Abstract
Dendritic cells (DC) are sensitive to their local environment and are affected by proximal cell death. This study investigated the modulatory effect of cell death on DC function. Monocyte-derived DC exposed to apoptotic Jurkat or primary T cells failed to induce phenotypic maturation of the DC and were unable to support CD4+ allogeneic T-cell proliferation compared with DC exposed to lipopolysaccharide (LPS) or necrotic cells. Apoptotic cells coincubated with LPS- or necrotic cell-induced mature DC significantly suppressed CD80, CD86 and CD83 and attenuated LPS-induced CD4+ T-cell proliferation. Reduced levels of interleukin-12 (IL-12), IL-10, IL-6, tumour necrosis factor-α and interferon-γ (IFN-γ) were found to be concomitant with the suppressive activity of apoptotic cells upon DC. Furthermore, intracellular staining confirmed IFN-γ expression by DC in association with apoptotic environments. The specific generation of IFN-γ by DC within apoptotic environments is suggestive of an anti-inflammatory role by the induction of indoleamine 2,3-dioxygenase (IDO). Both neutralization of IFN-γ and IDO blockade demonstrated a role for IFN-γ and IDO in the suppression of CD4+ T cells. Moreover, we demonstrate that IDO expression within the DC was found to be IFN-γ-dependent. Blocking transforming growth factor-β (TGF-β) also produced a partial release in T-cell proliferation. Our study strongly suggests that apoptosis-induced DC suppression is not an immunological null event and two prime mediators underpinning these functional effects are IFN-γ-induced IDO and TGF-β.
Keywords: apoptosis, dendritic cells, interferon, necrosis, tolerance
Introduction
Dendritic cells (DC) are professional antigen-presenting cells that play a critical role in the induction of immunity and tolerance. Regulation of these outcomes depends on several factors, such as the occurrence of specialized DC subsets and the maturation state of the DC.1–3 Immature DC (iDC) sample their local environment and efficiently capture antigen but are ineffective in T-cell priming because of their low expression of major histocompatibility complex (MHC) class I and II and costimulatory molecules and therefore contribute to the induction of tolerance. Upon receipt of a maturation signal, mature DC (mDC) show a decreased ability to capture antigen, concurrent with an upregulation of MHC and costimulatory molecules, which facilitates T-cell activation and subsequently the ability to induce an immune response.
Apoptosis is an active phenomenon regulating cellular homeostasis. In contrast, necrosis, associated with pathological tissue injury, is characterized by rapid, disorganized swelling and subsequent release of intracellular components into the local environment.4 It is suggested that the different pathways leading to cell death may give rise to distinct immunological responses.4,5 In support of this, earlier reports demonstrate that phagocytosis of necrotic cells (NC), but not apoptotic cells (AC), induces phenotypic and functional maturation of DC.6,7
Apoptotic cells are a preferential source of many autoantigens and a growing body of evidence indicates that DC ingesting dying cells maintain self-tolerance by constantly sampling peripheral self-antigens and presenting them in a tolerogenic way to the adaptive immune system.8–11 Ingestion of AC by macrophages and DC renders these cells unable to stimulate T-cell proliferation,7,10 but may influence the induction of T regulatory cells promoting tolerance.4,6 Conceivably, the ability of DC to present self-antigens unchecked might initiate autoimmunity, as a result of defects in apoptosis or dead cell clearance mechanisms.11
The mechanism by which AC are swiftly recognized and phagocytosed by DC is the subject of intense investigation. Phosphatidylserine and its receptor play a fundamental role12 in this process;9 however, studies have shown roles for numerous other receptors.10,13 Recognition and engulfment of AC by DC is known to modulate cytokine production, costimulatory receptor expression and ability to stimulate T-cell proliferation.14,15 Therefore, the AC effect is not an immunologically null event, but by the inhibition of DC maturation may contribute to the downregulation of responses to AC-derived self-antigens and ultimately to the maintenance of self-tolerance. Although the exact mechanisms of immunosuppression are not fully understood, a widely accepted view is that iDC, with low level costimulation, would induce anergy or deletion of an interacting T cell while mDC secreting an array of proinflammatory cytokines, including the potent stimulatory cytokine interleukin-12 (IL-12), along with high levels of costimulatory molecules would induce strong adaptive immunity.16
The DC cytokine profile plays an important role in the regulation of immune tolerance. As such, macrophage engulfment of AC induces transforming growth factor-β (TGF-β) and suppression of proinflammatory cytokines.15 Similarly the engulfment of AC by DC inhibits IL-12 production,17,18 which has a role in establishing tolerance.19 Cytokines, such as granulocyte–macrophage colony-stimulating factor (GM-CSF) and interferon-γ (IFN-γ), are also able to upregulate monocyte phagocytosis of dying cells, ensuring their quick and efficient removal.20
Previous work has implicated the expression of the enzyme indoleamine 2,3-dioxygenase (IDO) as an additional mechanism by which antigen-presenting cells may regulate T-cell responses. IDO, expressed by a variety of cells in an inactive or active form, is responsible for the catabolism of the essential amino acid tryptophan, the metabolites of which are known to inhibit T-cell function.21 Further evidence for IDO as an immune modulator is the fact that its transfection into non-antigen-presenting cell lines22 and its presence within human monocyte-derived macrophages23 and DC24,25 confers the ability to inhibit T-cell proliferation either by the depletion of tryptophan or through the accumulation of toxic and immunosuppressive tryptophan metabolites, acting to induce T-cell apoptosis or T-cell anergy.26In vivo studies demonstrate a role for IDO in maternal tolerance, in the control of T cells in autoimmune disorders and in the suppression of immune responses to tumours.21,26
Much work has demonstrated that IFN-γ is responsible for the induction of IDO in DC although IL-1β, tumour necrosis factor-α (TNF-α) and IL-12 have also recently been implicated.27,28 Further evidence for IFN-γ induction of IDO is supported by studies revealing IFN-γ-dependent IDO generation as a result of reverse signalling from the T cell into the DC via cytotoxic T-lymphocyte antigen 4 (CTLA-4) and CD80/CD86.29,30 It is therefore apparent that mechanisms controlling the activation of IDO are likely to be a fine balance between numerous environmental factors. We postulate that IFN-γ- induced IDO expression in DC plays a role in the immunosuppressive ability of apoptosis-conditioned DC observed in vitro.
