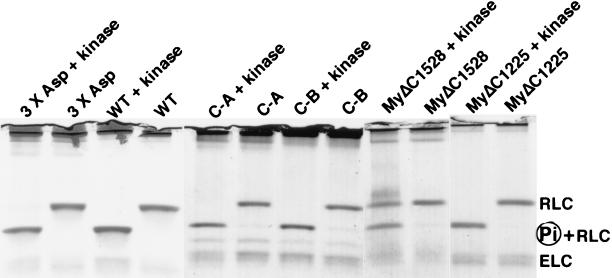
MyΔC1225 was truncated at Ala-1224 lacking approximately two-thirds of the tail, and MyΔC1528 was truncated at Ser-1527 with approximately one-third of the tail deleted (Fig. 1). S1 was truncated at Val-865. The mutant myosin heavy chain genes were individually expressed in cells devoid of the endogenous copy of the myosin heavy chain gene (19), extracted, and purified to electrophoretic homogeneity (not shown). The extent of RLC phosphorylation was checked by urea-SDS gel electrophoresis (Fig. 2). The levels of endogenous RLC phosphorylation of myosin and its fragments prepared by using these protocols were undetectable.
Figure 1.
Schematic representation of Dictyostelium myosins used in this study. The top line shows the relative scale of the tail region.
Figure 2.
Urea-SDS-glycerol/PAGE analysis of RLC phosphorylation. RLC, and P + RLC indicate unphosphorylated and phosphorylated RLC, respectively. All myosins were poorly phosphorylated before kinase treatment and, with the exception of MyΔC1528, were well phosphorylated after the treatment with kinase. C-A, chimera A; C-B, chimera B.
ATPase activities of each of the myosins were measured under the conditions described by Ruppel et al. (19). In the wild-type, unphosphorylated RLC is inhibitory and phosphorylation relieved the inhibition (Table 1), as reported previously (16–21). The extent of activation by RLC phosphorylation was 6- to 7-fold. Among the truncated myosins, unphosphorylated RLC no longer inhibited S1 (Table 1). This result is the same as that with smooth muscle S1 (2). Both MyΔC1225 and MyΔC1528 were less efficiently regulated than the wild-type by RLC phosphorylation (Table 1), although the extent of activation (≈1.5-fold) was reproducibly and significantly above 1. Because both MyΔC1225 and MyΔC1528 lacked the assembly domain (35), they must be in the monomer state in the reaction mixture. That the assembly-incompetent truncation mutant myosins are only inefficiently regulated sharply contrasts with the case of truncated smooth muscle myosin, which is well regulated even if the tail is as short as 105-aa residues (3, 5, 36).
Table 1.
Steady–state actin-dependent ATPase activities
| Myosins | Actin-dependent MgATPase
|
Fold activation by RLC phosphorylation | |
|---|---|---|---|
| No kinase treatment | Kinase treatment | ||
| Wild-type | 0.13 | 0.71 | 5.5 |
| 0.10 | 0.60 | 6.0 | |
| MyΔC1225 | 0.57 | 0.81 | 1.4 |
| 0.55 | 0.76 | 1.4 | |
| MyΔC1528 | 0.46 | 0.74 | 1.6 |
| 0.31 | 0.38 | 1.2 | |
| S1 | 0.89 | 0.78 | 0.9 |
| 1.20 | 1.20 | 1.0 | |
| 3xAsp | 0.23 | 0.57 | 2.5 |
| 0.33 | 1.10 | 3.3 | |
Activities were measured in the presence of 24 μM actin and are shown as Pi liberated per head per s. Activities of wild-type and 3xAsp myosins were measured at a myosin concentration of 100 μg/ml. Data were from assay of two independently prepared samples.
