Abstract
Naturally-occurring regulatory T cells (Tregs) are emerging as key regulators of immune responses to self-tissues and infectious agents. Insight has been gained into the cell types and the cellular events that are regulated by Tregs. Indeed, Tregs have been implicated in the control of initial activation events, proliferation, differentiation and effector function. However, the mechanisms by which Tregs disable their cellular targets are not well understood. Here we review recent advances in the identification of distinct mechanisms of Treg action and of signals that enable cellular targets to escape regulation. Roles for inhibitory cytokines, cytotoxic molecules, modulators of cAMP and cytokine competition have all been demonstrated. The growing number of inhibitory mechanisms ascribed to Tregs suggests that Tregs take a multi-pronged approach to immune regulation. It is likely that the relative importance of each inhibitory mechanism is context dependent and modulated by the inflammatory milieu and the magnitude of the immune response. In addition, the target cell may be differentially susceptible or resistant to distinct Treg mechanisms depending on their activation or functional status at the time of the Treg encounter. Understanding when and where each suppressive tool is most effective will help to fine tune therapeutic strategies to promote or constrain specific arms of Treg suppression.
Keywords: autoimmunity, inflammation, regulatory T cell
Introduction
Central to an efficient adaptive immune response is the ability to tightly regulate immune activation to prevent responses to self-antigens, permit responses to foreign antigens and limit collateral damage. Regulation occurs early in T-cell development, thymically, with the selection of an immune repertoire purged of many self-reactive T cells. In the periphery, strict T-cell signalling requirements intrinsically regulate T-cell activation while extrinsic regulation comes in part from specialized regulatory T cells (Tregs).1 The importance of active regulation of self-reactivity was brought to the fore, once again,2,3 around 1990 with the ability to induce a number of organ-specific autoimmune diseases in rodent strains that do not normally develop autoimmunity by procedures that rendered the animals partially T-cell deficient.4–6 Significantly, the transfer of a CD25+ CD4+ T-cell subset, from syngeneic healthy mice, prevented the development of autoimmunity on transfer to these lymphopenic animals,7 indicating that an intact immune system contains cells with the capacity to prevent the activation of autoreactive cells. Therefore, self-tolerance, at least to tissue-specific self-antigens, represents an active, dynamic state in which autoreactive cells are held in check by Tregs. More recently, Tregs have been shown to also limit immune responses to foreign antigen and can help or hinder transplantation and pathogen/tumour clearance.8–10 Given their broad suppressive action, it is unclear how the activity of Tregs is regulated to ensure that beneficial immune responses to infectious stimuli proceed unabated while other responses are suppressed.
The discovery that CD25+ CD4+ Tregs expressed the transcription factor Foxp3 gave credence to the notion that regulatory T cells represented a distinct T-cell lineage.11–14 In humans, disruption of Foxp3 function leads to an immune dysfunction, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome characterized by autoimmune disease, allergy and inflammatory bowel disease.15 Similarly in mice, natural or conditional deletion of Foxp3+ cells leads to multi-organ autoimmune disease, highlighting its key role in the function of Tregs.16–18 Recent studies on the gene targets under Foxp3 regulation should provide insight into the function of Foxp3 in the Tregs themselves.19–21 While it has been known for some time that these naturally-occurring Tregs develop in the thymus,6,22,23 the thymic signals that confer lineage specificity have not been fully determined. Nonetheless, thymic support of Tregs may depend on antigen expression on thymic epithelium and the engagement of high-affinity, T-cell receptors (TCR).24,25 There is current debate on the specificity of Tregs (self or non-self);23 in two independent studies CD25+ Foxp3+ Tregs in the periphery were shown to have TCRs with increased avidity for self-peptide when compared to CD25− non-regulatory T cells26,27 but this remains controversial.28,29 Maintenance of Tregs in the periphery is aided by signals via interleukin-2 (IL-2), CD28 and transforming growth factor-β (TGF-β).23 Additionally, Tregs may require interactions with their cognate ligand in the periphery to further mature and/or survive.30 Once in the periphery, Tregs can vigorously expand in response to antigen and homeostatic proliferative signals,31–33 in contrast to their anergic phenotype in vitro.
