Abstract
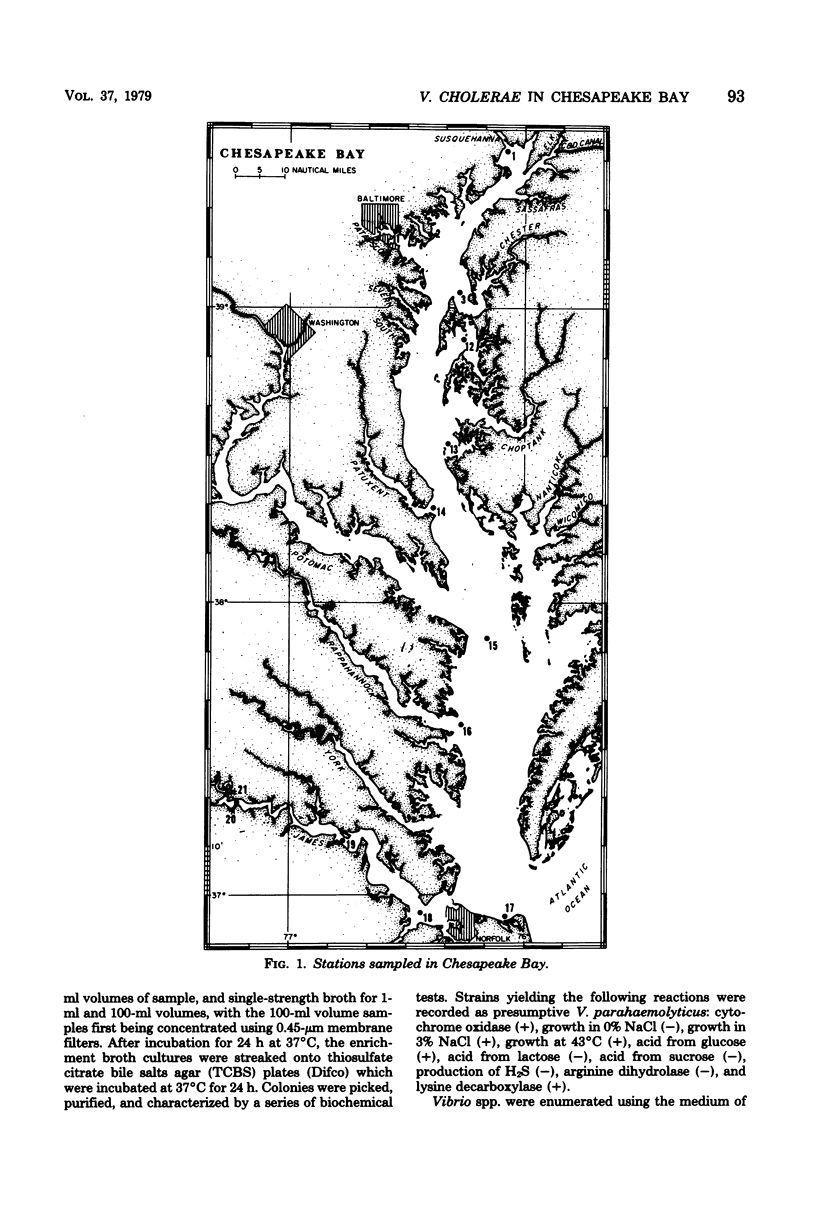
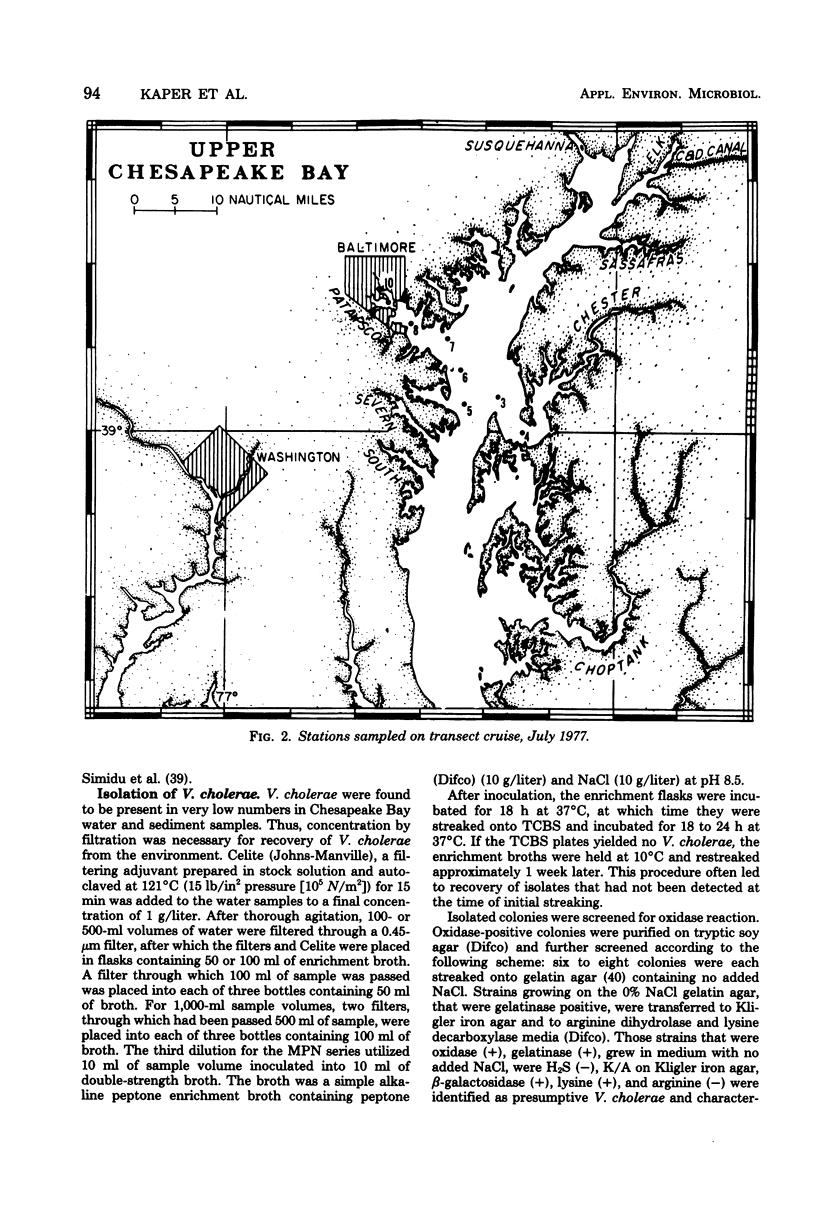
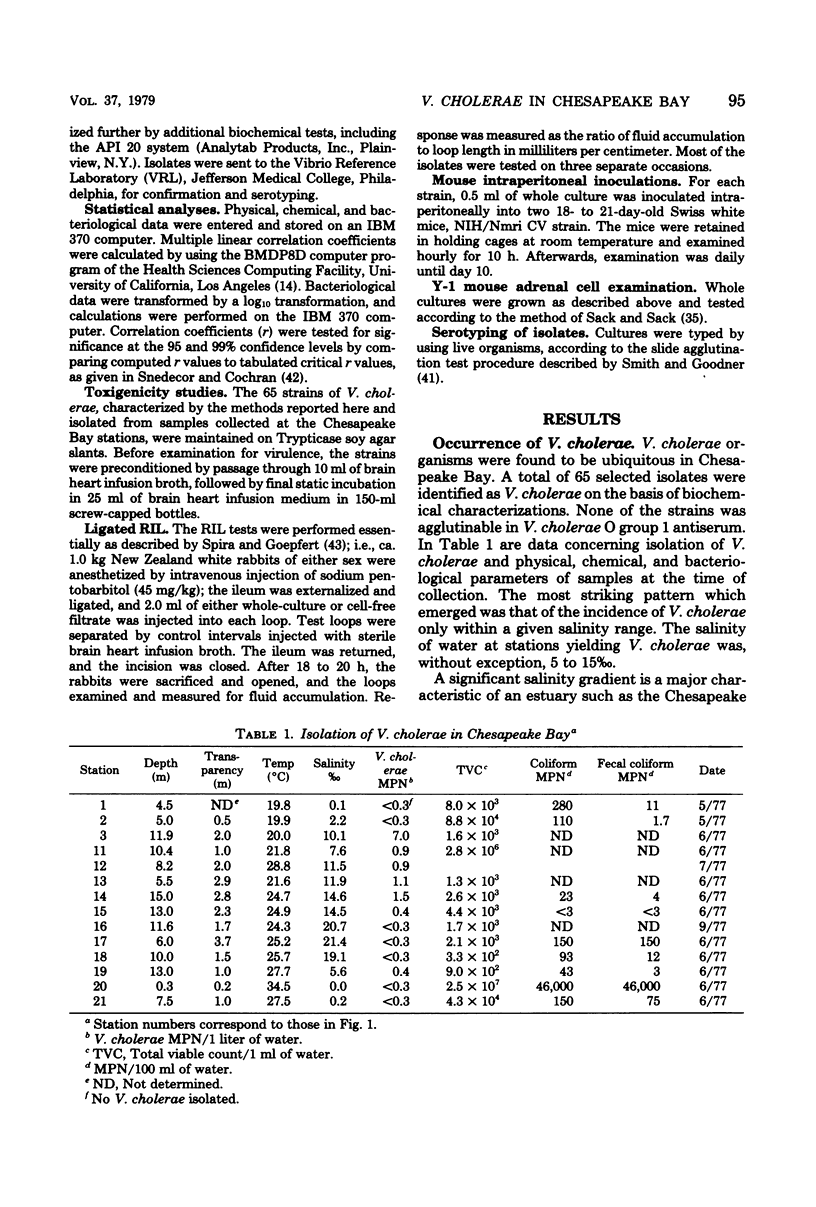
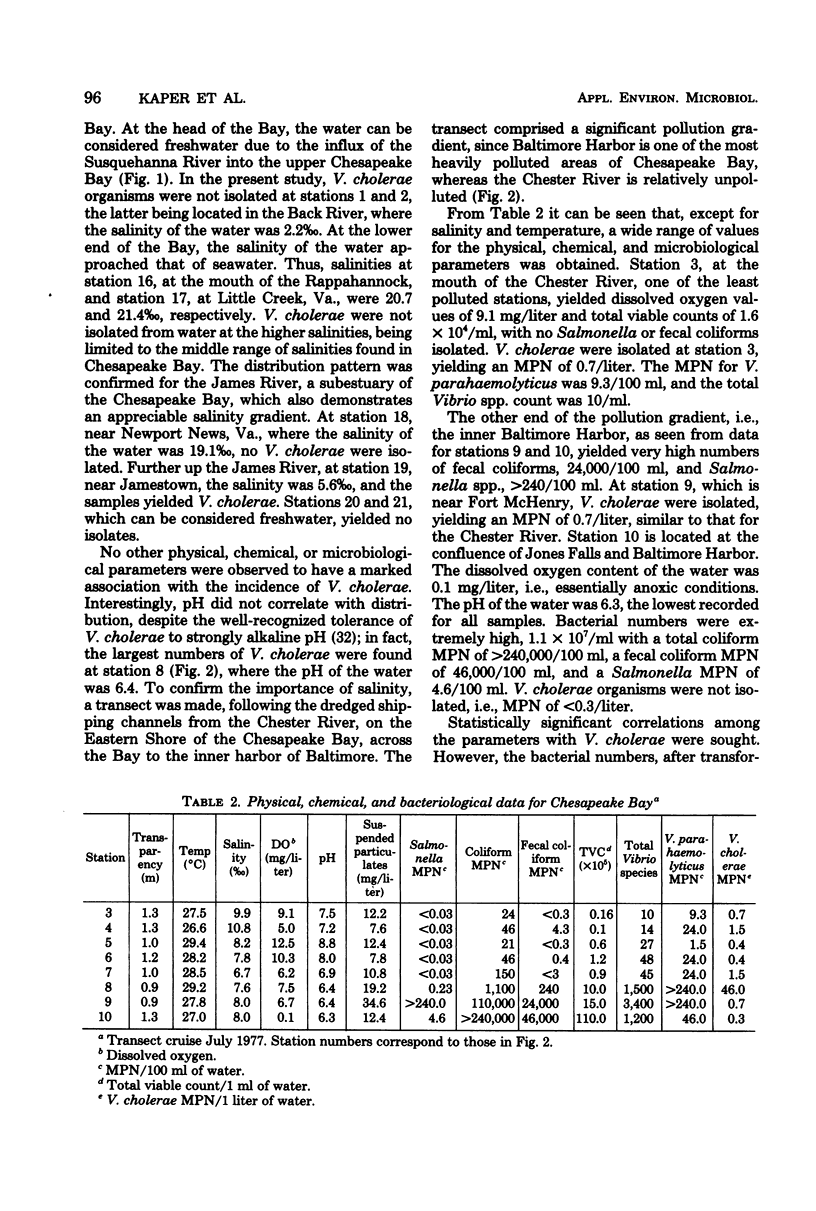
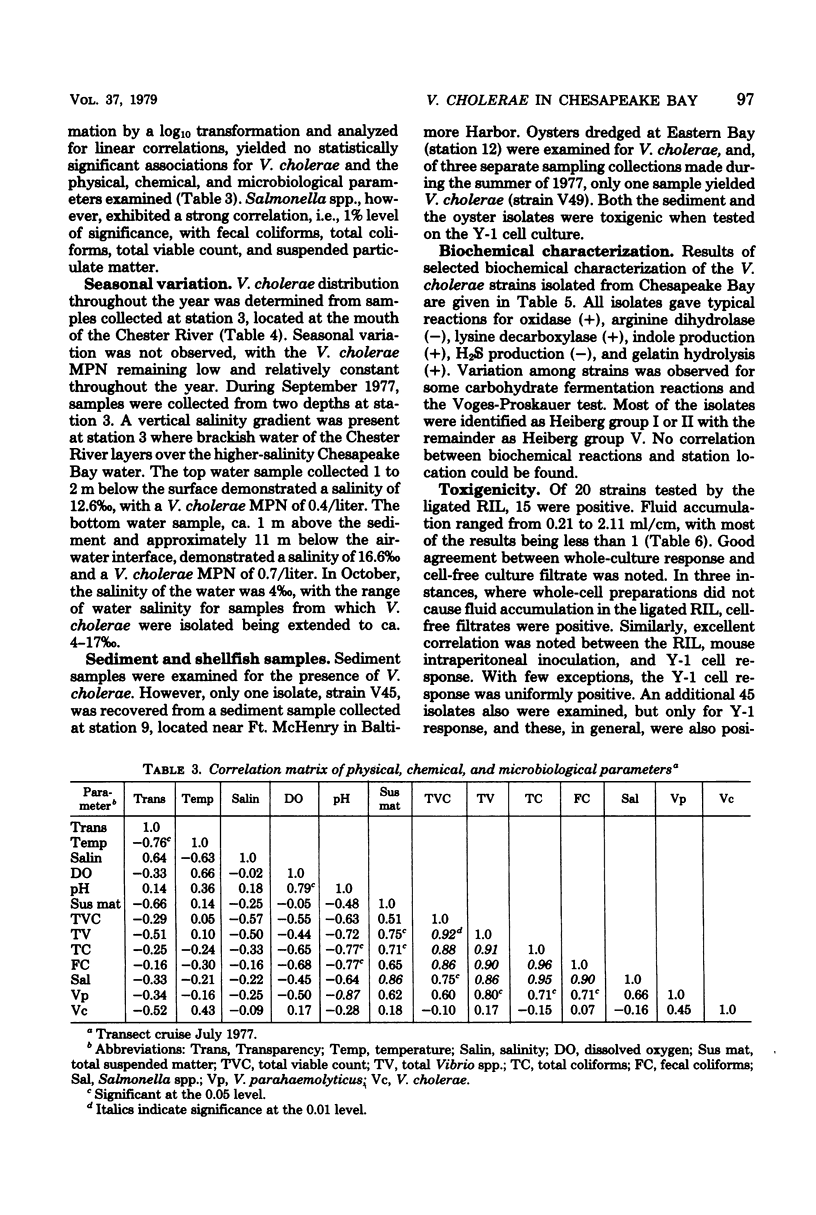
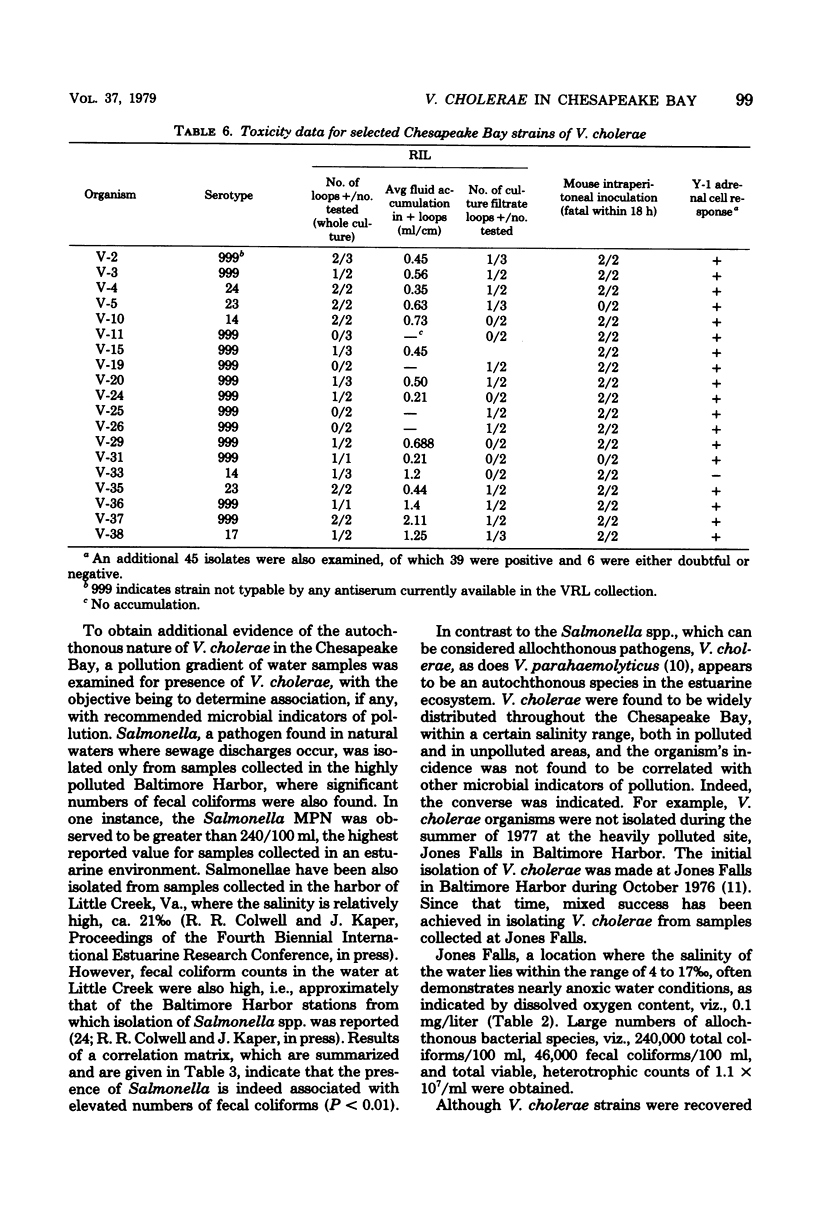
A total of 65 isolates of Vibrio cholerae, serotypes other than O--1, have been recovered from water, sediment, and shellfish samples from the Chesapeake Bay. Isolations were not random, but followed a distinct pattern in which salinity appeared to be a controlling factor in V. cholerae distribution. Water salinity at stations yielding V. cholerae (13 out of 21 stations) was 4 to 17 0/00, whereas the salinity of water at stations from which V. cholerae organisms were not isolated was less than 4 or greater than 17 0/00. From results of statistical analyses, no correlation between incidence of fecal coliforms and V. cholerae could be detected, whereas incidence of Salmonella species, measured concurrently, was clearly correlated with fecal coliforms, with Salmonella isolated only in areas of high fecal coliform levels. A seasonal cycle could not be determined since strains of V. cholerae were detectable