Materials and methods
Generation of monocyte-derived DC
All experiments were carried out in accordance with local ethical guidelines. Peripheral blood mononuclear cells (PBMC) were isolated from fresh peripheral blood following the method of McLeod et al.31 Monocytes were isolated from the PBMC population using the Monocyte Isolation Kit II (Miltenyi Biotec, Surrey, UK) and cultured following an established method.32 Briefly, cells were cultured at 2 × 106/ml at 37°, 5% CO2 for 5 days in RPMI-1640 supplemented with fetal calf serum (10%), penicillin and streptomycin solution (100 U/ml and 100 μg/ml) and l-glutamine (2 mm; all Invitrogen, Loughborough, UK) in the presence of GM-CSF (800 U/ml) and IL-4 (500 U/ml both Peprotech EC, London, UK). Cells were fed every other day through the replenishment of half the volume of fresh medium and cytokines. At day 5, cells were characterized and considered to be iDC by the low expression of CD14, CD40, CD80, CD86 and CD83. PBMC depleted of monocytes, predominantly consisting of T cells and subsequently referred to as primary T cells, were cultured at 1 × 106/ml and stimulated with phytohaemagglutinin (5 μg/ml; Sigma Aldrich, Poole, UK) for up to 5 days.
DC co-culture
Immature DC were resuspended at 1 × 106/ml in complete medium plus cytokines (as detailed above) and cultured for 48 hr in the presence of apoptotic or necrotic Jurkat cells or primary autologous or allogeneic T cells at a 1 : 5 ratio of DC : dying cells. As controls, DC were cultured alone or in the presence of lipopolysaccharide (LPS; 5 μg/ml) or monocyte-conditioned medium (MCM).33 Dendritic cells were also cultured with AC in the presence or absence of LPS. To determine the effects of TGF-β, IFN-γ and IDO in DC cultured with AC, in the presence or absence of LPS, co-cultures were set up with or without TGF-βLAP (20 ng/ml; e-Bioscience, Wembley, UK), anti-IFN-γ neutralizing antibody (5 μg/ml; e-Bioscience) or 1-methyl tryptophan (200 μm; Sigma Aldrich). After 48 hr, DC were stained for cell surface expression of CD80, CD86 and CD83 or re-isolated by flow cytometric sorting before incorporation into CD4+ T-cell proliferation assays.
Induction of apoptosis and necrosis
The TNF-α-induced apoptosis was induced by incubation with human recombinant TNF-α (10 ng/ml; R&D Systems, Abingdon, UK) plus cycloheximide (10 μg/ml; Sigma Aldrich) while all other cells were induced to undergo apoptosis by incubation with an anti-Fas antibody (0·1 μg/ml; Upstate Biotechnology, Dundee, UK) for 2 hr at 37° before incorporation into DC co-cultures. Apoptosis of cells was subsequently confirmed by means of an Annexin V (BD Bioscience, Oxford, UK) and propidium iodide (Sigma Aldrich) viability assay. Cells were induced to undergo necrosis following four 1-minute freeze–thaw cycles in liquid nitrogen.
Immunophenotyping of cells
Cell surface staining of DC was performed by incubating the cells for 30 min at 4° with primary antibodies recognizing; CD14 (5 μl stock solution; Sigma Aldrich), CD40 (1 μg/ml), CD80 (0·5 μg/ml), CD86 (4 μg/ml), CD83 (10 μg/ml) and human leucocyte antigen DR (2 μg/ml; all BD Bioscience) followed by incubation with a fluorescein isothiocyanate (FITC) -conjugated anti-mouse secondary antibody (Sigma Aldrich) for 30 min at 4°. Stained cells were analysed on a BD FACSVantage SE using CellQuest software (BD Becton and Dickinson, Oxford, UK). The geometric mean fluorescence intensity (GMFI) of cells relative to DC alone was used in analysis unless stated otherwise.
Allogeneic CD4+ T-cell proliferation assay
Allogeneic CD4+ T cells were isolated from human peripheral blood using the CD4+ CD25+ Regulatory T-cell kit (Miltenyi Biotec). The PBMC were obtained as described previously.31 Briefly, CD4+ T cells were isolated from PBMC using a process of negative isolation and CD4+ CD25+ cells were further depleted by a process of positive selection. Throughout this paper the CD4+ population of T cells depleted of T regulatory cells (CD4+ CD25+) will be referred to as CD4+ cells. These CD4+ T cells were fluorescently labelled with carboxyfluorescein succinimidyl ester (CFSE; 8 μm; Molecular Probes, Paisley, UK) as described previously.34 Then, 2 × 105 CD4+ T cells were incubated at a 10 : 1 ratio in triplicate with DC previously conditioned by culture alone or in the presence of apoptotic or necrotic Jurkat cells or LPS. The specific proliferation of CFSE+ T cells was quantified at day 5 by means of the Weighted Division Index34 using a BD FACSVantage SE. At day 5, cell viability was confirmed using an Annexin V and propidium iodide viability assay and flow cytometry.
Cytokine array
Cell-free supernatants from DC co-cultures were collected after 10, 24 and 48 hr and stored at −20° until analysis. Levels of IL-1α, IL-1β, IL-2, IL-4, GM-CSF, IL-6, IL-7, IL-8, IL-10, IL-12, TNF-α and IFN-γ were quantified using the Proteoplex cytokine array (Merck Bioscience, Nottingham, UK) in accordance with the manufacturer's instructions.
Intracellular staining
DC were stained with DiI (1 μl/1 × 106 cells; Molecular Probes) and cultured with LPS (5 μg/ml) in the presence or absence of AC. Intracellular IFN-γ staining was performed at 8 hr, adding brefeldin A (10 μg/ml; Sigma Aldrich) for the final 4 hr of culture to prevent IFN-γ secretion. Cells were fixed and permeabilized using commercially available buffers (e-Bioscience) and stained with an anti-IFN-γ FITC-conjugated antibody (1 μg/ml; e-Bioscience) or isotype control. Intracellular DC IDO expression was determined at 24 and 48 hr using a monoclonal anti-IDO antibody (1 μg/ml; Upstate Biotechnology) and an anti-mouse FITC-conjugated secondary antibody in the presence or absence of an IFN-γ neutralizing antibody (5 μg/ml) or retinoic acid (10 μm; Sigma Aldrich).