In essence, any cell that differentially secretes or consumes key cytokines can regulate the function of other effector cells that are activated in close proximity. However, this review focuses on the natural Foxp3+ regulatory T-cell lineage whose primary function appears to be one of down-regulating immune function. It has also become clear in the past 5 years that regulation of immune responses is under the control of multiple regulatory T-cell subsets.34 Naive CD4+ CD25− T cells can be induced to become regulatory in the periphery to a variety of signals35,36 including antigen exposure in the presence of immunosuppressive cytokines like IL-10 and TGF-β37,38 and/or retinoic acid,39–41 activation by immature dendritic cells42 and perhaps activation in the context of naturally-occurring Tregs.43 Many of these regulatory subsets act by secretion of the immunosuppressive cytokines IL-10 and/or TGF-β. In addition, CD8 ‘suppressor’ T cells, natural killer (NK) T cells and γδ T cells have also been shown to regulate immunity.44,45 The relative roles played by these subsets and co-operativity in a given immune response are not well understood.
Immune functions regulated by Tregs
Tregs appear to modulate a variety of immune functions from initial T-cell and B-cell activation to effector function in the target tissue (Fig. 1). Here we discuss the stages at which lymphocytes appear vulnerable to Treg suppression. Although not the focus of this review, many of these events in vivo may be mediated via modulation of antigen-presenting cell (APC) function. The classic in vitro suppression assay measures the ability of Tregs to abrogate lymphocyte proliferation in response to antigen stimulation.46,47 Suppression does not occur when Tregs and targets are separated in a trans-well system, suggesting that the inhibitory event requires close cellular proximity. Analysis of the kinetics of Treg action in vitro has revealed that the target T cells undergo initial activation (up-regulate CD69 and CD25) and secrete IL-2 but that the presence of Tregs leads to the premature termination of the activation programme between 6 and 12 hr,48 resulting in a down-regulation of IL-2 transcription.48,49 Although the down-regulation of IL-2 is often used as a readout of Treg function it is not clear at present if IL-2 is the primary molecular target of suppression. Indeed, gene expression profiling of CD4 T-cell targets undergoing Treg suppression provided evidence that Tregs induce a unique pattern of altered gene expression in the target T cells.50 The Treg-imposed gene programme was exemplified by the induction of many genes associated with growth arrest or inhibition of proliferation.50 In keeping with an early block in T-cell activation, recent data in vitro (explanted lymph nodes) and in vivo (intravital) using two-photon microscopy support the notion that Tregs limit T-cell signal duration: in two independent TCR transgenic systems the presence of Tregs led to a reduction in the duration of contacts between naive antigen-specific CD4+ T cells and antigen-loaded dendritic cells.51,52 Studies on the kinetics of Treg action and the disruption of stable T-cell–APC contacts are particularly interesting given accumulating data that suggest that long-term T-cell–APC interactions (> 10 hr) are essential for subsequent effector function.53,54 Therefore these long-term T-cell interactions with APCs promote ‘fitness’ in a developing immune response.55 It is intriguing therefore that Tregs may interfere with this period of prolonged activation.
Figure 1.
Regulatory T cells (Tregs) inhibit multiple stages of target cell activity. (a) Tregs appear to be unable to inhibit the early activation events (up-regulation of CD25 and CD69) of the first 6–10 hr of target CD4+ T-cell activation. (b) Tregs suppress proliferation of multiple immune cell types possibly via attenuation of interleukin-2 production. (c) Suppression of CD4+ T-cell differentiation by limiting the duration of T-cell receptor signalling or inhibiting the induction of the lineage specific transcription factors GATA3 and Tbet. (d) Treg suppression of effector T-cell cytokine production (interferon-γ and interleukin-4 by T helper type 1 and type 2 cells, respectively); inhibition of lytic granule release by CD8 effectors; inhibition of B-cell antibody production, directly or via blockade of CD4 help.