at low levels (ca. 1 to 10 cells/liter) throughout the year. Although none of the Chesapeake Bay isolates was agglutinable in V. cholerae O group 1 antiserum, the majority for Y-1 adrenal cells. Furthermore, rabbit ileal loop and mouse lethality tests were also positive for the Chesapeake Bay isolates, with average fluid accumulation in positive ileal loops ranging from 0.21 to 2.11 ml/cm. Serotypes of the strains of V. cholerae recovered from Chesapeake Bay were those of wide geographic distribution. It is concluded from the data assembled to date, that V. cholerae is an autochthonous estuarine bacterial species resident in Chesapeake Bay.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Baumann L. Biology of the marine enterobacteria: genera Beneckea and Photobacterium. Annu Rev Microbiol. 1977;31:39–61. doi: 10.1146/annurev.mi.31.100177.000351. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Rosenberg M. L., Costa J. B., Ferreira P. S., Guimaraes C. L., Gangarosa E. J. Cholera in Portugal, 1974.I. Modes of transmission. Am J Epidemiol. 1977 Apr;105(4):337–343. doi: 10.1093/oxfordjournals.aje.a112391. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Citarella R. V., Colwell R. R. Polyphasic taxonomy of the genus Vibrio: polynucleotide sequence relationships among selected Vibrio species. J Bacteriol. 1970 Oct;104(1):434–442. doi: 10.1128/jb.104.1.434-442.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R. R., Kaper J., Joseph S. W. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science. 1977 Oct 28;198(4315):394–396. [PubMed] [Google Scholar]
- Colwell R. R. Polyphasic taxonomy of the genus vibrio: numerical taxonomy of Vibrio cholerae, Vibrio parahaemolyticus, and related Vibrio species. J Bacteriol. 1970 Oct;104(1):410–433. doi: 10.1128/jb.104.1.410-433.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskovicová M., Karolcek J., Winkler Experimental Toxigenicity of NAG Vibrios. Zentralbl Bakteriol Orig A. 1977 Feb;237(1):65–71. [PubMed] [Google Scholar]
- Dutt A. K., Alwi S., Velauthan T. A shellfish-borne cholera outbreak in Malaysia. Trans R Soc Trop Med Hyg. 1971;65(6):815–818. doi: 10.1016/0035-9203(71)90097-6. [DOI] [PubMed] [Google Scholar]
- Fearrington E. L., Rand C. H., Jr, Mewborn A., Wilkerson J. Letter: Non-cholera vibrio septicemia and meningoencephalitis. Ann Intern Med. 1974 Sep;81(3):401–401. doi: 10.7326/0003-4819-81-3-401_1. [DOI] [PubMed] [Google Scholar]