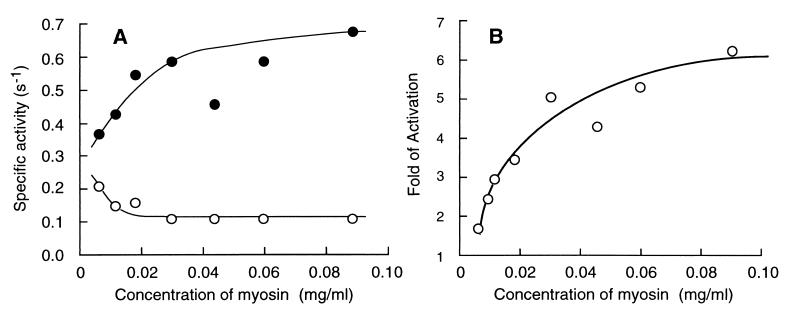
The above results imply that the regulation of Dictyostelium myosin by RLC phosphorylation depends on filament assembly. To test this hypothesis, we made a series of dilution of the concentrated myosin solution to obtain a gradual increase in the fraction of the monomer or oligomer forms in each reaction mixture and then assayed the actin-dependent ATPase activities. The actin-dependent activity of the phosphorylated form decreased whereas the activity of the unphosphorylated form increased 2-fold as the sample was diluted to 2.85 μg/ml (Fig. 3A), showing progressive loss of regulation as myosin became more and more diluted (Fig. 3B). In a parallel experiment, the extent of filament assembly was estimated by measuring fraction of pelletable molecules after a high speed centrifugation at 230,000 × g for 10 min at 20°C. At 120 μg/ml in the assay buffer containing 1 mM ATP, 39.0 ± 0.9% (average ± SD, n = 3) of myosin was pelletable, whereas only 7.6 ± 1.3% was pelletable at 2.85 μg/ml. In the absence of ATP, 93.1 ± 2.4% was pelletable at a concentration of 120 μg/ml, indicating that ATP strongly inhibits filament assembly of Dictyostelium myosin as reported previously (22).
Figure 3.
(A) Correlation between the actin-dependent MgATPase activities and concentration of wild-type myosin in the reaction mixture. The activities were measured in the presence of 24 μM actin at 30°C. ○, unphosphorylated form; •, phosphorylated form. (B) Dependence of activation by RLC phosphorylation on the concentration of wild-type myosin in the reaction mixture. The activation was calculated by dividing the actin-dependent MgATPase activity of the phosphorylated form by that of the unphosphorylated form.
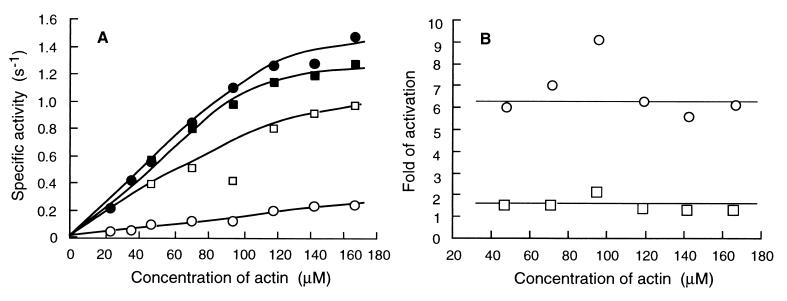
We then measured the activity at two fixed myosin concentrations and various actin concentrations. When the myosin concentration was 120 μg/ml, unphosphorylated RLC significantly inhibited the activity in the range of actin concentration from 48 μM to 167 μM (Fig. 4A). Myosin was activated ≈6-fold by RLC phosphorylation in this range of actin concentrations (Fig. 4B). When myosin concentration was reduced to 4.8 μg/ml (25-fold dilution), the difference of activities between phosphorylated and unphosphorylated forms became much smaller with the fold of activation of ≈1.5 (Fig. 4), suggesting that the molecules under such conditions are not efficiently regulated by RLC phosphorylation.
Figure 4.
(A) Actin-dependent MgATPase activity of the wild-type in the presence of various concentrations of actin. The activity was measured at 30°C. •, phosphorylated form at concentration of 120 μg/ml in the reaction mixture; ○, unphosphorylated form at a concentration of 120 μg/ml in the reaction mixture; ■, phosphorylated form at a concentration of 4.8 μg/ml in the reaction mixture; □, unphosphorylated form at a concentration of 4.8 μg/ml in the reaction mixture. (B) Fold of activation by RLC phosphorylation at various actin concentrations. ○, myosin at a concentration of 120 μg/ml in the reaction mixture; □, myosin at a concentration of 4.8 μg/ml in the reaction mixture.