In addition to disruption of early activation events, Tregs have also been shown to block T-cell and B-cell differentiation and effector function.56–62 Most strikingly, in vivo studies show that Tregs can modulate established inflammation in a variety of autoimmune and inflammatory settings. Indeed, for many of these examples Tregs failed to control the initial expansion of antigen-specific cells in the lymph nodes but did halt the execution of their effector function at sites of inflammation. In an elegant set of studies using Tregs from mice deficient in the ligands for E/P-selectin,63 Tregs that could not migrate to inflamed sites were unable to suppress T helper type 1-mediated inflammation in the skin, suggesting a key role for Tregs at the effector stage within the inflamed tissue. Moreover, fascinating studies on the control of CD8 responses highlighted the exquisite selectivity of the inhibition of effector function by Tregs: cytolytic function was terminated but not the expression of effector molecules such as interferon-γ.64,65 It is likely that the context in which the Treg encounters its target will determine which facet of the immune response is susceptible to Treg suppressive actions. Thus the anatomical location (lymph node or tissue), the make-up of the inflammatory milieu, antigen load, target cell number and activation status of the target cells will all influence the degree and mechanism of Treg immune regulation.
Mechanisms of Treg suppression
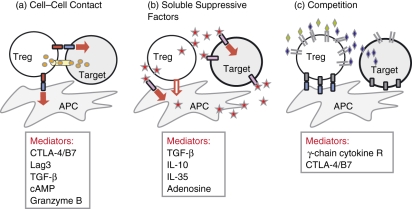
The mechanisms of Treg action remain poorly understood and contentious. Differences between in vitro and in vivo requirements, particularly with regard to the inhibitory cytokines IL-10 and TGF-β, have fuelled the controversy. Many of the discrepancies may arise because, unlike the controlled environment of in vitro suppression, in any given in vivo model the stage of activation or anatomical location of successful target T-cell–Treg interactions has been poorly defined. The latest identification of new (and revamped) inhibitory mechanisms may bridge the in vitro and in vivo divide. As recently reviewed,66 suppressive mechanisms can be divided into three categories: cell–cell contact, local secretion of inhibitory cytokines and local competition for growth factors (Fig. 2). In each category there are multiple examples of inhibitory pathways that are probably not mutually exclusive.
Figure 2.
Mechanisms of regulatory T-cell (Treg) suppression. (a) Cell–cell contact. Tregs may suppress target cells via direct interaction of receptor–ligand pairs on Tregs and target cells; delivery of suppressive factors via gap junctions including cyclic adenosine monophosphate (cAMP); direct cytolysis; membrane-bound suppressive cytokines such as transforming growth factor-β (TGF-β); and/or indirectly via modulating the antigen-presenting cell (APC) through cell–cell contact, possibly through reverse signalling via Treg–cytotoxic T-lymphocyte antigen-4 (CTLA-4) engagement of B7 on dendritic cells. (b) Soluble suppressive factors. Tregs can directly secrete interleukin-10 (IL-10), TGF-β and IL-35 or induce APCs to secrete such factors. Expression of CD73/CD39 by Tregs facilitates the local generation of adenosine that can down-modulate immune function. (c) Competition. Tregs may compete for some cytokines that signal via receptors that contain the common γ-chain (IL-2, IL-4 and IL-7). Additionally they may compete for APC costimulation via constitutive expression of CTLA-4. Red arrow indicates an inhibitory signal.
Cell–cell contact
There is perhaps most disagreement surrounding the mechanisms put forth for cell–cell contact-dependent suppression because the studies have not been robustly reproduced in multiple experimental settings. Membrane-bound TGF-β was shown to contribute to suppression67 and this remains consistent with the inability to suppress in an in vitro transwell system and with the in vivo importance of TGF-β-mediated regulation.68 Other cell surface molecules implicated in suppression include cytolytic molecules (Fas and Granzyme B),69–71 LAG3 72 and cytotoxic T-lymphocyte antigen 4 (CTLA-4). Tregs constitutively express CTLA-4 but its requirement for Treg activity is not clear. Various mechanisms of Treg CTLA-4-dependent regulation have been described, including delivery of ‘outside-in’ signals via B7 on the activated target T cells73,74 and/or B7 ligation on dendritic cells activating indoleamine 2,3-dioxygenase (IDO).75In vivo, a clear role for CTLA-4/B7 interactions in Treg control of colitis can be demonstrated with antibody blockade or CTLA-4 immunoglobulin treatment.76 However, CTLA-4-deficient Tregs still confer protection in that same in vivo model77 so the relationship between CTLA-4 expression and Treg function requires further examination. Data from the in vivo models have suggested that while CTLA-4−/− Tregs retain regulatory capacity they may inhibit responses using a qualitatively distinct mechanism to that of CTLA-4-sufficient Tregs, being more dependent on IL-10 for suppression.77,78 It is possible that individual regulatory mechanisms are differentially redundant depending on the immune setting.