Tryptophan and kynurenine determination
Supernatants were harvested from DC co-cultures and diluted with 10% (v/v) methanol. Kynurenine and tryptophan were detected by high-performance liquid chromatography (HPLC) as described elsewhere35 with minor modifications. Briefly 50 μl was injected into an Amersham reverse-phase C18 column and eluted with KH2PO4 buffer (0·01 m KH2PO4 and 0·15 mm EDTA) containing 10% methanol at a flow rate of 1 ml/min. The spectrophotometer was set at 365 and 285 nm to detect kynurenine and tryptophan production, respectively, using standard preparations (Sigma Aldrich). The concentrations of kynurenine (in μmol/l) and tryptophan (μmol/l) were used to calculate the kynurenine : tryptophan ratio (μmol/μmol), as a measure of IDO activity.
Statistical analysis
All data, where possible, are expressed as means ± SEM. Data were statistically analysed using Student's t-test. Statistical significance was determined at P ≤ 0·05.
Results
DC mature in response to NC but not AC environments
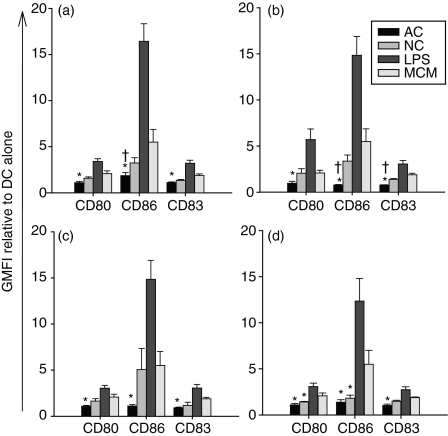
Initial experiments set out to investigate the phenotypic response of DC to different forms of cell death as determined by the upregulation of maturation-associated markers. DC were shown to be responsive to both MCM- and LPS-induced maturation, as observed by an upregulation of expression of CD80, CD86 and CD83 (Fig. 1a–d). By contrast, DC responses were minimal in response to apoptotic Jurkat cells (Fig. 1a,b) irrespective of the mode of apoptosis induction. As seen in Fig. 1 both Fas- and TNFα-induced apoptosis, generated a similar DC profile. Exposure to NC induced an upregulation of all surface markers similar to that observed with MCM-induced maturation (Fig. 1a–d). This was particularly evident with CD86, which was significantly different from AC-induced levels of expression. To determine the effects of cell type on DC maturation, DC were exposed to AC or NC allogeneic or autologous primary T cells. The DC response to both autologous and allogeneic primary T cells was similar to that observed with Jurkat cells (Fig. 1c,d).
Figure 1.
Apoptotic cells (AC) do not induce phenotypic maturation of dendritic cells (DC). The DC were cultured with lipopolysaccharide (LPS; 5 μg/ml) or AC or necrotic cells (NC) at a 1 : 5 ratio for 48 hr before staining for CD80, CD86 and CD83. The DC cultured with Fas- (a) or TNF-α- (b) induced AC Jurkat cells or Fas-induced AC primary autologous (c) or allogeneic (d) T cells do not upregulate CD80, CD86 and CD83, displaying a phenotype similar to DC cultured alone. DC exposed to NC Jurkat cells and primary autologous and allogeneic T cells demonstrate increases in CD80, CD86 and CD83. Changes in surface expression markers were detected by flow cytometry and are represented as the geometric mean fluorescence intensity (GMFI) relative to DC alone. Bars represent means of four or more independent experiments ± SEM. *P ≤ 0·05 and †P ≤ 0·05 when compared with DC + monocyte-conditioned medium (MCM) and DC + NC respectively.
AC suppress DC responses to NC and LPS
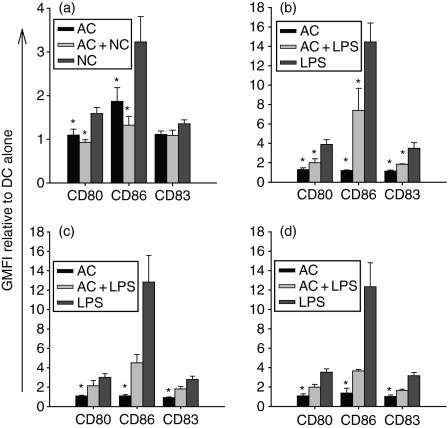
To further assess the effects of AC upon DC maturation we investigated the effects of AC upon DC maturation in response to known stimuli. The addition of apoptotic Jurkat cells to NC-stimulated (Fig. 2a) or LPS-stimulated (Fig. 2b) mDC resulted in a reduction in the level of CD80, CD86 and CD83, a response which was also observed with apoptotic autologous (Fig. 2c) and allogeneic (Fig. 2d) primary T cells. The ability of AC to suppress DC maturation was independent of AC engulfment because reduced levels of surface markers were observed in the entire DC population irrespective of phagocytosis (data not shown).
Figure 2.
Apoptotic cells (AC) inhibit phenotypic maturation of dendritic cells (DC). The DC were cultured with AC simultaneously with necrotic cells (NC) or lipopolysaccharide (LPS) for 48 hr before staining for surface expression of CD80, CD86 and CD83. Addition of AC Jurkat cells to NC- (a) or LPS- (b) stimulated mature DC (mDC) results in reduced expression of CD80, CD86 and CD83 when compared with controls. Apoptotic autologous (c) or allogeneic (d) primary T cells reduced LPS-stimulated mDC expression of CD80, CD86 and CD83. Surface expression of markers was determined by flow cytometry and is represented as geometric mean fluorescence intensity (GMFI) relative to DC alone. Values expressed are means of three or more independent experiments ± SEM. *P ≤ 0·05 DC + NC or DC + LPS.