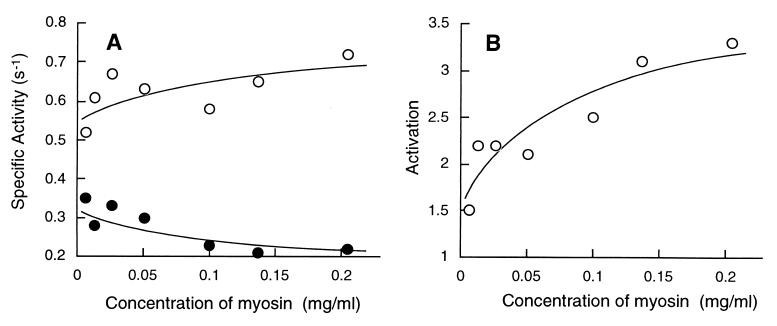
It is known that phosphorylation of three Thr residues in the distal region of the heavy chain reduces the tendency of Dictyostelium myosin to assemble into filament structure (21–23). In the 3xAsp mutant myosin, the three Thr residues were substituted with Asp residues to mimic the phosphorylated, assembly-incompetent state (23). The 3xAsp myosin was purified to electrophoretic homogeneity (not shown) and was quantitatively phosphorylated at RLC (Fig. 2). The ATPase activities of 3xAsp varied significantly from preparation to preparation. However, the extent of its regulation by RLC phosphorylation was always ≈2- to 3-fold, which is roughly one-half of that of the wild-type (Table 1). Fig. 5 shows that increase in concentration of myosin enhanced the extent of regulation similarly to wild-type but only up to ≈3.2-fold at a myosin concentration of 0.2 mg/ml.
Figure 5.
(A) Correlation between the actin-dependent MgATPase activities and concentration of 3xAsp myosin in the reaction mixture. The activities were measured in the presence of 24 μM actin at 30°C. •, unphosphorylated form; ○, phosphorylated form. (B) Fold of activation by RLC phosphorylation on the concentration of 3xAsp myosin in the reaction mixture.
To examine whether filament formation is sufficient for efficient regulation by RLC phosphorylation, we assayed two chimeric myosins, called chimera A and chimera B. In chimera A, the head and approximately one-third of S2 from Dictyostelium myosin (amino acid residues 1–940) was fused at the corresponding site to chicken skeletal myosin tail, keeping the frame of 28-aa residue repeats. Chimera B was made up of Dictyostelium myosin HMM and chicken skeletal myosin light meromyosin, fused at the amino acid residue 1,223 of the Dictyostelium myosin heavy chain. Table 2 shows that the actin-dependent ATPase activities of both chimeric myosins are much higher than the fully phosphorylated wild-type and are only slightly inhibited by the unphosphorylated RLC. The result clearly indicates that the two chimeric myosins are not efficiently regulated by RLC phosphorylation and that the filament structure of skeletal muscle myosin cannot substitute for the native filament structure of Dictyostelium myosin.
Table 2.
Steady–state actin-dependent ATPase activities of chimeric myosins
| Myosins | Actin-dependent MgATPase
|
Fold activation by RLC phosphorylation | |
|---|---|---|---|
| No kinase treatment | Kinase treatment | ||
| Wild-type | 0.11 | 0.85 | 7.7 |
| Chimera A | 1.9 | 2.3 | 1.2 |
| Chimera B | 1.7 | 2.0 | 1.2 |
Activities were measured in the presence of 24 μM actin and 85 μg/ml myosin and are shown as Pi liberated per head per s.
DISCUSSION
We have shown that the filament structure is essential for efficient regulation of Dictyostelium myosin by phosphorylation of RLC. This conclusion was drawn based on the observation that regulation was impaired under three independent conditions that affect filament assembly: removal of the assembly domain by truncation mutations, altering the assembly equilibrium by a set of point mutations in the assembly regulatory domain, and reduction of myosin concentration in the reaction mixture. It is not clear, however, how the filament structure is involved in the regulatory switch of Dictyostelium myosin. Our preliminary kinetic analysis (X.L. and T.Q.P.U., unpublished data) suggested that the regulation of wild-type myosin by RLC phosphorylation involves alteration of both Vmax and Kapp for actin, whereas the inefficient regulation of truncation mutants affects only Kapp. It appears that the filament structure is involved in efficient regulation by changing Vmax.
In Acanthamoeba myosin, heavy chain phosphorylation is hypothesized to increase the flexibility of the hinge region between S2 and light meromyosin and thereby decrease the actin-dependent ATPase activity, implying that a specific filament structure is essential for switching of that molecule (11). RLC phosphorylation is shown to change the filament structure in skeletal muscle myosin (37). It is thus possible that RLC phosphorylation changes flexibility of the filament structure of Dictyostelium myosin and thus activates its activity. In other words, Dictyostelium and Acanthamoeba myosins may share a similar regulatory mechanism although the former is phosphorylated on the RLC and the latter on the heavy chain. Alternatively, Dictyostelium myosin may be similar to smooth muscle myosin in that it is regulated by physical interactions between the two heads and the amino terminal S2, except that such interaction in Dictyostelium myosin requires a specific filament structure. Unphosphorylated smooth muscle myosin forms a folded conformation under low ionic strength conditions, two heads bending toward the tail (8). It should be pointed out that such bent conformations around the head/tail junction have not been observed by electron microscopy in Dictyostelium myosin under low ionic strength conditions and even in the absence of salts (22).