- Gallut J., Quiniou J. Interactions de Vibrio cholerae classique, V. cholerae biotype E1 Tor et vibrions NAG. Bull World Health Organ. 1970;42(3):464–466. [PMC free article] [PubMed] [Google Scholar]
- Hsieh H., Liu P. V. Serological identities of proteases and alkaline phosphatases of the so-called nonagglutinable (NAG) vibrios and those of Vibrio cholerae. J Infect Dis. 1970 Mar;121(3):251–259. doi: 10.1093/infdis/121.3.251. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Hollis D. G., Gangarosa E. J., Weaver R. E. Non-cholera vibrio infections in the United States. Clinical, epidemiologic, and laboratory features. Ann Intern Med. 1978 May;88(5):602–606. doi: 10.7326/0003-4819-88-5-602. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Sayler G. S., Baldini M. M., Colwell R. R. Ambient-temperature primary nonselective enrichment for isolation of Salmonella spp. from an estuarine environment. Appl Environ Microbiol. 1977 Apr;33(4):829–835. doi: 10.1128/aem.33.4.829-835.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE O. R., FEELEY J. C., GREENOUGH W. B., 3rd, BENENSON A. S., HASSAN S. I., SAAD A. DIARRHEA CAUSED BY NON-CHOLERA VIBRIOS. Am J Trop Med Hyg. 1965 May;14:412–418. doi: 10.4269/ajtmh.1965.14.412. [DOI] [PubMed] [Google Scholar]
- MUKERJEE S. RECENT INCIDENCE OF CHOLERA OUTSIDE INDIA. Indian J Med Res. 1964 Aug;52:771–776. [PubMed] [Google Scholar]
- McIntyre O. R., Feeley J. C. Characteristics of non-cholera Vibrios isolated from cases of human diarrhoea. Bull World Health Organ. 1965;32(5):627–632. [PMC free article] [PubMed] [Google Scholar]
- Merson M. H., Martin W. T., Craig J. P., Morris G. K., Blake P. A., Craun G. F., Feeley J. C., Camacho J. C., Gangarosa E. J. Cholera on Guam, 1974: epidemiologic findings and isolation of non-toxinogenic strains. Am J Epidemiol. 1977 Apr;105(4):349–361. doi: 10.1093/oxfordjournals.aje.a112393. [DOI] [PubMed] [Google Scholar]
- Müller G. Befunde an nicht-agglutinierenden, cholera-ähnlichen Vibrionen (NAG's) in Abwasser, Flusswasser und Meerwasser. Zentralbl Bakteriol Orig B. 1977 Dec;165(5-6):487–497. [PubMed] [Google Scholar]