AC suppress iDC- and mDC-mediated T-cell proliferation
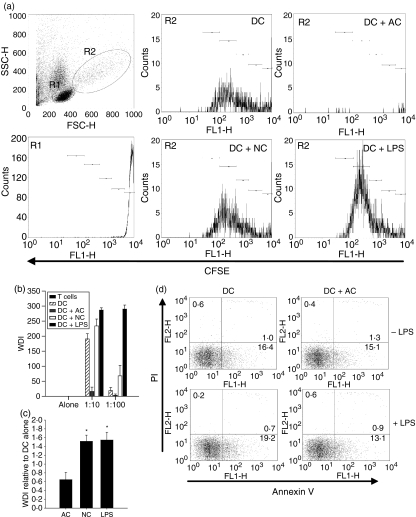
We next investigated the ability of DC, conditioned by different forms of cell death, to stimulate allogeneic CD4+ T-cell proliferation. The iDC supported T-cell proliferation as measured by a CFSE dilution analysis; this was suggestive of a mixed lymphocyte reaction. DC stimulated by NC supported allogeneic T-cell proliferation to levels comparable to that of LPS-induced mDC (Fig. 3a–c) while DC cultured within apoptotic environments did not support allogeneic CD4+ T-cell proliferation, a response which was not attributed to decreased T-cell viability (Fig. 3d).
Figure 3.
Apoptotic cell (AC) conditioned immature DC (iDC) do not support CD4+ T-cell proliferation. Dendritic cells (DC) were cultured with either AC, necrotic cells (NC) or lipopolysaccharide (LPS) for 48 hr before incorporation into T-cell proliferation assays. (a) and (b) show representative results including CFSE plots of R1 and R2 gates of T-cell cultures (a) and DC : T-cell ratios of 1 : 10 and 1 : 100 (b). (c) shows mean data relative to DC showing that AC suppress iDC to support T-cell proliferation. Proliferation is represented as the weighted division index (WDI) or the WDI relative to DC alone. T-cell apoptosis was quantified at day 5 using flow cytometry and an annexin V and propidium iodide viability assay (d). The percentage of positive cells for the given quadrant are shown. Bars represent the mean of triplicate wells for the representative assay or of four independent experiments ± SEM for collated data. Histograms and dot plots represent one independent experiment of three. *P ≤ 0·001 when compared with DC + AC.
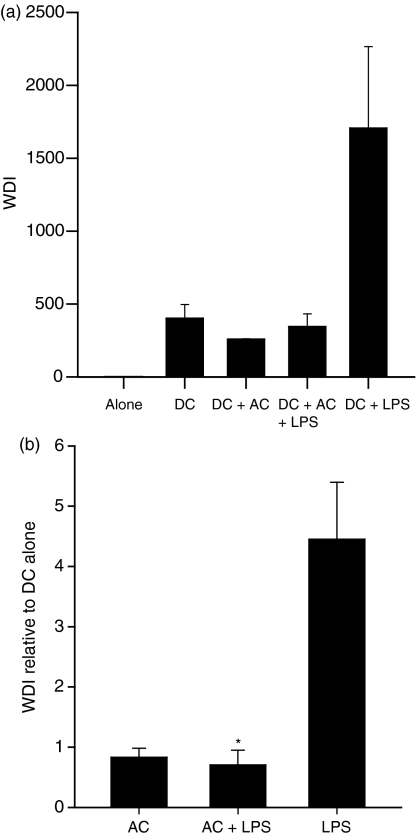
Our phenotypic analysis revealed that AC were able to inhibit DC upregulation of cell surface CD80, CD86 and CD83 in response to a known inducer of maturation. We therefore investigated the ability of these DC to support T-cell proliferation. Similarly to the effects on phenotypic maturation, the addition of AC to mDC reduced the ability of DC to induce T-cell proliferation (Fig. 4a,b). Preliminary results investigating the effector status of the proliferating T cells, using intracellular IFN-γ as a marker, showed that both iDC and mDC in the presence of AC showed an abrogated level of IFN-γ compared with cultures without AC (data not shown).
Figure 4.
Apoptotic cells (AC) suppress lipopolysaccharide (LPS)-stimulated dendritic cell (DC)-induced CD4+ T-cell proliferation. The DC were cultured with AC simultaneously with LPS before incorporation into T-cell proliferation assay at a 1 : 10 ratio. T-cell proliferation was quantified at day 5 by flow cytometry and CFSE dilution analysis. (a) shows one representative assay and (b) shows mean data expressed relative to DC alone. Proliferation is represented as the weighted division index (WDI) or the WDI relative to DC alone. Bars represent the mean of triplicate wells for the representative assay or of four independent experiments ± SEM for collated data. *P ≤ 0·001 when compared with DC + LPS.
AC induce IFN-γ production by DC and suppress LPS-stimulated cytokine responses
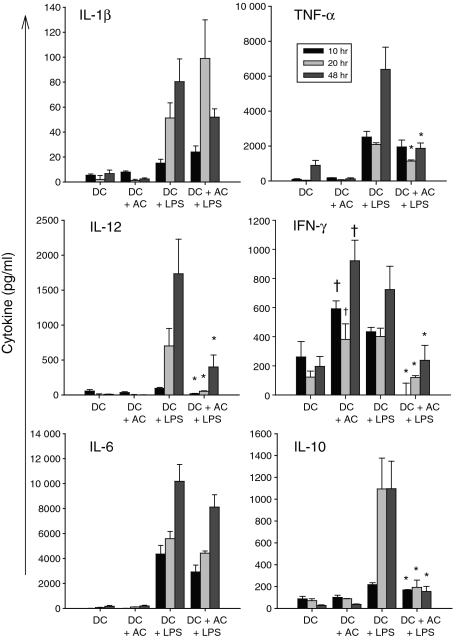
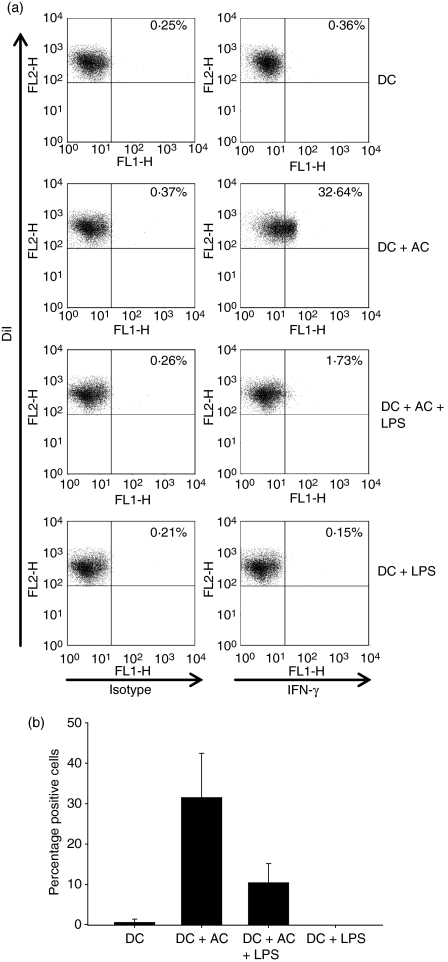
To identify the factors responsible for the AC-induced suppression, the cytokine profile of the differentially conditioned DC was investigated. As expected, mDC secreted high levels of the proinflammatory cytokines; IL-1α, IL-1β, IL-6, IL-8, IL-12, TNF-α, IFN-γ and also the immunoregulatory cytokine IL-10 in a time-responsive manner (Fig. 5, key data shown). The AC were found to significantly suppress LPS-induced IL-12, IFN-γ and IL-10, an effect also observed with TNF-α at the later time-points investigated. Immature DC conditioned with AC secreted minimal levels of all the cytokines investigated, with the exception of IFN-γ, which was detected at all time-points at levels comparable or higher than that induced by LPS. We subsequently confirmed by intracellular cytokine analysis that the IFN-γ observed in AC cultures was DC derived (Fig. 6a) with the greatest expression detected at 8 hr, the most pronounced production of which was determined in the absence of LPS (Fig. 6b).