An interesting inhibitory pathway to enter the Treg field is the modulation of cyclic adenosine monophosphate (cAMP) levels in the target cells. Elevation of cAMP levels has long been associated with inhibition of cellular proliferation and differentiation and in lymphocytes causes selective inhibition of cytokine gene expression, including IL-2 and interferon-γ, in part through protein kinase A (PKA)-blockade of nuclear factor-κB (NF-κB) activity79 or the activation of the transcriptional repressor ICER (inducible cAMP early repressor).80 Recent evidence indicates that Tregs can increase cAMP levels in the target cells through at least two mechanisms: directly by delivery of cAMP and indirectly by the local generation of adenosine. In a provocative set of experiments it was suggested that Tregs, which express high levels of cAMP, transfer cAMP into the activated target cells via gap junctions.81 Data using a fluorescent dye system to detect transfer of material from Tregs to targets nicely demonstrated the potential of Tregs to directly deliver material to T-cell targets. Moreover, partial blockade of either cAMP or gap junctions attenuated Treg suppression of IL-2. At this stage, the specificity of material transferred and the degree to which this is a Treg-specific phenomenon remain to be elucidated. In parallel, two groups described the generation of adenosine by Tregs via the surface expression of the ectonucleotidases CD73 and CD39.82,83 Binding of adenosine to the adenosine A2A receptor can increase intracellular cAMP84 and in these current studies Treg-generated adenosine suppressed proliferation and cytokine production by effector T cells. Importantly, the loss of CD39 or the addition of an A2A receptor antagonist abrogated Treg suppressive function in the presence of 5′AMP in vitro and in vivo.82,83 In addition, a recent study reported an association between the loss of CD39+ Tregs and multiple sclerosis, suggesting a regulatory role for this Treg subset in controlling autoimmune inflammation.85
It has not been determined how increasing cAMP mediates some of the inhibitory effects of Tregs. Increased cAMP levels may selectively inhibit immune responses based on differential cytokine gene sensitivity to cAMP and/or NF-κB: indeed early studies found that the IL-2 gene, but not the IL-4 gene, was inhibited by cAMP.86 More recently, a study has implicated the downstream transcriptional repressor ICER.74 Regardless of the specific mechanism of cAMP action, Tregs appear to have co-opted a common pathway for tissue regulation of immune function: tissue-derived adenosine has been shown to both promote T-cell anergy and abrogate autoimmune tissue destruction.87
Secretion of inhibitory cytokines
In vivo, the suppressive cytokines TGF-β and IL-10 have been implicated as active players in the effector function of Tregs.88,89 The TGF-β plays a key role in lymphocyte homeostasis at many levels as dramatically demonstrated by the multi-organ immune pathology occurring in mice that are deficient in TGF-β or in the absence of signalling molecules required for TGF-β responsiveness.90 Target cells that are unable to respond to TGF-β escape Treg control in the colitis model.91 However, Tregs themselves do not have to make the TGF-β,91,92 implicating a mechanistic pathway that involves Treg induction of TGF-β production from other cell types, possibly APCs. Suppression via IL-10 production is also important in a number of in vivo models of Treg-controlled inflammation and homeostatic expansion,32,88,93 and once again Tregs may not need to produce the cytokine themselves. Neither IL-10 nor TGF-β, however, is required for in vitro suppression of proliferation. We suggest that some of the discrepancies between mechanisms used in suppression in vitro and in vivo might be explained by differences in the stage of T-cell activation being suppressed in each setting. Indeed, interesting studies from the Powrie group94 highlighted that while IL-10 was a key player in Treg inhibition of colitis mediated by antigen-experienced T cells, it was not essential for Treg control of colitis induced by naive T cells. Therefore IL-10 may be important in the Treg control of effector function within inflamed tissue but not in Treg control of T-cell activation and/or differentiation events in the draining lymph nodes in vivo.
