Abstract
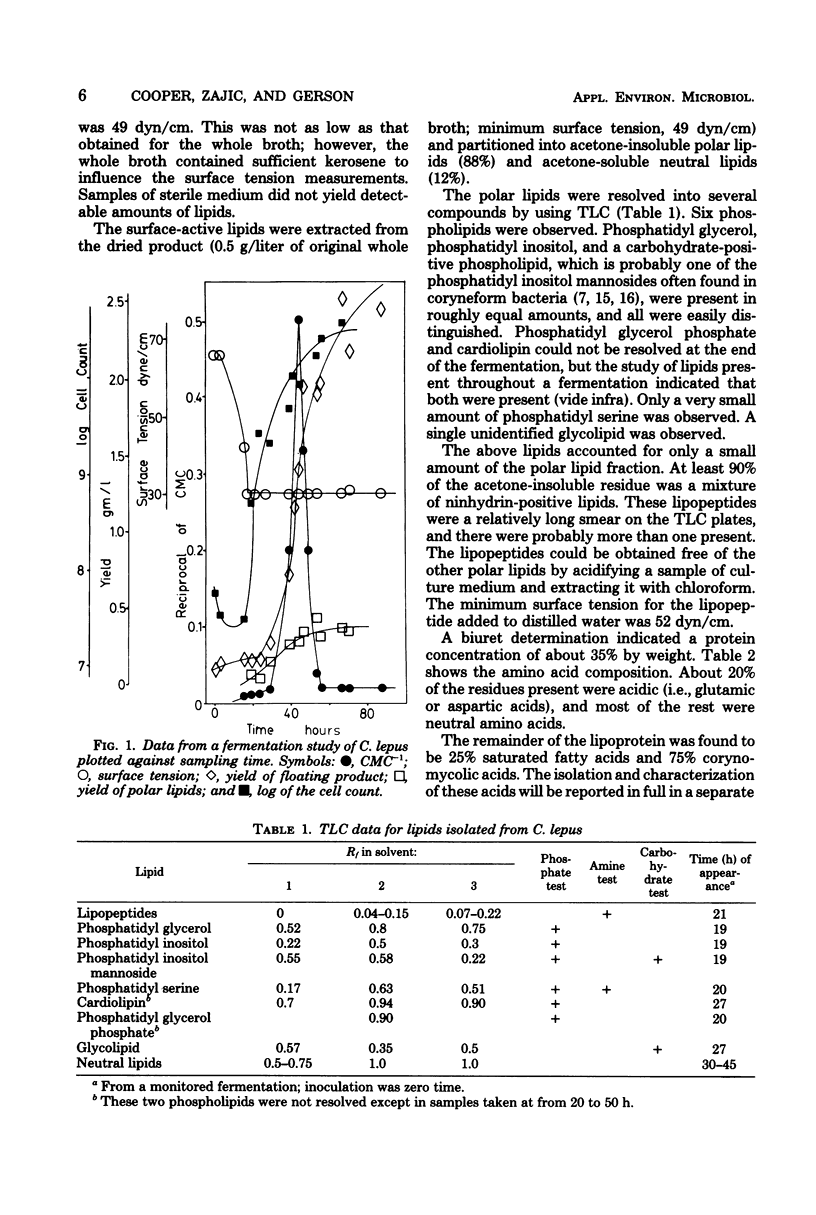
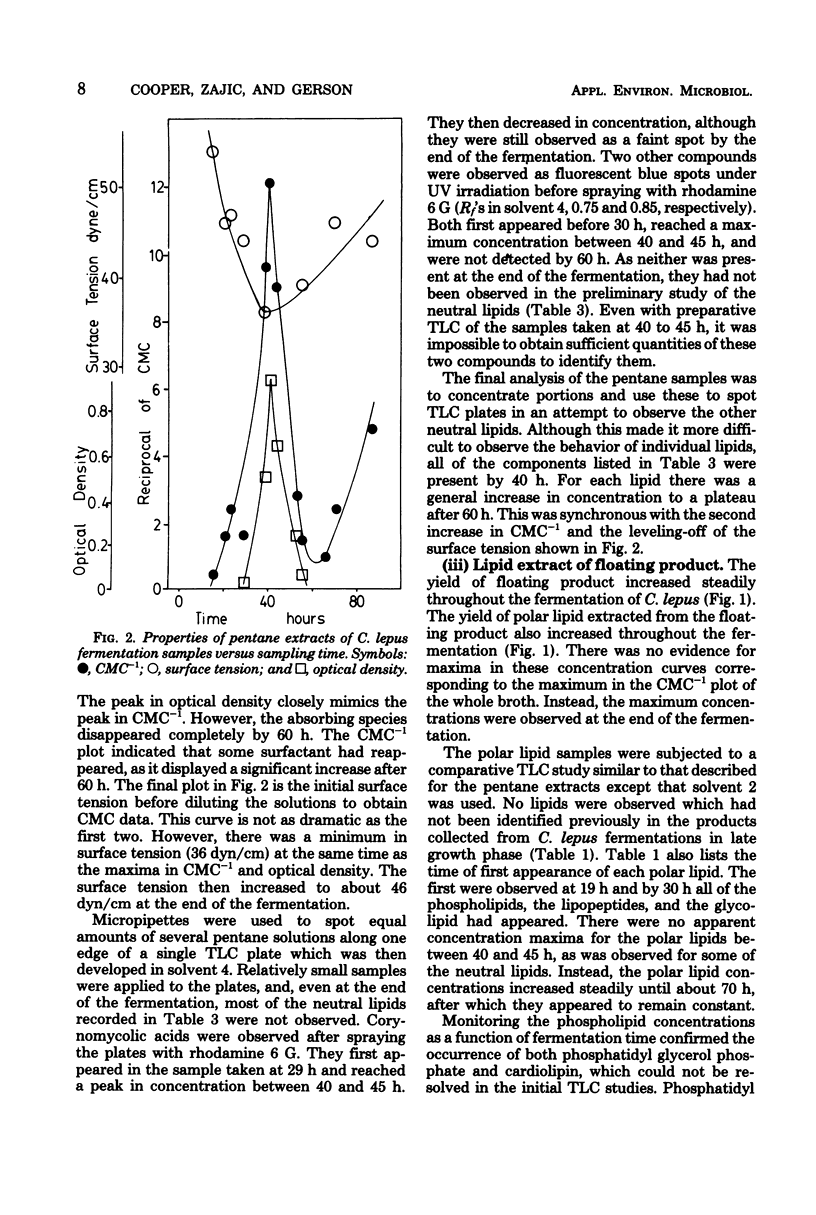
Corynebacterium lepus was grown in 20-liter batch fermentations with kerosene as the sole carbon source. Critical micelle concentration measurements indicated the production of appreciable quantities of biosurfactants. This surface activity of the culture medium was due to lipids, which were extracted and identified. Samples of C. lepus whole broth were taken during a fermentation and monitored for surface tension, amount of surfactant present, and lipid content. The changes in the surfactant measured correlated with concentration changes of several surface-active lipids. An early dramatic increase in surfactant concentration was attributed to the production of a mixture of corynomycolic acids (beta-hydroxy alpha-branched fatty acids). Surface activity at the end of the fermentation was due to a lipopeptide containing corynomycolic acids plus small amounts of several phospholipids and neutral lipids which were identified by thin-layer chromatography.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Kakinuma A., Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun. 1968 May 10;31(3):488–494. doi: 10.1016/0006-291x(68)90503-2. [DOI] [PubMed] [Google Scholar]
- Beebe J. L., Umbreit W. W. Extracellular lipid of Thiobacillus thiooxidans. J Bacteriol. 1971 Oct;108(1):612–614. doi: 10.1128/jb.108.1.612-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Lehane D. P. The phospholipids of corynebacteria. Lipids. 1971 Jun;6(6):401–409. doi: 10.1007/BF02531377. [DOI] [PubMed] [Google Scholar]