Figure 5.
Apoptotic cells (AC) induce interferon-γ (IFN-γ) production by dendritic cells (DC) and suppress lipopolysaccharide (LPS)-stimulated DC cytokine secretion. DC were cultured alone, with LPS or with AC in the presence or absence of LPS for up to 48 hr. Supernatants were analysed by cytokine array, the concentration of which are shown as pg/ml. AC specifically induce IFN-γ production by DC and suppress LPS-induced cytokine production of interleukin-12 (IL-12), IL-6, IL-10, tumour necrosis factor-α (TNF-α) and IFN-γ. Data represents the mean of quadruplicate spots from three or more independent experiments ± SEM. *P ≤ 0·05 and †P ≤ 0·01 when compared with DC + LPS or DC alone.
Figure 6.
Apoptotic cells (AC) induce dendritic cell (DC) production of interferon-γ (IFN-γ) at 8 hr. The DC were cultured with AC at a 1 : 5 ratio with or without lipopolysaccharide (LPS) for up to 48 hr. Intracellular IFN-γ production was determined in DiI+ DC by the addition of Brefeldin A (10 μg/ml) 4 hr before staining. At 8 hr, IFN-γ was produced by DC in response to AC maximally observed in the absence of LPS (a) as determined by flow cytometric analysis. Values represent the percentage of isotype or IFN-γ+ DiI+ cells. (b) shows the mean percentage of IFN-γ+ DiI+ DC of three independent experiments ± SD.
AC-induced suppression of T-cell proliferation is mediated in part by TGF-β, IFN-γ and IDO
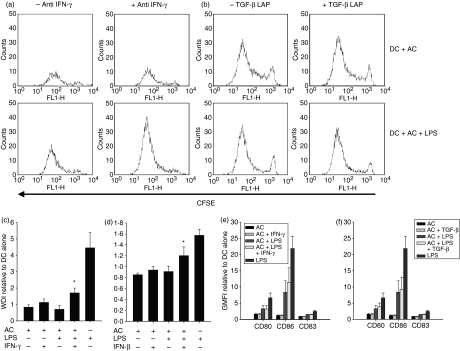
The specific production of IFN-γ by DC within AC environments suggests a key role for IFN-γ in this context. We therefore further investigated the functional role of IFN-γ in AC-mediated suppression, alongside the role of TGF-β, because of its immunoregulatory nature. Blocking IFN-γ (Fig. 7a,c) or TGF-β (Fig. 7b,d) caused a partial but significant release in the apoptotic inhibition of mDC-induced CD4+ allogeneic T-cell proliferation and a subtle release in the apoptotic inhibition of the mixed lymphocyte reaction. In contrast, blocking these cytokines demonstrated little effect upon the release of AC-associated suppression of cell surface marker expression (Fig. 7e, f).
Figure 7.
Interferon-γ (IFN-γ) and transforming growth factor-β (TGF-β) modulate mature dendritic cell (mDC)-induced T-cell proliferation. DC were cultured with apoptotic cells (AC) at a 1 : 5 ratio, in the presence or absence of lipopolysaccharide (LPS), interferon-γ (IFN-γ) neutralizing antibody or TGF-β LAP for 48 hr, analysed phenotypically or incorporated into a T-cell proliferation assay. AC suppression of mDC-induced CD4+ T-cell proliferation was partially released by neutralizing IFN-γ (a, representative plot and c) and by blocking TGF-β (b, representative plots and d). Representative CFSE plots are of the proliferating blast (R2 region) cells (region gates and a representative R1 CFSE plot are shown in Fig. 3a). Representative T-cell proliferation was determined by flow cytometry and CFSE dilution analysis and is represented by the weighted division index (WDI) relative to DC alone. The effects of blockade of IFN-γ and TGF-β were independent of effects upon DC phenotype (e, f). Changes in expression of surface markers were determined by flow cytometry and are represented as GMFI relative to DC alone. Bars represent the means of three or more independent experiments ± SEM for collated data. *P ≤ 0·05 when compared with DC + AC + LPS.
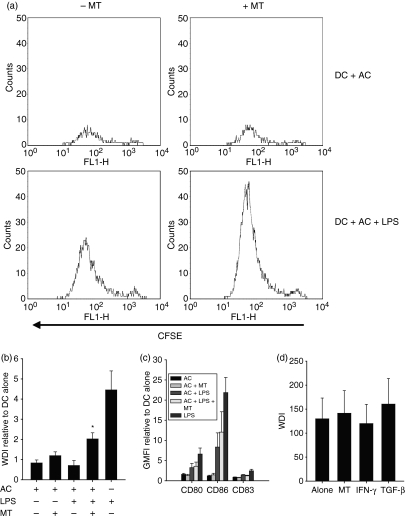
The suppressive properties of IFN-γ are considered to be the result of its ability to activate IDO. To investigate the association of IDO with the effects observed functionally, studies set out to assess the effects of IDO blockade. As with blocking IFN-γ and TGF-β, the AC-associated inhibition of mDC stimulated T-cell proliferation was significantly reduced in part by blocking IDO activity (Fig. 8a,b) with a subtle effect upon the AC inhibition of the mixed lymphocyte reaction. Blockade of IDO, IFN-γ or TGF-β showed no effect upon mDC-stimulated CD4+ T-cell proliferation (Fig. 8d). Consistent with the blocking of IFN-γ and TGF-β, blocking of IDO also had a minimal effect on DC phenotypic maturation (Fig. 8c). Together these results suggest a role for TGF-β, IFN-γ and IDO in the AC suppression of T-cell proliferation.
