Abstract
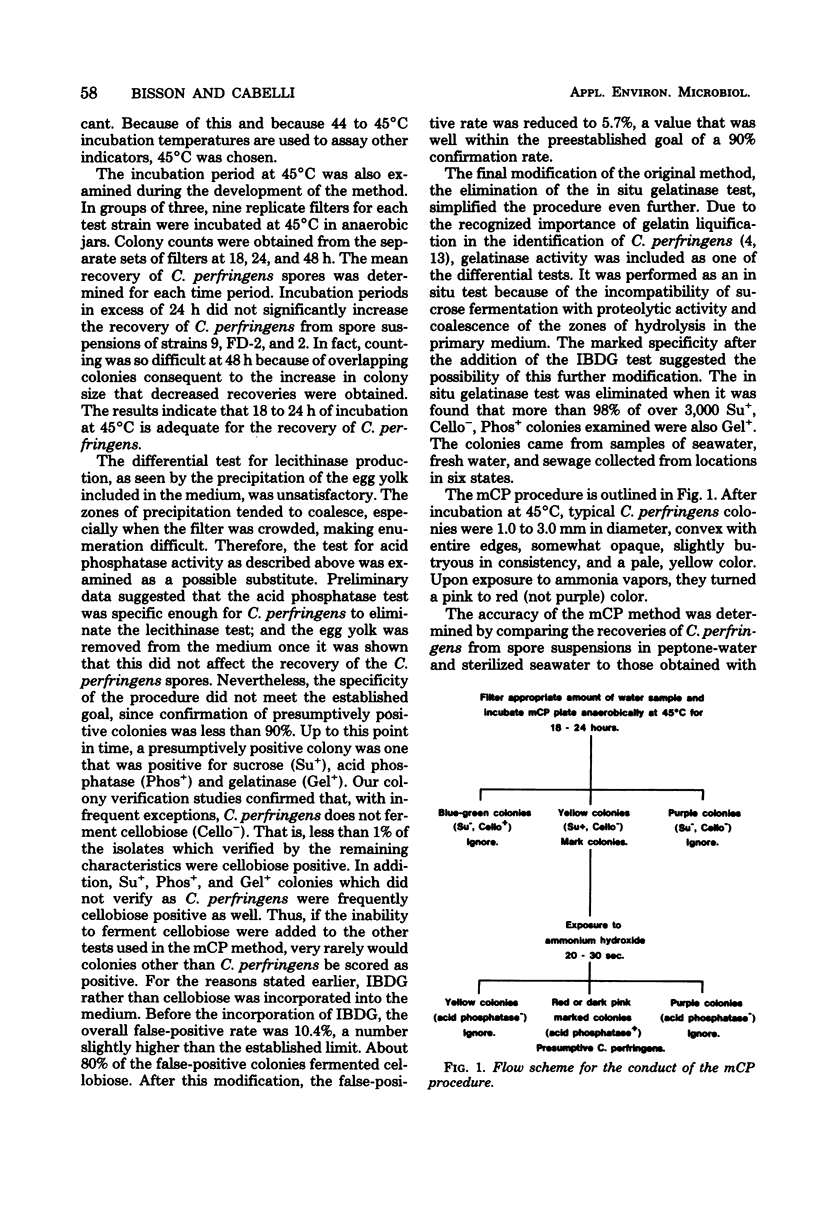
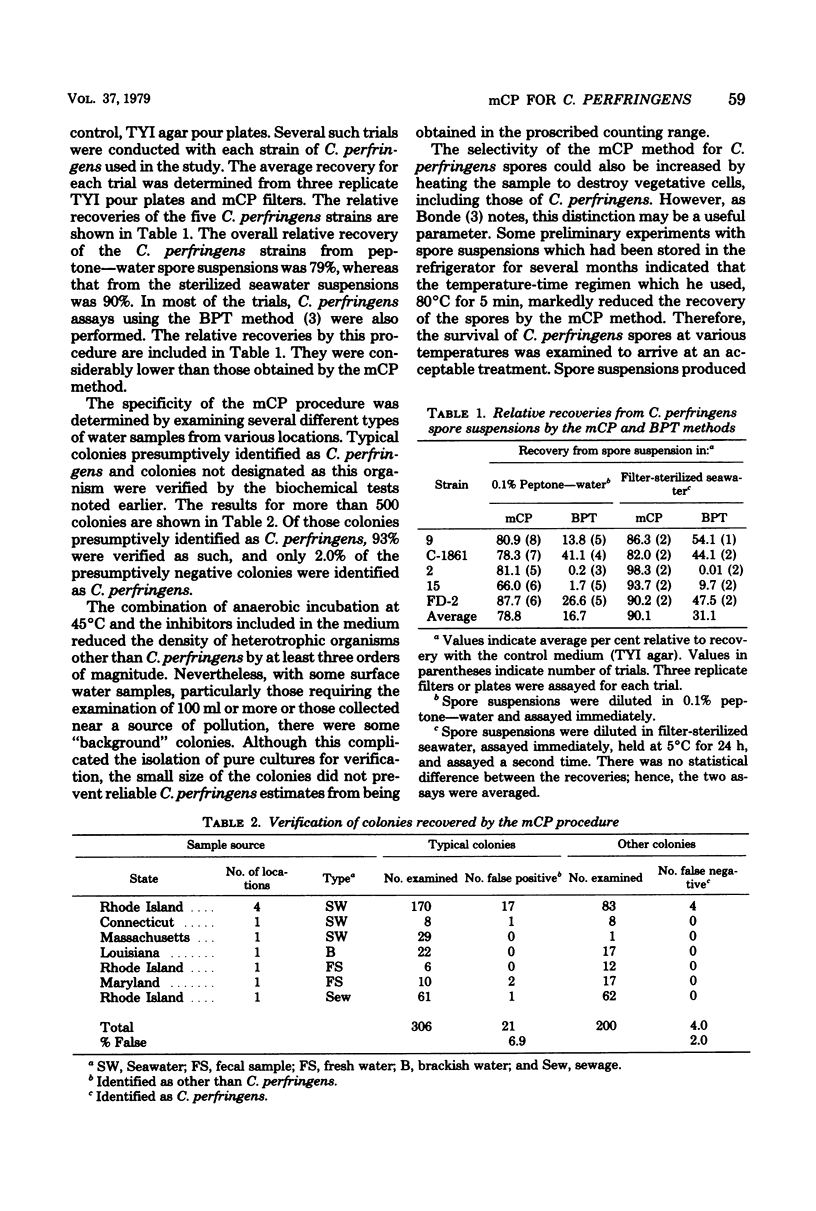
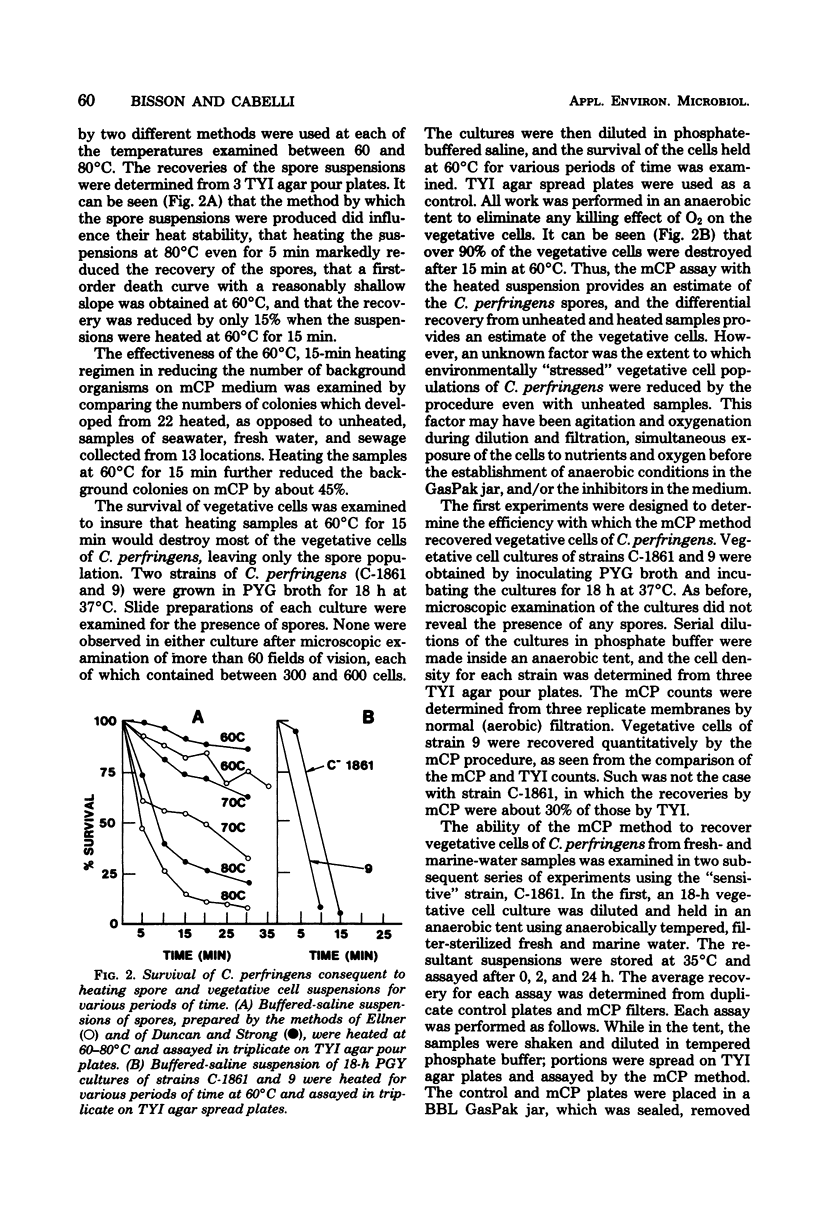
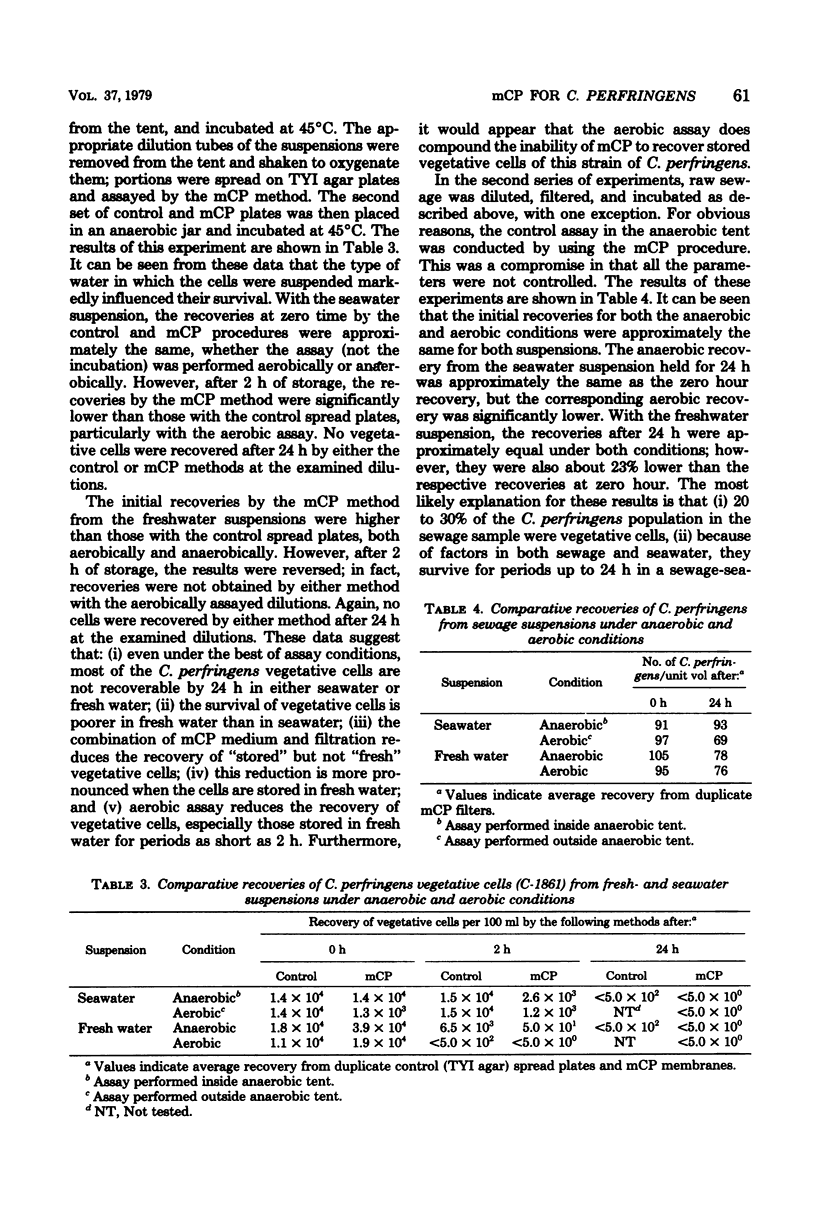
A membrane filter procedure has been developed for the rapid quantitation of C. perfringens in the aquatic environment. Background growth is inhibited by the use of D-cycloserine, polymyxin B sulfate, and incubation at 45 degrees C. Differential characteristics include the fermentation of sucrose, production of acid phosphatase, and the absence of beta-D-glucosidase activity. The medium is prepared as follows (in grams per 100 ml of distilled water): tryptose, 3.0; yeast extract, 2.0; sucrose, 0.5; L-cysteine, 0.1; MgSO4. 7H2O, 0.01; bromocresol purple, 0.004; and agar, 1.5. The ingredients are dissolved, and the pH is adjusted to 7.6. After autoclaving at 121 degrees C for 15 min, the medium is allowed to cool at 50 degrees C, and the following are added per 100 ml: D-cycloserine, 40 mg; polymyxin B sulfate, 2.5 mg; indoxyl-beta-D-glucoside, 60 mg; 2.0 ml of a filter-sterilized 0.5% phenolpthalein diphosphate solution; and 0.2 ml of a