The Vignali group recently described an exciting new inhibitory cytokine, IL-35, that appears to contribute to Treg suppression.95 The Epstein–Barr-virus-induced gene 3 (Ebi3) was identified in genomic screens as being overrepresented in mouse CD4+ CD25+ T cells. Ebi3 encodes the IL-27 β-chain and pairs with IL-27α to form IL-27 or with IL-12α to form the new cytokine IL-35. The Tregs express both Ebi3 and IL-12α. Unlike IL-10 and TGF-β, IL-35 seems to be required for suppressive function both in vitro and in vivo. Ebi3−/− Tregs have reduced suppressive capacity in in vitro assays of proliferation and in vivo in the control of homeostatic expansion and colitis. Moreover, ectopic expression of IL-35 in T cells confers regulatory properties. Interestingly, Ebi3 appears to be a downstream target of Foxp3, perhaps explaining its preferential expression in Tregs; however, many immune cell types that make IL-12α could also express IL-35 and modulate immune responses. It remains to be determined, therefore, whether IL-35 is a Treg-specific suppressive factor and which cell types are receptive to its inhibitory actions.96 Nonetheless, IL-35 may represent an important therapeutic target for the modulation of immunity.
Competition for growth factors
The constitutive expression of CD25 by Tregs gives them an initial competitive advantage for the consumption of IL-2 over naive T cells, which express CD25 only after TCR stimulation. Indeed, Tregs clearly deprive effector T cells of IL-2 in cocultures in vitro.97,98 More recently, Pandiyan et al.99 highlight the functional consequence of Treg competition for cytokines. They demonstrated that Treg-mediated competition for growth factors leads to cytokine deprivation-induced apoptosis in the target effector cells both in vitro and in vivo. The apoptotic death induced in the targets was shown to be Bcl-2 interacting mediator of cell death (BIM)-dependent and perforin/Fas-independent. The significant degree of target cell death measured in this study has rarely been seen in other in vitro experimental set-ups for Treg suppression; but at face value it does show that in certain circumstances cytokine consumption by Tregs can have profound consequences on the fate of concurrently activated T cells. Presumably this is not a Treg-specific phenomenon but could be mediated by any previously activated effector cell with high cytokine receptor levels as previously suggested in models of T-cell competition.100
However, Treg suppression cannot be entirely explained by the competitive consumption of IL-2 in particular. The Tregs can suppress the autoimmune response of IL-2 receptor-deficient T cells, excluding an essential role for this mechanism.101 Moreover, bypassing competition for IL-2, by provision of exogenous IL-2 in in vitro coculture, enables the target T cells to proliferate in the presence of Tregs while endogenous target T-cell production of IL-2 remains suppressed.48,49 In support of additional suppressive mechanisms, the altered gene profile of target T cells following a Treg encounter bears little similarity to the gene profile induced with IL-2 deprivation.50 Nonetheless, competition for IL-2, and possibly other growth factors, is certainly a component part of Treg action and may be a dominant mechanism in some situations. In turn, IL-2-uptake activates Tregs and may also modulate their activity through enhanced IL-10 production.97,98
This recent cytokine deprivation study, along with the identification of IL-35 as a Treg effector molecule, challenges the popular interpretation of transwell experiments that cell–cell contact is required for Treg suppression. Indeed, there has been no formal demonstration that Tregs need direct physical contact with target cells. More likely, these mechanisms require local cytokine delivery or uptake in a finely balanced microenvironment. Indeed, it has long been known that CD4+ T-cell cytokines are secreted in a polar fashion, presumably to target cytokine delivery and limit collateral damage.102,103 It is therefore not difficult to imagine that Tregs also work in a targeted fashion and may have a defined suppressive reach; the scope of which may differ according to the nature of the local milieu.