Figure 8.
IDO modulates mature dendritic cell (mDC)-induced T-cell proliferation. Dendritic cells (DC) were cultured for 48 hr with apoptotic cells (AC) at a ratio of 1 : 5 in the presence or absence of LPS or 1-methyl tryptophan before staining or incorporation into a T-cell proliferation assay. AC suppression of lipopolysaccharide (LPS) -stimulated DC CD4+ T-cell proliferation is partially released by blocking IDO (a, representative plots and b) independent of effects upon the DC phenotype (c). Representative CFSE plots are of the proliferating blast (R2 region) cells (region gates and a representative R1 CFSE plot are shown in Fig. 3a). (d) shows the effects of blockade of IDO, IFN-γ and TGF-β upon mDC-induced T-cell proliferation. T-cell proliferation was determined by flow cytometry and CFSE dilution analysis and is represented by the weighted division index (WDI) or the WDI relative to DC alone. Changes in DC phenotype were determined by flow cytometry and are represented as GMFI relative to DC alone. Bars represent the mean of three or more independent donors ± SEM for the pooled data. *P ≤ 0·01 when compared with DC + AC + LPS.
AC co-culture induces upregulation of IFN-γ-induced IDO expression by DC
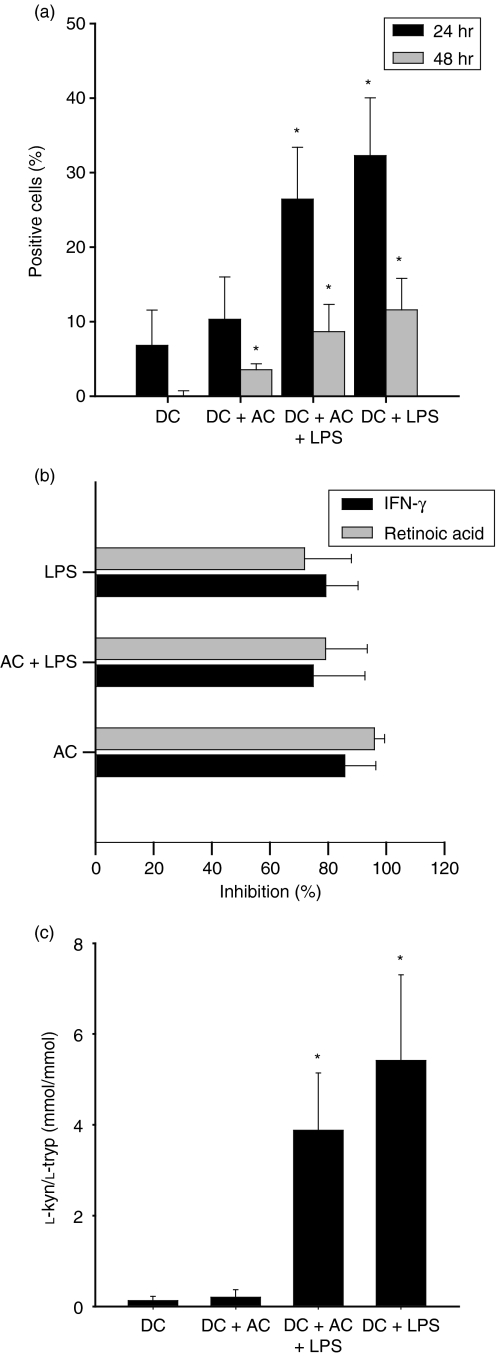
Given that our studies clearly demonstrate a functional role for IDO, particularly apparent in the AC suppression of mDC effects, we sought to confirm DC-induced IDO expression by intracellular staining. Mature DC in the presence or absence of AC demonstrated a significant increase in IDO expression compared with iDC (Fig. 9a) at both 24 and 48 hr, while iDC exposed to AC showed increased levels of IDO, significantly by 48 hr in comparison with iDC alone. To investigate whether IFN-γ was responsible for AC-associated IDO, expression was investigated in the absence of IFN-γ. Inhibition of IFN-γ using a neutralizing antibody and also retinoic acid resulted in reduced levels of IDO expression in all conditions tested (Fig. 9b). These data support the role of DC in IDO production and in its regulation of expression by IFN-γ.
Figure 9.
Dendritic cells (DC) express functional IDO. The DC were stained with DiI and cultured alone, with lipopolysaccharide (LPS) alone or simultaneously with AC at a 1 : 5 ratio and stained for intracellular IDO (a). Peak intracellular IDO expression at 24 hr was confirmed at low levels in iDC and increased levels in response to LPS. Data are expressed as the percentage of cells positive for IDO as determined by flow cytometry. IDO expression at 24 hr was inhibited in the presence of a neutralising IFN-γ antibody and retinoic acid (b). Percentage inhibition of expression represents the percentage change of the percentage of cells positive for IDO as determined by flow cytometry. Enzymatic activity of IDO was determined at 24 hr as a ratio of kynurenine production to tryptophan catabolism by HPLC analysis (c). IDO activity was greatest in LPS-stimulated DC cultures but was reduced in the presence of apoptotic cells (AC). Results represent the mean of three independent experiments ± SEM. *P ≤ 0·05 when compared with DC alone.
AC environments induce active IDO
As IDO expression was detected in mDC cultures in the presence and absence of AC, but functional evidence for IDO activity was detected only in AC-suppressed mDC, we subsequently investigated whether the IDO expression observed was of the active or inactive form. The DC exposed to AC in the presence of LPS or to LPS alone showed a significantly higher l-kynurenine : l-tryptophan ratio compared with DC or AC-conditioned DC, indicating strong functioning IDO enzymatic activity in mDC environments (Fig. 9c).
Discussion
Optimal presentation of antigens acquired from dying cells by DC requires two steps; first, the efficient acquisition of dying cells via phagocytosis when DC are in their immature state and, second, the receipt of a maturation signal that can be provided by standard maturation stimuli, i.e. MCM or LPS.1 The current study therefore investigated the differential effects of two types of cell death upon DC function and the mechanisms by which this is achieved.