Decrease in myosin concentration nearly halved the activity of the phosphorylated form and doubled the activity of unphosphorylated form, resulting in loss of regulation (Fig. 3). More myosin molecules are in the unpelletable form such as monomers or oligomers under diluted conditions. Thus the increase of the activity of unphosphorylated form under diluted conditions can be explained by release from inhibition by unphosphorylated RLC due to filament disassembly. The fact that only 39% of the molecules were pelleted by a high speed centrifugation under our standard assay conditions suggests the possibility that the extent of regulation of Dictyostelium myosin by RLC phosphorylation may be much higher than the observed 6- to 7-fold if most molecules are incorporated into filaments.
In the monomer form, the tail of Dictyostelium myosin tends to form a kink, particularly when heavy chain phosphorylation sites are phosphorylated (22, 38). It may be possible that such a kink in the tail interferes with efficient interaction with actin, contributing to the reduced activity of phosphorylated form under diluted conditions. Myosin filaments attract actin filaments in their close proximity to form aggregates, decreasing Kapp for actin and consequently increasing actin-dependent ATPase activities when the actin concentration is not saturating. This also would contribute to the decrease of activity of phosphorylated form as it is diluted in the reaction mixture. These two inactivating factors should similarly affect activities of unphosphorylated form under diluted conditions. Therefore, the true extent of increase in activity due to release from inhibition by unphosphorylated RLC under diluted conditions must be larger than 2-fold, and may reach 4-fold if simply doubled according to the 2-fold reduction of the activity of the phosphorylated form. This value is reasonable in a sense that it is close to the 6-fold activation by RLC phosphorylation under normal conditions.
The fact that two chimeric myosins were poorly regulated by RLC phosphorylation suggests that Dictyostelium myosin needs a specific structure in the filament for efficient regulation. In both chimera A and B, majority of the tail sequences, including the S2-light meromyosin hinge and the assembly domain, were of chicken skeletal myosin. These chimeric myosins are readily incorporated into filaments of skeletal muscle myosin tail, whereas native Dictyostelium myosin is not (T. Okumura, H. Tanaka, T. Kusumoto, S.M., A.H.-I., et al., unpublished observations). This suggests that the filament structure of the skeletal muscle myosin and the chimeric myosins differs from that of wild-type Dictyostelium myosin and that the subtle structural difference may impair the inhibitory interaction between the heads and the tail in the chimeric myosins. It would be of interest to compare filament structure between the wild-type and two chimeric myosins.
Our study suggests that there are some differences in the regulatory mechanism of RLC phosphorylation between Dictyostelium and smooth muscle myosins. The extent of activation of Dictyostelium myosin is only 6-fold, which is one order of magnitude smaller than that of smooth muscle myosin (9). Most strikingly, the complete regulatory switch of Dictyostelium myosin depends on filament assembly and can be easily nullified by changes within the filament structure, whereas smooth muscle HMM is insensitive to such perturbations (2, 4, 36). Taken together, Dictyostelium myosin has an apparently distinctive regulatory mechanism by RLC phosphorylation. It is yet to be examined whether any one of these mechanisms is seen more commonly among diverse species of myosin and whether there is a common underlying principle among the apparently diverse regulatory mechanisms.
Acknowledgments
We are most grateful to Dr. Tetsuya Tateishi for his support throughout this work and thank Dr. Keiko Hirose for critical reading of the manuscript. K.I. was supported by a fellowship from New Energy and Industrial Technology Development Organization of Japan. Supported in part by Grants-in-Aid to T.Q.P.U. from the Ministry of Education, Science and Culture of Japan.
