Abstract
Redox-sensitive transcriptional regulator redox factor-1 (Ref-1) is induced by oxidative stress and protects cells against it. However, the function of Ref-1 in regulating nitric oxide (NO) synthesis in macrophages has not been defined. We investigated the role of Ref-1 related to the regulation of NO synthesis in lipopolysaccharide (LPS)-stimulated macrophage RAW 264.7 cells. LPS stimulates the up-regulation and nuclear translocation of Ref-1 in macrophages. Importantly, Ref-1-deficient macrophages using a small interfering RNA did not stimulate inducible NO synthase (iNOS) expression as well as nuclear factor-κB nuclear translocation by stimulation with LPS. When the cells were pretreated with diphenyleneiodonium or p47phox small interfering RNA for inhibition of NADPH oxidase activity, LPS did not stimulate the nuclear translocation of Ref-1. We next asked whether reactive oxygen species are sufficient for the nuclear translocation of Ref-1 in macrophages. The direct use of H2O2 stimulated the translocation to the nucleus of nuclear factor-κB, but not Ref-1 and antioxidant N-acetyl cysteine did not inhibit the LPS-stimulated nuclear translocation of Ref-1. These data suggest that Ref-1 nuclear translocation in LPS-stimulated macrophages requires the activation of other signalling molecules aside from reactive oxygen species followed by the activation of NADPH oxidase.
Keywords: nitric oxide, lipopolysaccharide, redox factor-1, reactive oxygen species, NADPH oxidase
Introduction
Activated macrophages release nitric oxide (NO), which is a multifunctional biomolecule involved in a variety of physiological and pathological processes.1,2 In contrast to the effects of protection and anti-inflammation, massive production of NO has been associated with oxidative stress3,4 and detrimental effects have occurred during septic shock, autoimmune disease and persistent local inflammatory processes.2,5,6 Moreover, NO mediates reactions with proteins and nucleic acids, and causes apoptosis of macrophages. Nitric oxide-mediated apoptosis is generally considered to occur through DNA or mitochondrial damage.7 Irrespective of numerous studies on the signalling network for NO synthesis, the intracellular mechanism involved in the pivotal double-edged role of NO is still to be elucidated.
Inducible NO synthase (iNOS) expression is regulated mainly at the transcription level by the activation of several transcription factors that bind to the promoter region of the iNOS gene, such as nuclear factor-κB (NF-κB), signal transducer and activator of transcription-1 (STAT1) and interferon regulatory factor-1 (IRF-1).8–10 In particular, NF-κB acts as a critical activator in the expression of iNOS,8,11 the DNA binding activity of which is regulated by a redox (reduction/oxidation) mechanism.12 In macrophages, the bacterial product lipopolysaccharide (LPS) causes the release of reactive oxygen species (ROS) through the assembly of NADPH oxidase. This multimeric protein complex is composed of membrane-bound proteins (p91phox and p22phox) and cytosolic factors (GTPase Rac1, p67phox, and p47phox)13 and is essential for macrophage function.
Redox factor-1 (Ref-1), also known as APE,14 HAP115 and APEX,16 is a ubiquitous bifunctional protein with both apurinic/apyrimidinic endonuclease DNA repair activity and nuclear reducing activity.7,17 Ref-1 plays a fundamental role in DNA repair and its activity is considered to be the rate-limiting step in the process of base excision repair after oxidative damage.18 In addition to playing a role in DNA repair, Ref-1 functions as a nuclear reducing factor that promotes the DNA-binding properties of many redox-sensitive transcription factors, such as NF-κB, AP-1, p53, Egr-1 and c-Myb.19–21 The ability of Ref-1 to activate the proteins involved in the cellular response to various stresses suggests that Ref-1 may play a role in cell survival signalling pathways.
Interestingly, Ref-1 is regulated by various stresses such as hypoxia, radical species, ionizing radiation, and DNA-damaging drugs. Several studies have reported that stress signals lead to increased expression of Ref-1 and/or its nuclear translocation.18,22,23 Although Ref-1 has a nuclear localizing signal at its N terminus, numerous studies have also demonstrated its distribution in the cytoplasm.14,18 Moreover, differential cellular and subcellular expression patterns of Ref-124,25 suggest that Ref-1 also has a potential physiological role in the cytoplasm. Notably, Ref-1 has been observed in the cytoplasm of metabolically active cells such as spermatocytes and hippocampal cells. Ref-1 was also distributed in the cytoplasm of cells, including macrophages, which are constantly confronted with oxidative stress. These data possibly reflect a significant cytoplasmic role for Ref-1 in cellular responses to the balance of the redox milieu.
However, the role of Ref-1 in the regulation of iNOS expression and its importance in macrophages are not well known. The present study was undertaken to elucidate the exact molecular mechanisms underlying the regulation of the signalling network by Ref-1 during macrophage activation. We demonstrate that NADPH oxidase activity is responsible for LPS-induced Ref-1 nuclear translocation, not only ROS, and that Ref-1 regulates LPS-induced NO synthesis via regulating the activation of a redox-sensitive transcription factor NF-κB in RAW 264.7 macrophages.