Many of the suppressive mechanisms that we have discussed here are not essential mediators of Treg activity and may play a supportive role to an, as yet, unidentified, master regulatory function. To accommodate the variety of immune functions regulated by Tregs (Fig. 1), Tregs may use a suppressive mechanism that targets an essential and common step in lymphocyte activation. For instance a mechanism that focused on disrupting a key component of proximal B-cell and T-cell receptor signalling could disrupt function at any stage of immune activation (Fig. 1): proliferation, differentiation or effector function. The quantity and quality of the activation signals would then determine target T-cell sensitivity to Treg actions.
Target cell escape from regulation
Given such a diverse array of suppressive mechanisms, Treg activity needs to be attenuated to mount effective immune responses to infection. Signals that mitigate regulatory T-cell activity either negatively regulate the Treg itself or positively arm the target cells against Treg suppressive mechanisms. Treg function can be modulated by a variety of proinflammatory signals including Toll-like receptor triggering104 and direct inhibition by tumour necrosis factor-α (TNF-α).105 Here we focus on the signals that enable target cells to escape Treg actions. Identifying signal components that render target cells resistant to suppression could provide clues to the molecular targets of Treg suppressive mechanisms.
The up-regulation of B7 expression (CD80 and CD86) by APC represents a central event in the activation of naive T cells to infectious agents and may serve as a mechanism to disrupt regulatory T-cell tolerance by rendering effector T cells unresponsive to suppression. Indeed, elevating the strength of target T-cell activation signals in a variety of ways, through increasing antigen dose or provision of costimulatory signals, has been shown to ‘abrogate’ Treg activity.46,47,106 However, in many cases it has been hard to determine if the stimulus is providing positive signals to the target to escape suppression or if the same signals are negatively regulating the Tregs. Nevertheless, distinct signals delivered to the target T cells have been shown to enable them to resist regulation, independent of effects on Tregs. Indeed, CD28 signalling directed to the target T cell enables them to resist Treg suppression of early IL-2 production.48 Similarly, positive signals from OX-40 and glucocorticoid-induced tumour necrosis factor receptor (GITR) also help target cells to evade suppression.107–109 In addition, Toll-like receptor-induced IL-6 production by dendritic cells can also render target cells resistant to suppression independent of costimulation.110 Therefore it appears that the kind of activation signals delivered by a fully mature dendritic cell following exposure to pathogens are the same signals that enable naive T cells to escape suppression. Intuitively for the maintenance of self-tolerance, it follows that presentation of self-antigen in the absence of such potent costimulatory signals or inflammatory cytokines would leave effector T cells more vulnerable to suppression.
At the molecular level, a number of signalling components have been identified that can impact target T-cell sensitivity to Treg suppression. Mice deficient in the signalling molecules Cbl-b, NFATc1, c3, and TRAF6 all develop multi-organ inflammatory disease, which highlights the important roles of these molecules in T-cell homeostasis. The loss of each signalling molecule did not impact on the function of Tregs but rendered the non-regulatory CD4+ population resistant to Treg suppression.111–113 Mechanistically, these signal deficiencies all result in a similar phenotype: deficient T cells display CD28-independent proliferation probably mediated via hyperactivation of the phosphatidyl inositol 3-kinase (PI3K)/ the serine/threonine protein kinase (AKT) pathway. Indeed, in the TRAF6 studies, overexpression of the PI3K-inhibitor, phosphatase and tensin homolog (PTEN) restored the ability of the target T cells to be suppressed113 so the degree of activation of the PI3K pathway appears to regulate target T-cell sensitivity to Treg suppression.114 It is tempting to speculate that IL-6 may also confer target T-cell resistance to suppression via this same pathway given the well-established ability of IL-6 to activate AKT via PI3K.115
In all three examples of signalling deficiencies that confer resistance to Treg suppression there is a T-cell hyperactivity component, therefore the data need to be interpreted with caution. The coincident loss of signalling molecules and the loss of suppression could indicate that those signal components are critical in the targets to transduce the Treg suppressive signal. For instance, nuclear factor of activated T cells (NFAT) signalling in isolation confers an anergic state on T cells as the result of the expression of a distinct group of NFAT target genes, including E3 ligases.116,117 Thus Tregs may qualitatively modulate TCR signals to alter target T-cell gene expression.50 Alternatively, the loss of the signalling molecules may simply alter the activation kinetics of the deficient targets, enabling them to escape suppression. Indeed, wild-type target T cells are susceptible to suppression in a narrow kinetic window, the first 6–12 hr of activation, but become resistant thereafter.48 Therefore a more rapid time to activation of deficient target T cells may give them a competitive advantage.
