Abstract
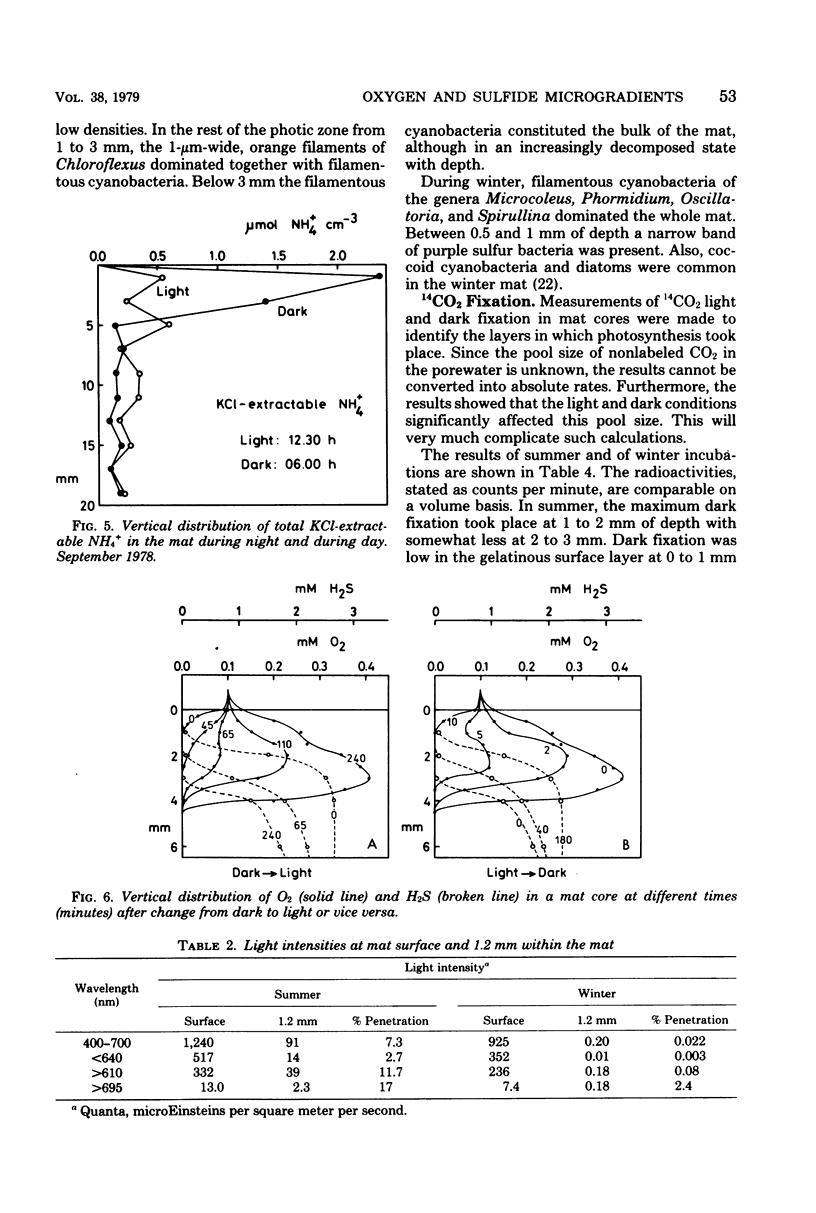
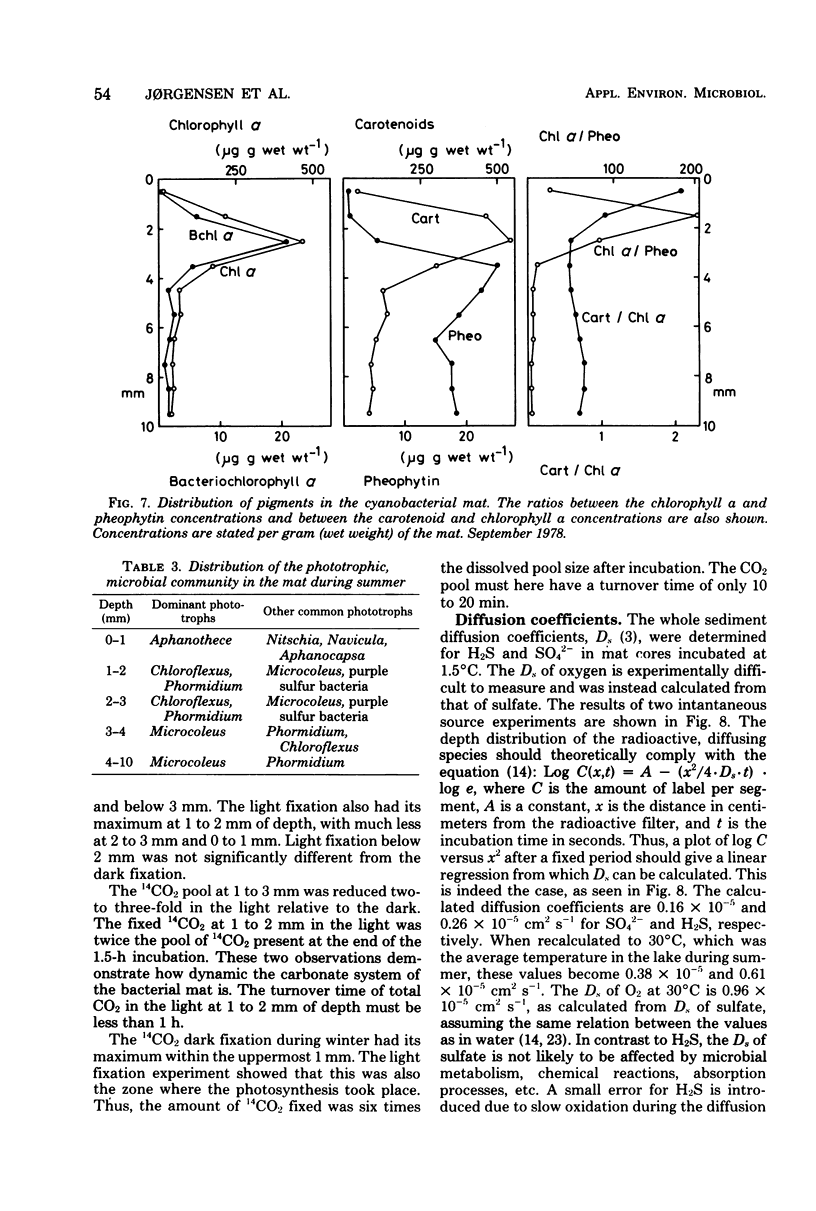
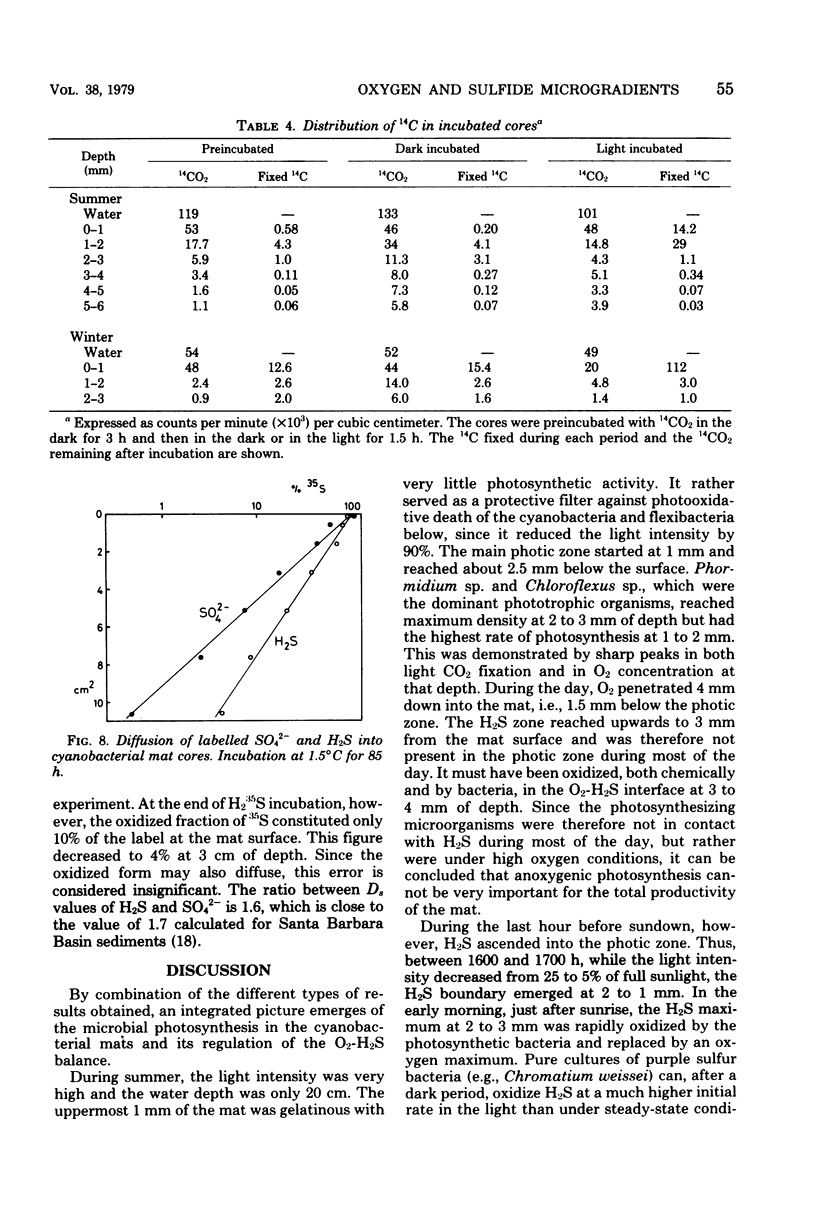
The diurnal variation in the microgradients of O2, H2S, and Eh were studied in the benthic cyanobacterial mats of a hypersaline desert lake (Solar Lake, Sinai). The results were related to light intensity, light penetration into the mat, temperature, pH, NH4+, photosynthetic activity, pigments, and the zonation of the microbial community. Extreme diurnal variation was found, with an O2 peak of 0.5 mM at 1 to 2 mm of depth below the mat surface during day and a H2S peak of 2.5 mM at 2 to 3 mm of depth at night. At the O2-H2S interface, the two compounds