Abstract
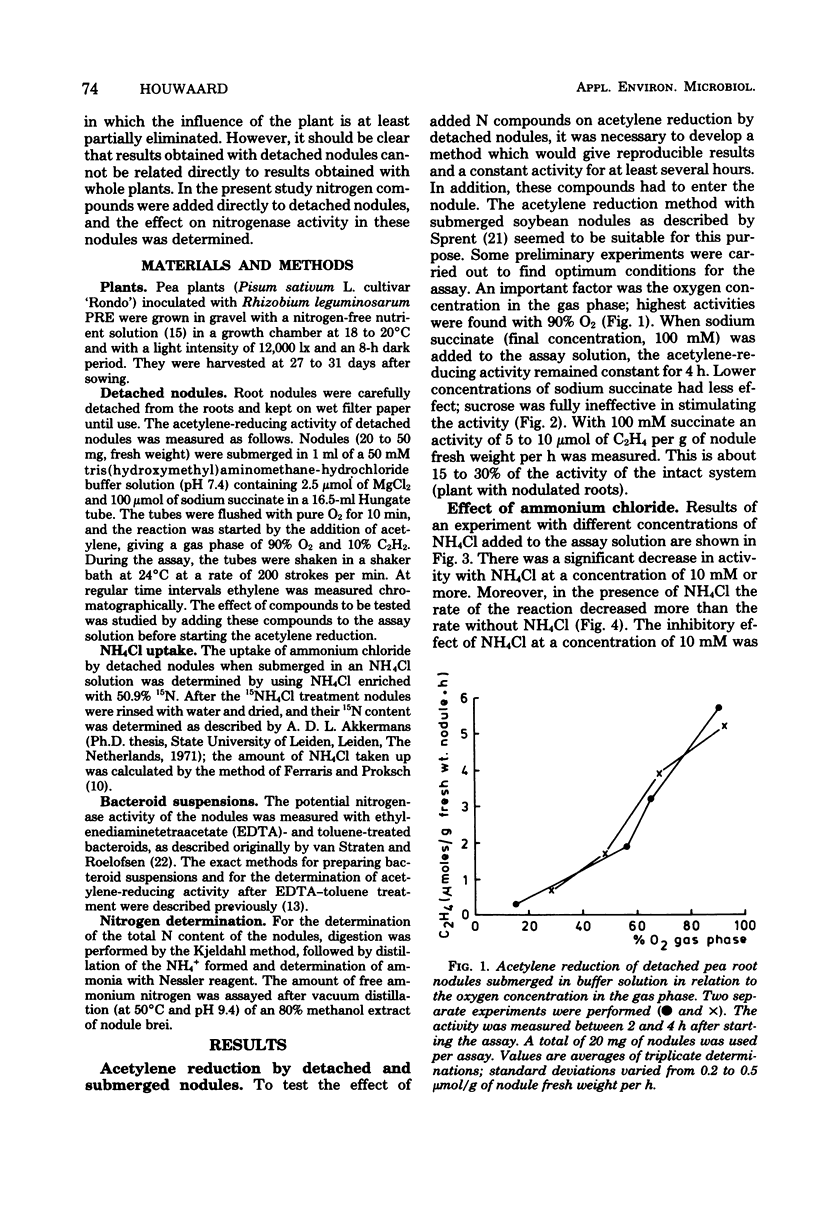
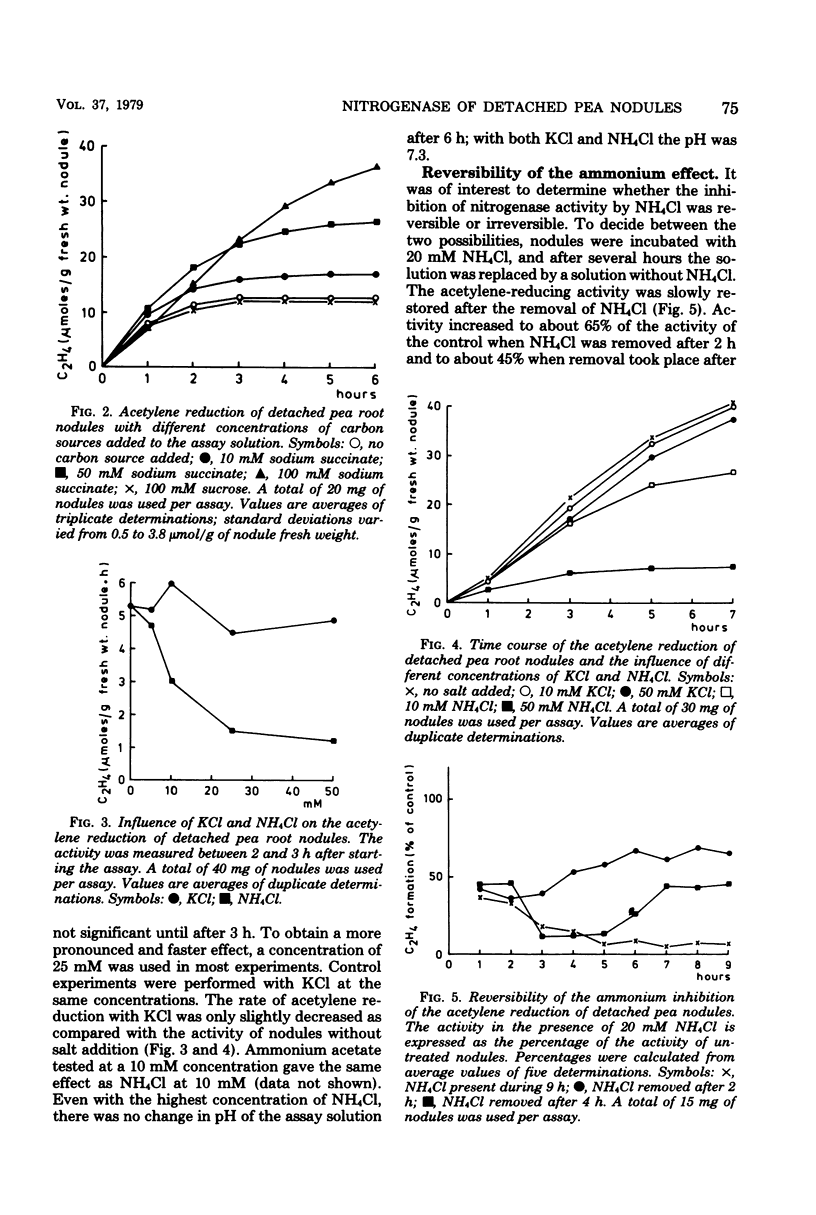
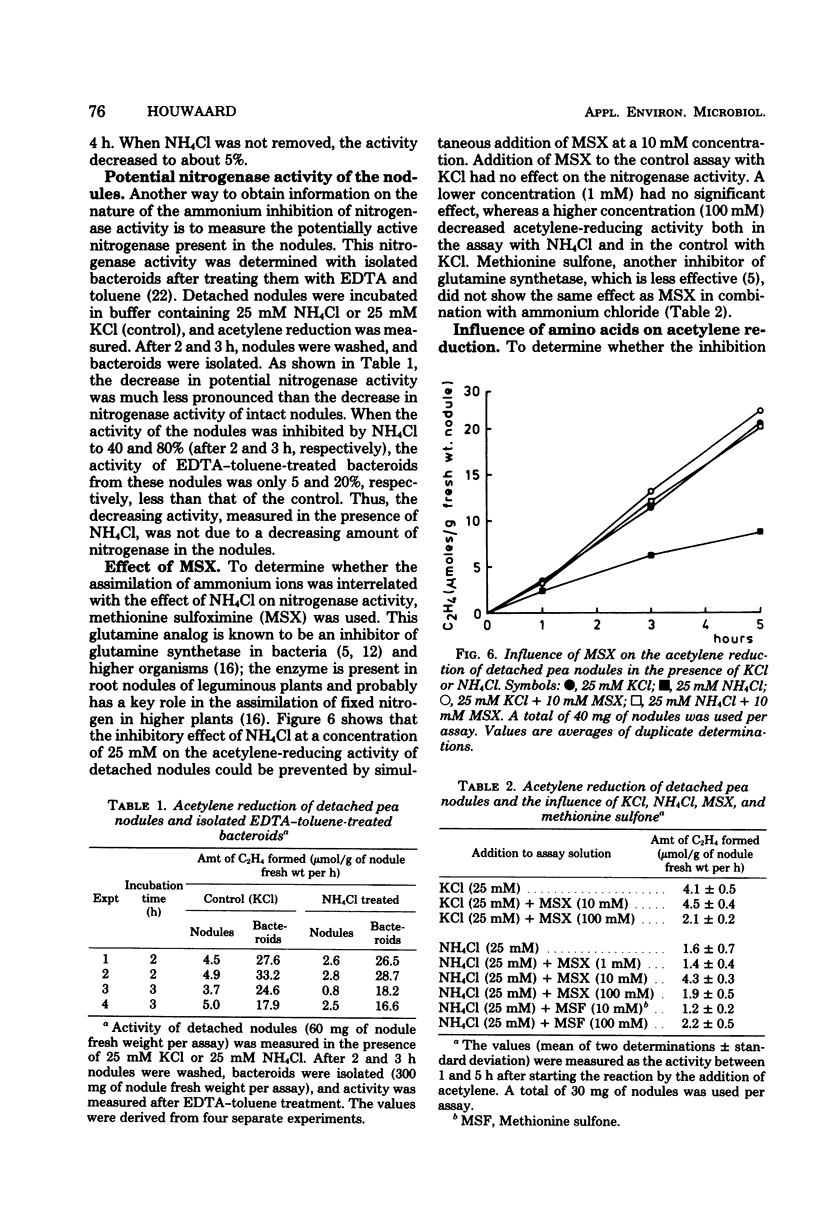
Acetylene-reducing activity of detached pea nodules was determined by submerging the nodules in buffer solution [tris(hydroxymethyl)aminomethane-hydrochloride, pH 7.4] containing 100 mM sodium succinate and incubating under a gas phase of 90% O2 and 10% C2H2. The nitrogenase activity was 4 to 8 μmol of C2H4 formed per g of nodule fresh weight per h and remained constant for at least 4 h. Addition of NH4Cl to the buffer solution (at a concentration of 10 mM or more) resulted in a significant decrease of nitrogenase activity, which was more pronounced at higher concentrations of ammonium chloride. The inhibition of nitrogenase activity by NH4Cl was reversible; when the NH4Cl-containing buffer solution was replaced by buffer without NH4Cl, the original activity was partly restored. Treatment of the nodules with NH4Cl had almost no effect on the amount of nitrogenase, as measured by the acetylene-reducing activity of ethyl-enediaminetetraacetate-toluene-treated bacteroid suspensions. The effect of NH4Cl was largely eliminated by simultaneous addition of 10 mM methionine sulfoximine to the assay solution. This suggests that the assimilation of ammonium ions by glutamine synthetase controls the functioning of nitrogenase activity in the nodules. However, no effect of glutamine, glutamate, or aspartate on the acetylene reduction by detached nodules could be detected.