Physiological relevance
The most informative data on signals that modulate Treg function will most likely come from in vivo situations where immune responses are either disregulated (tissue-specific autoimmunity) or overly regulated (tumours). Over the past few years there have been a plethora of studies on Treg number and function in autoimmune diseases in mice and man but global Treg deficiencies have not been widespread. However, more recently a number of reports have suggested that Treg activity may indeed be altered at sites of inflammation. The cytokines IL-6 and TNF are emerging as major negative regulators of Treg function either by directly inhibiting Tregs or rendering target cells resistant to suppression. In experimental autoimmune encephalomyelitis, Tregs could be found in the central nervous system but, although these Tregs suppressed naive T-cell activation, they failed to control IL-6- and TNF-secreting central nervous system-derived encephalitogenic effector T cells.118 Similarly, Treg function was compromised in patients with active systemic lupus erythematosus but not in those patients whose disease was inactive.119 Strikingly, the functional defects were reversed if the Tregs were first activated in vitro.119 These reversible Treg defects suggest that Tregs are not intrinsically defective in systemic lupus erythematosus but function poorly during the systemic inflammation associated with active disease. TNF is a strong candidate for the dampening of Treg function in inflammatory sites. Indeed, compromised Treg function in rheumatoid arthritis can be reversed by anti-TNF-α therapy.120
Concluding remarks
In the past few years we have seen a number of exciting new studies that highlight distinct facets of Treg suppression. The relative contribution of these disparate inhibitory mechanisms to distinct immune responses remains to be elucidated. A target T cell may be differentially sensitive to distinct Treg mechanisms depending on when and where it encounters the Treg. Given that regulation by Tregs is a continuous process and and the result of multiple factors we suggest that the outcome of a given immune challenge will be controlled by the degree of target cell activation. Therefore a productive immune response requires the provision of signals that enable immune effectors to escape regulatory T-cell control.
Acknowledgments
D.J.F. is supported by grants from the Juvenile Diabetes Research Foundation and the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (R01 AI070826, U19 AI056390 Autoimmunity Centers of Excellence). D.K.S. is supported by NIH T32-A107285.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Special regulatory T cell review: how i became a T suppressor/regulatory cell maven. Immunology. 2008;123:3–5. doi: 10.1111/j.1365-2567.2007.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain RN. Special regulatory T-cell review: a rose by any other name: from suppressor T cells to Tregs, approbation to unbridled enthusiasm. Immunology. 2008;123:20–7. doi: 10.1111/j.1365-2567.2007.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penhale WJ, Irvine WJ, Inglis JR, Farmer A. Thyroiditis in T cell-depleted rats: suppression of the autoallergic response by reconstitution with normal lymphoid cells. Clin Exp Immunol. 1976;25:6–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J Autoimmun. 1996;9:211–20. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 6.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–36. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 8.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 9.Cobbold SP, Adams E, Graca L, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–55. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 18.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 20.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–5. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Seddon B, Mason D. The third function of the thymus. Immunol Today. 2000;21:95–9. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 23.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 25.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+ CD4+ CD25+ T cells. Immunity. 2006;25:249–59. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–41. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 30.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 33.Shen S, Ding Y, Tadokoro CE, Olivares-Villagomez D, Camps-Ramirez M, Curotto de Lafaille MA, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. J Clin Invest. 2005;115:3517–26. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia M, Blazar BR, Roncarolo MG. The puzzling world of murine T regulatory cells. Microbes Infect. 2002;4:559–66. doi: 10.1016/s1286-4579(02)01573-3. [DOI] [PubMed] [Google Scholar]
- 36.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–62. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZM, O'shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, Roncarolo MG, Blazar BR. IL-10 and TGF-beta induce alloreactive CD4+ CD25− T cells to acquire regulatory cell function. Blood. 2003;101:5076–83. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 38.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 39.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 42.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 43.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 45.Pennington DJ, Silva-Santos B, Silberzahn T, et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–7. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 46.Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 48.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–80. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- 49.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+ CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 50.Sukiennicki TL, Fowell DJ. Distinct molecular program imposed on CD4+ T cell targets by CD4+ CD25+ regulatory T cells. J Immunol. 2006;177:6952–61. doi: 10.4049/jimmunol.177.10.6952. [DOI] [PubMed] [Google Scholar]