ABBREVIATIONS
- RLC
regulatory light chain
- S
subfragment
- HMM
heavy meromyosin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Tan J L, Ravid S, Spudich J A. Annu Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- 2.Seidel J C. J Biol Chem. 1980;255:4355–4361. [PubMed] [Google Scholar]
- 3.Sellers J R. J Biol Chem. 1985;260:15815–15819. [PubMed] [Google Scholar]
- 4.Sweeney H L, Yang Z, Zhi G, Stull J T, Trybus K M. Proc Natl Acad Sci USA. 1994;91:1490–1494. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trybus K M, Freyzon Y, Faust L Z, Sweeney H L. Proc Natl Acad Sci USA. 1997;94:48–52. doi: 10.1073/pnas.94.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremo C R, Sellers J R, Facemyer K C. J Biol Chem. 1995;270:2171–2175. doi: 10.1074/jbc.270.5.2171. [DOI] [PubMed] [Google Scholar]
- 7.Matsu-ura M, Ikebe M. FEBS Lett. 1995;363:246–250. doi: 10.1016/0014-5793(95)00326-5. [DOI] [PubMed] [Google Scholar]
- 8.Onishi H, Wakabayashi T. J Biochem. 1982;92:871–879. doi: 10.1093/oxfordjournals.jbchem.a134001. [DOI] [PubMed] [Google Scholar]
- 9.Ikebe M, Koretz J, Hartshorne D J. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- 10.Trybus K M. J Muscle Res Cell Motil. 1994;15:587–594. doi: 10.1007/BF00121066. [DOI] [PubMed] [Google Scholar]
- 11.Brzeska H, Korn E D. J Biol Chem. 1996;271:16983–16986. doi: 10.1074/jbc.271.29.16983. [DOI] [PubMed] [Google Scholar]
- 12.Korn E D, Hammer J A., III Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- 13.Pato M D, Sellers J R, Preston Y A, Harvey E V, Adelstein R S. J Biol Chem. 1996;271:2689–2695. doi: 10.1074/jbc.271.5.2689. [DOI] [PubMed] [Google Scholar]
- 14.Wagner P D, George J N. Biochemistry. 1986;25:913–918. doi: 10.1021/bi00352a026. [DOI] [PubMed] [Google Scholar]
- 15.Ikebe M, Reardon S. Biochemistry. 1990;29:2713–2720. doi: 10.1021/bi00463a014. [DOI] [PubMed] [Google Scholar]
- 16.Griffith L M, Downs S M, Spudich J A. J Cell Biol. 1987;104:1309–1323. doi: 10.1083/jcb.104.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrow B D, Chen P, Chisholm R L. J Cell Biol. 1994;127:1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uyeda T Q P, Abramson P D, Spudich J A. Proc Natl Acad Sci USA. 1996;93:4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruppel K M, Uyeda T Q P, Spudich J A. J Biol Chem. 1994;269:18773–18780. [PubMed] [Google Scholar]
- 20.Uyeda T Q P, Spudich J A. Science. 1993;262:1867–1870. doi: 10.1126/science.8266074. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski E R, Spudich J A. Proc Natl Acad Sci USA. 1980;77:7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczmarski E R, Tafuri S R, Parysek L M. J Cell Biol. 1987;105:2989–2997. doi: 10.1083/jcb.105.6.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egelhoff T T, Lee R J, Spudich J A. Cell. 1993;75:363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- 24.De Lozanne A, Spudich J A. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 26.Egelhoff T T, Titus M A, Manstein D J, Ruppel K M, Spudich J A. Methods Enzymol. 1991;196:319–334. doi: 10.1016/0076-6879(91)96029-q. [DOI] [PubMed] [Google Scholar]
- 27.Sussman M. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- 28.Yumura S, Uyeda T Q P. Mol Biol Cell. 1997;8:2089–2099. doi: 10.1091/mbc.8.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Spudich J A, Watt S. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 31.Smith J L, Silveira L A, Spudich J A. EMBO J. 1996;15:6075–6083. [PMC free article] [PubMed] [Google Scholar]
- 32.Perrie W T, Perry S V. Biochem J. 1970;119:31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama T, Fukui K, Kometani K. J Biochem. 1986;99:1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- 34.Truong T, Medley Q G, Côte G P. J Biol Chem. 1992;267:9767–9772. [PubMed] [Google Scholar]
- 35.Lee R J, Egelhoff T T, Spudich J A. J Cell Sci. 1994;107:2875–2886. doi: 10.1242/jcs.107.10.2875. [DOI] [PubMed] [Google Scholar]
- 36.Sellers J R, Eisenberg E, Adelstein R S. J Biol Chem. 1982;257:13880–13883. [PubMed] [Google Scholar]
- 37.Levine R J C, Kensler R W, Yang Z, Stull J T, Sweeney H L. Biophys J. 1996;71:898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasternak C, Flicker P F, Ravid S, Spudich J A. J Cell Biol. 1989;109:203–210. doi: 10.1083/jcb.109.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]