coexisted over a depth interval of 0.2 to 1 mm and with a turnover time of a few minutes. The photic zone reached 2.5 mm into the mat in summer, and the main 14CO2 light fixation took place at 1 to 2 mm of depth. During winter, light and photosynthesis were restricted to the uppermost 1 mm. The quantitative dynamics of O2 and H2S were calculated from the chemical gradients and from the measured diffusion coefficients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doemel W. N., Brock T. D. Bacterial stromatolites: origin of laminations. Science. 1974 Jun 7;184(4141):1083–1085. doi: 10.1126/science.184.4141.1083. [DOI] [PubMed] [Google Scholar]
- Doemel W. N., Brock T. D. Structure, growth, and decomposition of laminated algal-bacterial mats in alkaline hot springs. Appl Environ Microbiol. 1977 Oct;34(4):433–452. doi: 10.1128/aem.34.4.433-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Brock T. D. Photosynthetic sulfide oxidation by Chloroflexus aurantiacus, a filamentous, photosynthetic, gliding bacterium. J Bacteriol. 1975 May;122(2):782–784. doi: 10.1128/jb.122.2.782-784.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Padan E. Induction of anaerobic, photoautotrophic growth in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1978 Feb;133(2):558–563. doi: 10.1128/jb.133.2.558-563.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]