Our observations that AC, but not NC, fail to induce maturation of DC,16 as determined by the upregulation of cell surface markers, are consistent with those of previous studies, irrespective of the mode of apoptosis induction or cell type.7,36–39 Conversely, however, one study40 suggests that AC induce the maturation of DC, which may be because the study used ‘late’ AC, which have been shown previously to elicit different responses to the ‘early’ apoptotic cells41,42 used in the present study. Despite the ability of DC to phagocytose both AC and NC, our data, and those of others, show that only NC induce maturation of DC, leading to subsequent activation of CD4+ allogeneic T cells, similar to that observed with LPS.7,14,36
We investigated whether DC responses towards AC were the result of suppression of DC maturation or an immunological null event. The data presented demonstrate that DC conditioned with AC actively downregulated phenotypic DC maturation, a response suggested to be the result of complement receptor binding.43,44 The effects of AC were also evidenced at a functional level by the inhibition of LPS-driven CD4+ T-cell proliferation. This is supported by the data of others,17 although it contrasts with another study,7 which may be explained by the use of syngeneic CD8+ T cells, and by differences in the cell ratios and maturation stimuli used. However, our data and those of others strongly suggest that the DC response to AC is one of active suppression, thus reinforcing the current perception that DC exposed to apoptotic environments play a major role in the presentation of self-antigens and the induction of immune tolerance.8,16,45
The involvement of TGF-β in the antigen-presenting cell response to AC environments is well accepted.14,15 Our results suggest that TGF-β had little effect on the DC phenotype but reduced suppression of mDC-induced T-cell proliferation by AC. The lack of cytokine-induced modulation of the DC phenotype is supported by the inability of AC supernatants to suppress LPS-induced DC maturation,14,15 although recent work shows TGF-β to have an inhibitory role.46 The ability of TGF-β to influence the DC phenotype may be the results of differences in cytokine priming before the addition of LPS, resulting in suppressed DC that are unable to respond maximally to further stimulus. These data, however, would suggest that, in our system, TGF-β, although not involved in the phenotypic maturation state of the DC, is a regulator of AC-induced suppression of CD4+ T-cell proliferation.
To further elucidate the role of cytokines in the suppressive action of the differentially conditioned DC, cytokine profiling was performed. The LPS-stimulated DC promoted an overall T helper type 1 (Th1) environment by the production of IFN-γ, TNF-α, IL-12, IL-1β and IL-6, together with an increase in IL-10. One explanation for increased IL-10, a classical immunoregulatory cytokine, may be the role of IL-12-induced IL-10 in a mechanism of negative feedback.47 Unlike previous reports,44 we were unable to detect decreases in basal DC production of TNF-α and IL-1 upon phagocytosis of AC because initial DC cytokine production was low. Of particular interest was the specific generation of IFN-γ by iDC co-cultured with AC; this suggested IFN-γ to be a key mediator in the AC-associated suppressive effect, a mechanism not reported previously.
Multiple signalling events within DC induced by co-culture of AC in combination with LPS promoted decreased mDC-induced cytokine responses, which may occur through the phagocytosis of AC.44 The tolerogenic potential of AC would support the inhibition of key proinflammatory cytokines because IL-12 is known to abrogate established tolerance18,19,48 and IFN-γ has been shown to override AC-induced suppression of TNF-α.15 Despite demonstrating that IFN-γ is a key cytokine in the iDC response to AC, we were unable to detect IFN-γ in AC-suppressed mDC cultures, possibly because of its rapid use. We therefore investigated the functional role of IFN-γ in iDC and mDC cultures and its effects on DC function.
One of the most interesting recent findings is that IFN-γ, classically a proinflammatory cytokine involved in Th1 immune responses, can also play an immunoregulatory role in the immune system via the induction of IDO.49 Our data demonstrate a statistically significant role for IFN-γ in AC-induced suppression of mDC-induced T-cell proliferation independent of phenotypic modulation of the DC. In contrast, neutralization of IFN-γ had no effect on mDC function, suggesting that IFN-γ is not an overt player in this proinflammatory context. The dichotomy of effects that IFN-γ has on T-cell proliferation depending on the DC environment may be the result of the Th1/Th2 balance. As proposed by others,50,51 increased IL-12 together with high levels of costimulation, as observed in response to LPS, suggests a Th1 environment. However, reduced IL-12 together with low expression of CD80/CD86, as seen in iDC cultures with AC, suggests an environment that is biased towards tolerance or Th2 cell generation, depending on the CD80/CD86 responsive threshold. As Th2 cells are sensitive to IFN-γ, because of the expression of both IFN-γ receptor 1 and IFN-γ receptor 2,52 the effect of co-culture with AC would enable IFN-γ to have an anti-inflammatory effect.
In our system, IDO mediated the partial suppression of mDC-induced CD4+ T-cell proliferation via IFN-γ-dependent mechanisms. Further evidence of a regulatory role of IFN-γ is demonstrated in vivo in models of human autoimmunity, including experimental autoimmune encephalomyelitis53 and experimental autoimmune uveoretinitis54, where an absence of IFN-γ results in exacerbated disease. The biological significance of our results, however, remains to be investigated. Residual expression of IDO following inhibition of IFN-γ suggests a role for other regulatory factors, together with IFN-γ.27,28
The enzyme responsible for tryptophan catabolism, IDO, is involved in various mechanisms of T-cell tolerance, including the induction of T-cell anergy, immune deviation and apoptosis of activated T cells. In addition, Fallarino et al.55 demonstrate a possible feedback loop through tryptophan-induced T-cell apoptosis inducing a ‘regulatory DC’ with subsequent effects on T-cell function. However, preliminary studies in our laboratory suggest apoptosis-related effects on DC irrespective of the cell type undergoing apoptosis and, indeed, no correlation of an IDO-related response with T-cell viability. IDO involvement in DC responsiveness has been documented in both tolerogenic and mDC environments.56,57 Explanations for the variation in expression and effect include the existence of an active and inactive enzyme58 although, our HPLC data suggest IDO activity in both contexts. In addition to its regulation by IFN-γ, IDO activity has recently been shown to be responsive to IL-6.59 In the cytokine milieu produced by mDC, IL-6 remains high in the presence of AC. Production of IL-6 by DC has been reported as a result of reverse signalling via CD80/CD86 and CD28 during DC–T-cell interactions,59 which counters the effects of reverse signalling via CTLA-4, previously shown to invoke IFN-γ production by DC.29,30,60,61 It is therefore possible that in mDC cultures, IL-6 regulation of IDO is overridden. Therefore, DC are affected by both the effects of T-cell ligation and their environment.
