Abstract
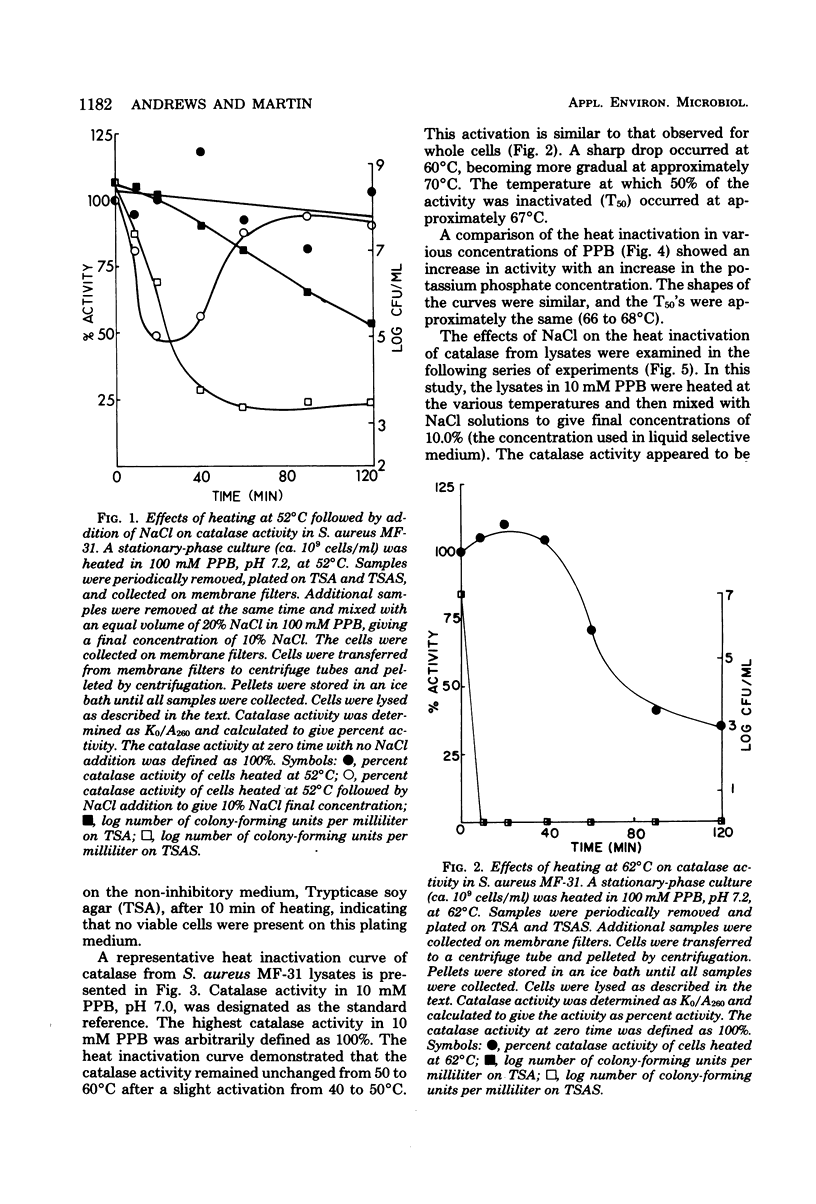
The effects of heat on catalase from Staphylococcus aureus lysates were examined. Catalase activity increased with increasing concentrations of potassium phosphate buffer, when heated at temperatures between 50 and 65 degrees C for 10 min. Inactivation of catalase by NaCl during heating was demonstrated. Extended heating of S. aureus cells at 52 degrees C resulted in a slight decrease in catalase activity of the resultant lysates. This decrease was more pronounced in the presence of salt. Heating at 62 degrees C caused a decrease in catalase activity, but not complete inactivation. These results implicate the combined effects of heat, and NaCl in the inactivation of catalase from S. aureus. The findings are consistent with the hypothesis that H2O2 may accumulate as a result of decreased catalase activity and be responsible for the decreased colony-forming ability of stressed S. aureus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C., Russell A. D. Mechanisms of thermal injury in nonsporulating bacteria. Adv Appl Microbiol. 1970;12:89–119. doi: 10.1016/s0065-2164(08)70583-5. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith M. J., Warby C. Protective effect of low and high molecular weight compounds on the stability of catalase subjected to freezing and thawing. Cryobiology. 1972 Apr;9(2):137–140. doi: 10.1016/0011-2240(72)90021-1. [DOI] [PubMed] [Google Scholar]
- Brewer D. G., Martin S. E., Ordal Z. J. Beneficial effects of catalase or pyruvate in a most-probable-number technique for the detection of Staphylococcus aureus. Appl Environ Microbiol. 1977 Dec;34(6):797–800. doi: 10.1128/aem.34.6.797-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyshire B. The influence of dehydration on catalase stability. A comparison with freezing effects. Cryobiology. 1974 Apr;11(2):148–151. doi: 10.1016/0011-2240(74)90304-6. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Feinstein R. N., Sacher G. A., Howard J. B., Braun J. T. Comparative heat stability of blood catalase. Arch Biochem Biophys. 1967 Nov;122(2):338–343. doi: 10.1016/0003-9861(67)90203-2. [DOI] [PubMed] [Google Scholar]
- Flowers R. S., Martin S. E., Brewer D. G., Ordal Z. J. Catalase and enumeration of stressed Staphylococcus aureus cells. Appl Environ Microbiol. 1977 May;33(5):1112–1117. doi: 10.1128/aem.33.5.1112-1117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Beneficial effect of catalase treatment on growth of Clostridium perfringens. Appl Environ Microbiol. 1976 Sep;32(3):409–416. doi: 10.1128/aem.32.3.409-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Recovery of clostridia on catalase-treated plating media. Appl Environ Microbiol. 1977 Apr;33(4):762–770. doi: 10.1128/aem.33.4.762-770.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács E., Lantos J., Mazarean H. H. The effect of phage infection on the catalase induction of the Staphylococcus aureus culture. Experientia. 1966 Dec 15;22(12):802–803. doi: 10.1007/BF01897425. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litchfield W. J. Inactivation of catalase by chloride. FEBS Lett. 1977 Nov 15;83(2):281–284. doi: 10.1016/0014-5793(77)81023-5. [DOI] [PubMed] [Google Scholar]
- Martin S. E., Flowers R. S., Ordal Z. J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976 Nov;32(5):731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M. K., Aris B., El Derea H. B. The effect of compounds which degrade hydrogen peroxide on the enumeration of heat-stressed cells of Salmonella senftenberg. Can J Microbiol. 1978 Jul;24(7):883–885. doi: 10.1139/m78-146. [DOI] [PubMed] [Google Scholar]
- Sinha A. K. Colorimetric assay of catalase. Anal Biochem. 1972 Jun;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]