- Nacescu N., Ciufecu C., Nocoara I., Florescu D., Konrad I. Morphological, cultural and biochemical characteristics of so called NAG vibrios isolated from human faeces and vomitus. Preliminary report. Zentralbl Bakteriol Orig A. 1974;229(2):209–215. [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Pesigan T. P., Plantilla J., Rolda M. Applied studies on the viability of El Tor vibrios. Bull World Health Organ. 1967;37(5):779–786. [PMC free article] [PubMed] [Google Scholar]
- Prats G., Mirelis B., Pericas R., Verger G. Letter: Non-cholera vibrio septicemia and meningoencephalitis. Ann Intern Med. 1975 Jun;82(6):848–849. doi: 10.7326/0003-4819-82-6-848_2. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Still C. S., Isaäcson M., Koornhof H. J., Appelbaum P. C., Scragg J. N. Pathogenic mechanisms of a non-agglutinable Vibrio cholerae strain: demonstration of invasive and enterotoxigenic properties. Infect Immun. 1977 Nov;18(2):542–545. doi: 10.1128/iai.18.2.542-545.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. L., Jr, GOODNER K. Detection of bacterial gelatinases by gelatin-agar plate methods. J Bacteriol. 1958 Dec;76(6):662–665. doi: 10.1128/jb.76.6.662-665.