- 51.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–11. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–55. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 54.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–65. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 56.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+ CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–51. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 57.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–66. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stassen M, Jonuleit H, Muller C, Klein M, Richter C, Bopp T, Schmitt S, Schmitt E. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol. 2004;173:267–74. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- 59.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+ CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 60.Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Becourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+ CD25+ cells in vivo. J Immunol. 2004;172:3391–8. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- 61.DiPaolo RJ, Glass DD, Bijwaard KE, Shevach EM. CD4+ CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–42. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 62.Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–64. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- 63.Siegmund K, Feuerer M, Siewert C, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–41. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–7. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+ CD25+ T cells lyse antigen-presenting B cells by Fas–Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–12. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 70.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+ CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bodor J, Fehervari Z, Diamond B, Sakaguchi S. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells. Eur J Immunol. 2007;37:884–95. doi: 10.1002/eji.200636510. [DOI] [PubMed] [Google Scholar]
- 75.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–23. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 76.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+ CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–83. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 79.Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 80.Bodor J, Bodorova J, Gress RE. Suppression of T cell function: a potential role for transcriptional repressor ICER. J Leukoc Biol. 2000;67:774–9. doi: 10.1002/jlb.67.6.774. [DOI] [PubMed] [Google Scholar]
- 81.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 83.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hershfield MS. New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. Eur J Immunol. 2005;35:25–30. doi: 10.1002/eji.200425738. [DOI] [PubMed] [Google Scholar]
- 85.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 86.Novak TJ, Rothenberg EV. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci USA. 1990;87:9353–7. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 91.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886–95. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 93.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 94.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–8. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 95.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 96.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–9. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 97.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 98.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, Stockinger B. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol. 2005;17:279–88. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 99.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4(+) T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 100.Ge Q, Bai A, Jones B, Eisen HN, Chen J. Competition for self-peptide–MHC complexes and cytokines between naive and memory CD8+ T cells expressing the same or different T cell receptors. Proc Natl Acad Sci USA. 2004;101:3041–6. doi: 10.1073/pnas.0307339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 102.Poo WJ, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–80. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- 103.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci USA. 1991;88:775–9. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu G, Zhao Y. Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology. 2007;122:149–56. doi: 10.1111/j.1365-2567.2007.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+ CD25 hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–11. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 107.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–9. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 108.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+ CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 109.Shevach EM, Stephens GL. The GITR–GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–8. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 110.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 111.Bopp T, Palmetshofer A, Serfling E, et al. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:181–7. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+ CD25+ regulatory T cells and TGF-beta in Cbl-b–/– mice. J Immunol. 2004;173:1059–65. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 113.King CG, Kobayashi T, Cejas PJ, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–92. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 114.Wohlfert EA, Clark RB. ‘Vive la Resistance!’– the PI3K-Akt pathway can determine target sensitivity to regulatory T cell suppression. Trends Immunol. 2007;28:154–60. doi: 10.1016/j.it.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem. 1999;274:23013–9. doi: 10.1074/jbc.274.33.23013. [DOI] [PubMed] [Google Scholar]
- 116.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–31. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 117.Heissmeyer V, Macian F, Im SH, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 118.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 120.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]