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J. Nitrogen fixation in legume root nodules: biochemical studies with soybean. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):401–416. doi: 10.1098/rspb.1969.0029. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., van den Bos R. C., van Kammen A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim Biophys Acta. 1978 Feb 13;539(1):1–11. doi: 10.1016/0304-4165(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. C., Phillips D. A. Induction of Root Nodule Senescence by Combined Nitrogen in Pisum sativum L. Plant Physiol. 1977 Mar;59(3):440–442. doi: 10.1104/pp.59.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daesch G., Mortenson L. E. Effect of ammonia on the synthesis and function of the N 2 -fixing enzyme system in Clostridium pasteurianum. J Bacteriol. 1972 Apr;110(1):103–109. doi: 10.1128/jb.110.1.103-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd J. W., Tubb R. S., Postgate J. R. A chemostat study of the effect of fixed nitrogen sources on nitrogen fixation, membranes and free amino acids in Azotobacter chroococcum. J Gen Microbiol. 1972 Nov;73(2):221–232. doi: 10.1099/00221287-73-2-221. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Houwaard F. Influence of ammonium chloride on the nitrogenase activity of nodulated pea plants (Pisum sativum). Appl Environ Microbiol. 1978 Jun;35(6):1061–1065. doi: 10.1128/aem.35.6.1061-1065.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Shanmugam K. T., Morandi C. Amino acids as repressors of nitrogenase biosynthesis in Klebsiella pneumoniae. Biochim Biophys Acta. 1976 Jul 21;437(2):322–332. doi: 10.1016/0304-4165(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M. Studies with detached lupin root nodules in culture: I. Maintenance and induction of acetylene reduction activity. Plant Physiol. 1975 Nov;56(5):665–670. doi: 10.1104/pp.56.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S., Postgate J. R. Control of nitrogenase synthesis in Klebsiella pneumoniae. J Gen Microbiol. 1973 Nov;79(1):103–117. doi: 10.1099/00221287-79-1-103. [DOI] [PubMed] [Google Scholar]
- Tubb R. S. Regulation of nitrogen fixation in Rhizobium sp. Appl Environ Microbiol. 1976 Oct;32(4):483–488. doi: 10.1128/aem.32.4.483-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Straten J., Roelofsen W. Improved method for preparing anaerobic bacteroid suspensions of Rhizobium leguminosarum for the acetylene reduction assay. Appl Environ Microbiol. 1976 Jun;31(6):859–863. doi: 10.1128/aem.31.6.859-863.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