filter-sterilized 4.5% FeCl3.6H2O solution. Enumeration of C. perfringens in a water sample is completed within 18 to 24 h. The verification of typical colonies was 93%. The average recovery from peptone-water spore suspensions of five strains was 79%, and that from filter-sterilized seawater suspensions was 90%. The precision of the method was approximately equal to that expected from random error alone. Confirmed recoveries of C. perfringens from water and sewage samples generally were greater than those by the Bonde pour tube method.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELOTTI R., HALL H. E., FOTER M. J., LEWIS K. H. Quantitation of Clostridium perfringens in foods. Appl Microbiol. 1962 May;10:193–199. doi: 10.1128/am.10.3.193-199.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLNER P. D. A medium promoting rapid quantitative sporulation in Clostridium perfringens. J Bacteriol. 1956 Apr;71(4):495–496. doi: 10.1128/jb.71.4.495-496.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhart C., Wilson P. W. STATISTICAL METHODS AND CONTROL IN BACTERIOLOGY. Bacteriol Rev. 1943 Jun;7(2):57–137. doi: 10.1128/br.7.2.57-137.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUCHS A. R., BONDE G. J. The nutritional requirements of Clostridium perfringens. J Gen Microbiol. 1957 Apr;16(2):317–329. doi: 10.1099/00221287-16-2-317. [DOI] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A., Peeler J. T. Comparison of media for the enumeration of Clostridium perfringens. Appl Microbiol. 1971 May;21(5):922–927. doi: 10.1128/am.21.5.922-927.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Evaluation and modifications of media for enumeration of Clostridium perfringens. Appl Microbiol. 1974 Jan;27(1):78–82. doi: 10.1128/am.27.1.78-82.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON R., HARMON S., KAUTTER D. METHOD TO FACILITATE THE ISOLATION OF CLOSTRIDIUM BOTULINUM TYPE E. J Bacteriol. 1964 Nov;88:1521–1522. doi: 10.1128/jb.88.5.1521-1522.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. A., Cabelli V. J. Membrane filter technique for enumeration of Pseudomonas aeruginosa. Appl Microbiol. 1972 Dec;24(6):864–870. doi: 10.1128/am.24.6.864-870.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL R. S., STEENBERGEN J. F., MCCLUNG L. S. RAPID TECHNIQUE FOR THE ENUMERATION OF CLOSTRIDIUM PERFINGENS. Appl Microbiol. 1965 Jul;13:559–563. doi: 10.1128/am.13.4.559-563.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porschen R. K., Spaulding E. H. Phosphatase activity of anaerobic organisms. Appl Microbiol. 1974 Apr;27(4):744–747. doi: 10.1128/am.27.4.744-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S. A., Ferguson A. R. New quantitative, qualitative, and confirmatory media for rapid analysis of food for Clostridium perfringens. Appl Microbiol. 1971 Mar;21(3):500–506. doi: 10.1128/am.21.3.500-506.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Fujii H., Marui T., Takahashi J., Sugitani T. Acid phosphatase in Clostridium perfringens. A new rapid and simple identification method. Jpn J Microbiol. 1970 Mar;14(2):171–173. [PubMed] [Google Scholar]
- Watkins W. D., Thomas C. D., Cabelli V. J. Membrane filter procedure for enumeration of Vibrio parahaemolyticus. Appl Environ Microbiol. 1976 Nov;32(5):679–684. doi: 10.1128/aem.32.5.679-684.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



