Abstract
Luminous bacteria in the Mediterranean Sea and the Gulf of Aqaba-Elat have different distribution patterns. In the Mediterranean Sea, Beneckea harveyi is present all year round, with different subtypes alternating in summer and winter; Photobacterium fischeri was only present during the winter. In the Gulf of Elat, P. leiognathi is present throughout the water column in similar densities during the entire year. This constancy in distribution is presumably due to the near-constancy in water temperature. In summer, Photobacterium leiognathi is replaced by B. harveyi in coastal surface waters. In the hypersaline Bardawil lagoon, only B. harveyi types are present. P. fischeri, a major component of the Mediterranean Sea winter communities, is absent from the lagoon. Luminous Beneckea strains show a great diversity in properties, e.g. temperature range for growth, sensitivity to infection by phages, sensitivity to attack by Bdellovibrio strains, and differences in tolerance to high-salinity shock. Therefore, subdivision of the taxonomic cluster of B. harveyi into subtypes is indicated. The composition of the luminous bacteria communities may serve as indicators of different marine water bodies. The symbiotic luminous bacteria of the light organ of the common Gulf of Elat fish, Photoblepharon palbebratus steinitzi, is different from any of the types described.
Full text
PDF


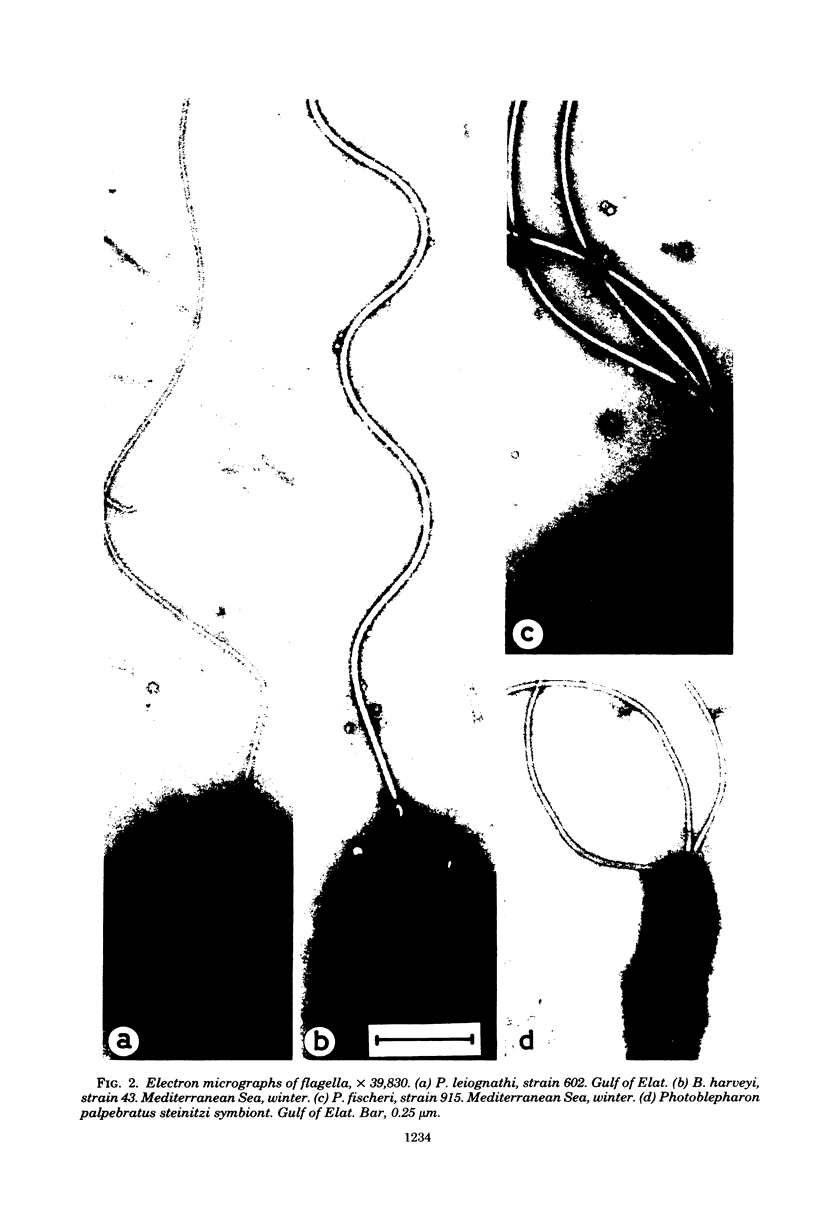


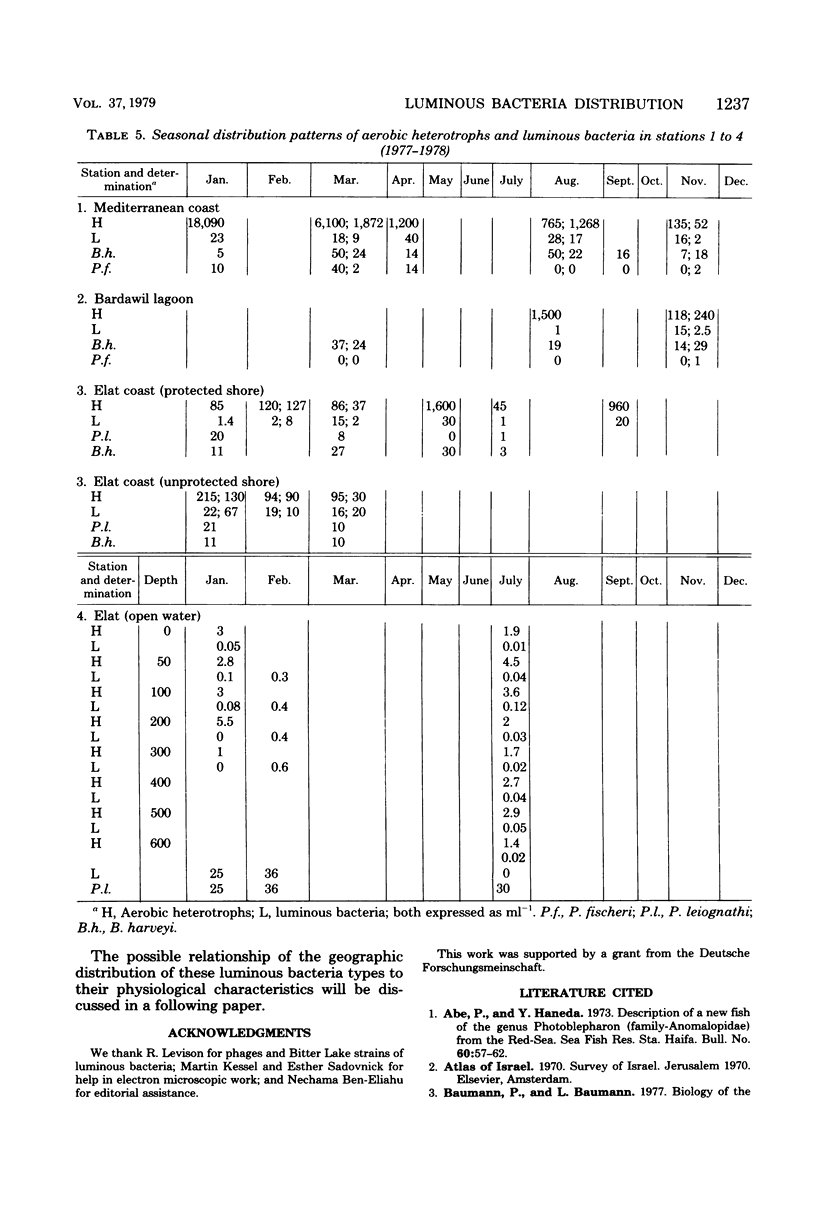

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol. 1973 Jan;113(1):24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katznelson R., Ulitzur S. Control of luciferase synthesis in a newly isolated strain of Photobacterium leiognathi. Arch Microbiol. 1977 Dec 15;115(3):347–351. doi: 10.1007/BF00446462. [DOI] [PubMed] [Google Scholar]
- Marbach A., Shilo M. Dependence of marine bdellovibrios on potassium, calcium, and magnesium ions. Appl Environ Microbiol. 1978 Jul;36(1):169–177. doi: 10.1128/aem.36.1.169-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Reichelt J. L., Baumann P., Baumann L. Study of genetic relationships among marine species of the genera Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch Microbiol. 1976 Oct 11;110(1):101–120. doi: 10.1007/BF00416975. [DOI] [PubMed] [Google Scholar]
- SIERRA G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek. 1957;23(1):15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]