- Hackett J. A., Brennan P. J. The mannophosphoinositides of Corynebacterium aquaticum. Biochem J. 1975 May;148(2):253–258. doi: 10.1042/bj1480253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES G. E., BENSON A. A. PHOSPHATIDYL GLYCEROL IN THIOBACILLUS THIOOXIDANS. J Bacteriol. 1965 Jan;89:260–261. doi: 10.1128/jb.89.1.260-261.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie R. M., Cure G. L. The cell wall composition and distribution of free mycolic acids in named strains of coryneform bacteria and in isolates from various natural sources. J Appl Bacteriol. 1977 Apr;42(2):229–252. doi: 10.1111/j.1365-2672.1977.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Khuller G. K., Brennan P. J. Further studies on the lipids of corynebacteria. The mannolipids of Corynebacterium aquaticum. Biochem J. 1972 Apr;127(2):369–373. doi: 10.1042/bj1270369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERER E., PUDLES J. Sur l'isolement et la constitution chimique d'un hydroxyacide ramifié du bacille diphtérique. Bull Soc Chim Biol (Paris) 1951;33(8):1003–1011. [PubMed] [Google Scholar]
- Makula R. A., Lockwood P. J., Finnerty W. R. Comparative analysis of the lipids of Acinetobacter species grown on hexadecane. J Bacteriol. 1975 Jan;121(1):250–258. doi: 10.1128/jb.121.1.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. Lipid composition as a guide to the classification of bacteria. Adv Appl Microbiol. 1974;17(0):63–108. doi: 10.1016/s0065-2164(08)70555-0. [DOI] [PubMed] [Google Scholar]
- Walker R. W., Prome J. C., Lacave C. S. Biosynthesis of mycolic acids. Formation of a C32 beta-keto ester from palmitic acid in a cell-free system of Corynebacterium diphtheriae. Biochim Biophys Acta. 1973 Oct 17;326(1):52–62. doi: 10.1016/0005-2760(73)90027-1. [DOI] [PubMed] [Google Scholar]