In conclusion, these findings strongly implicate IFN-γ and IDO in a role in the immunosuppressive effect observed upon DC within AC environments, supporting a role for IDO in self-antigen presentation and immune tolerance. The essential role of apoptosis in cellular homeostasis makes it vital that, upon phagocytosis of AC, DC do not mature or initiate an immune response. One possibility is that the activation of IDO through IFN-γ is a mechanism by which the body ensures immune tolerance to self-antigens, thereby reducing the risk of autoimmunity. Despite this evidence, it is clear that IDO is not the sole factor and the role of numerous factors, e.g. TGF-β, remains to be elucidated. Investigations into these processes will increase our understanding of the mechanisms controlling peripheral self-tolerance, providing new strategies for antigen delivery that maintain tolerance rather than immunity.
Acknowledgments
This work was funded by the Engineering and Physical Sciences Research Council.
Glossary
Abbreviations
- AC
apoptotic cells
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- DC
dendritic cells
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- iDC
immature DC
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
interferon-γ
- IL-12
interleukin-12
- LPS
lipopolysaccharide
- MCM
monocyte-conditioned medium
- mDC
mature DC
- MHC
major histocompatibility complex
- NC
necrotic cells
- PBMC
peripheral blood mononuclear cells
- TGF-β
transforming growth factor-β
- Th1
T helper type 1
- TNF-α
tumour necrosis factor-α
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 4.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–31. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 5.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–30. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 6.Reis ES. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 7.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 10.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 11.Rosen A, Casciola-Rosen L. Clearing the way to mechanisms of autoimmunity. Nat Med. 2001;7:664–5. doi: 10.1038/89034. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann PR, Kench JA, Vondracek A, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 13.Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155:501–4. doi: 10.1083/jcb.200110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 15.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 16.Gad M, Claesson MH, Pedersen AE. Dendritic cells in peripheral tolerance and immunity. APMIS. 2003;111:766–75. doi: 10.1034/j.1600-0463.2003.11107808.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–94. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–53. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Riemann H, Loser K, Beissert S, Fujita M, Schwarz A, Schwarz T, Grabbe S. IL-12 breaks dinitrothiocyanobenzene (DNTB)-mediated tolerance and converts the tolerogen DNTB into an immunogen. J Immunol. 2005;175:5866–74. doi: 10.4049/jimmunol.175.9.5866. [DOI] [PubMed] [Google Scholar]
- 20.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154:2366–74. [PubMed] [Google Scholar]
- 21.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Tredget EE, Ghaffari A, Lin X, Kilani RT, Ghahary A. Local expression of indoleamine 2,3-dioxygenase protects engraftment of xenogeneic skin substitute. J Invest Dermatol. 2006;126:128–36. doi: 10.1038/sj.jid.5700022. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 24.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 25.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 26.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–8. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 27.Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23:413–21. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 30.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 31.McLeod JD, Walker LS, Patel YI, Boulougouris G, Sansom DM. Activation of human T cells with superantigen (staphylococcal enterotoxin B) and CD28 confers resistance to apoptosis via CD95. J Immunol. 1998;160:2072–9. [PubMed] [Google Scholar]
- 32.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–84. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 33.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 34.Angulo R, Fulcher DA. Measurement of Candida-specific blastogenesis: comparison of carboxyfluorescein succinimidyl ester labelling of T cells, thymidine incorporation, and CD69 expression. Cytometry. 1998;34:143–51. [PubMed] [Google Scholar]
- 35.Yong S, Lau S. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr. 1979;175:343–6. doi: 10.1016/s0021-9673(00)89443-1. [DOI] [PubMed] [Google Scholar]
- 36.Kacani L, Wurm M, Schwentner I, Andrle J, Schennach H, Sprinzl GM. Maturation of dendritic cells in the presence of living, apoptotic and necrotic tumour cells derived from squamous cell carcinoma of head and neck. Oral Oncol. 2005;41:17–24. doi: 10.1016/j.oraloncology.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 38.Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12:919–30. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newton PJ, Weller IV, Katz DR, Chain BM. Autologous apoptotic T cells interact with dendritic cells, but do not affect their surface phenotype or their ability to induce recall immune responses. Clin Exp Immunol. 2003;133:50–8. doi: 10.1046/j.1365-2249.2003.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi AA. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–71. [PubMed] [Google Scholar]
- 41.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 42.Pietra G, Mortarini R, Parmiani G, Anichini A. Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res. 2001;61:8218–26. [PubMed] [Google Scholar]
- 43.Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N. The apoptotic cell receptor CR3, but not αvβ5, is a regulator of human dendritic cell immunostimulatory function. Blood. 2006;108:947–55. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 45.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation – friend or foe? Immunity. 2001;14:357–68. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 46.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–70. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 47.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–82. [PubMed] [Google Scholar]
- 48.Ushio H, Tsuji RF, Szczepanik M, Kawamoto K, Matsuda H, Askenase PW. IL-12 reverses established antigen-specific tolerance of contact sensitivity by affecting costimulatory molecules B7-1 (CD80) and B7-2 (CD86) J Immunol. 1998;160:2080–8. [PubMed] [Google Scholar]
- 49.Frucht DM, Fukao T, Bogdan C, Schindler H, O'shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–60. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 50.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 51.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–8. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 52.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183–7. doi: 10.1016/j.it.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 54.Caspi RR, Chan CC, Grubbs BG, et al. Endogenous systemic IFN-gamma has a protective role against ocular autoimmunity in mice. J Immunol. 1994;152:890–9. [PubMed] [Google Scholar]
- 55.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 56.Fujigaki S, Saito K, Sekikawa K, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–8. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 57.Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–71. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terness P, Chuang JJ, Opelz G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 2006;27:68–73. doi: 10.1016/j.it.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Orabona C, Grohmann U, Belladonna ML, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–42. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 60.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 61.Mellor AL, Baban B, Chandler P, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–5. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]