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Nelson J. D., Jr, Justice A., Colwell R. R. Distribution and significance of fecal indicator organisms in the Upper Chesapeake Bay. Appl Microbiol. 1975 Oct;30(4):625–638. doi: 10.1128/am.30.4.625-638.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Goepfert J. M. Bacillus cereus-induced fluid accumulation in rabbit ileal loops. Appl Microbiol. 1972 Sep;24(3):341–348. doi: 10.1128/am.24.3.341-348.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turova T. P., Antonov A. S. Skhodstvo polinukleotidnykh posledovatel'nostei DNK kholernykh i tak nazyvaemykh neaggliutiniruiushchikhsia vibrionov. Zh Mikrobiol Epidemiol Immunobiol. 1977 Jul;(7):47–50. [PubMed] [Google Scholar]
- Weissman J. B., DeWitt W. E., Thompson J., Muchnick C. N., Portnoy B. L., Feeley J. C., Gangarosa E. J. A case of cholera in Texas, 1973. Am J Epidemiol. 1974 Dec;100(6):487–498. doi: 10.1093/oxfordjournals.aje.a112061. [DOI] [PubMed] [Google Scholar]
- Zafari Y., Rahmanzadeh S., Zarifi A. Z., Fakhar N. Diarrhoea caused by non-agglutinable Vibrio cholerae (non-cholera Vibrio). Lancet. 1973 Aug 25;2(7826):429–430. doi: 10.1016/s0140-6736(73)92285-x. [DOI] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]