Materials and methods
Materials
Antibodies for Ref-1, iNOS, NF-κB p65, and p47phox were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phenol-extracted Salmonella enteritidis LPS, protease inhibitor cocktail, bicinchoninic acid, sulphanilamide, N-(1-naphthyl)-ethylenediamine dihydrochloride, phosphoric acid (H3PO4), sodium nitrite (NaNO2), hydrogen peroxide (H2O2), N-acetyl cysteine (NAC), and diphenyleneiodonium (DPI) were all obtained from Sigma-Aldrich Chemical Co. (St Louis, MO). RPMI containing l-arginine (200 mg/l), Opti-MEM media, fetal bovine serum (FBS) and other tissue culture reagents were purchased from Life Technologies (Gaithersburg, MD) and siPORT Amine was obtained from Ambion (Austin, TX). Diaminofluorescein-2 diacetate (DAF2-DA) and 2′-7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) were obtained from Molecular Probes (Eugene, OR). The secondary fluorescein isothiocyanate (FITC)-conjugated antibodies and enhanced chemiluminescence (ECL) Western blotting kits were obtained from Amersham Pharmacia Biotech (Piscataway, NJ).
Macrophage culture
The murine monocyte/macrophage cell line RAW 264.7 was cultured in 75-cm2 plastic flasks (Falcon-Becton Dickinson Labwares, Franklin Lakes, NJ) and maintained at 37° with a 5% CO2 in air atmosphere in RPMI-1640 supplemented with 10% (volume/volume) heat-inactivated FBS and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). The cells were grown to 80% confluence, scraped and then cultured in either 24-well plates (5 × 105 cells/well) or six-well plates (2 × 106 cells/dish) for 12–18 hr before an experiment.
Measurement of NO production
To measure fluorescence intensity, LPS-stimulated cells were washed twice with Krebs–Ringer phosphate (KRP) buffer (120 mm NaCl; 4·8 mm KCl; 0·54 mm CaCl2; 1·2 mm MgSO4; 11 mm glucose and 15·9 mm sodium phosphate, pH 7·2), and then KRP buffer containing 10 μm DAF2 and 1 mm l-arginine in the presence or absence of the NOS inhibitor l-NAME (1 mm) was added.26 After incubation for 1 hr, supernatants were collected and the fluorescence was measured with a fluorescence microplate reader (Titertek Fluoroscan II; Flow Laboratories, North Ryde, Australia) calibrated for excitation at 485 nm and emission at 538 nm. To measure the NO metabolite nitrite (), 100 μl macrophage culture supernatant was collected, mixed with an equal volume of the Griess reagent (1% sulphanilamide; 0·1%N-(1-naphthyl)-ethylenediamine dihydrochloride; 2·5% H3PO4) and incubated for 10 min at room temperature. Nitrite concentration was determined by measuring the absorbance at 540 nm in an Emax 96-well microtest plate spectrophotometer (Molecular Devices, Menlo Park, CA), as described previously.27
Preparation and transfection of small interfering RNA (siRNA)
Twenty-one-nucleotide RNA with 3′-dTdT overhangs was synthesized to interfere exclusively with Ref-1 and p47phox mRNA by Ambion (Austin, TX) in the ‘ready-to-use’ option. As a negative control, the same nucleotides were scrambled to form a non-genomic combination (controlled by a basic local alignment search tool) (scrambled RNA; SCR). The AA-N19 mRNA targets were Ref-1 target sequence (5′-CUUCGAGCCUGGAUUAAGA-3′) and p47phox target sequence (5′-UAACGUAGCUGACAUCACA-3′). Cells in the exponential phase of growth were plated in six-well plates at 5 × 105 cells/well, grown for 24 hr, and then transfected with 30 nm siRNA per well using siPORT Amine and Opti-MEM media according to the manufacturer's recommended protocol. The concentration of siRNA was chosen based on dose–response studies.
Measurement of intracellular ROS
Intracellular ROS was detected by monitoring changes in the fluorescence of the ROS-sensitive fluorophore H2DCF-DA as previously described.28 The fluorescence was measured after 10 min of incubation with H2DCF-DA using a Zeiss LSM 510 laser-scanning confocal microscope (Göttingen, Germany). Absolute fluorescence intensities were determined from the same numbers of cells in a randomly selected area.
Immunofluorescence staining
Sorted cells were cultured on collagen-coated four-well glass chamber slides for 48 hr in RPMI with 10% FBS. Cells were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) followed by permeabilization in 0·1% saponin/PBS. Primary antibodies, rabbit polyclonal anti-Ref-1 (0·13 μg/ml in 1% BSA/PBS) or mouse monoclonal anti-NF-κB p65 (0·13 μg/ml in 1% donkey serum/0·3% Tween-20/PBS), were added to the cells for 30 min at 37°. For secondary labelling, cells were incubated with FITC-conjugated secondary antibody or Texas Red-conjugated secondary antibody for 30 min at 37°. Nuclei were counterstained using propidium iodide (PI). After staining, coverslips were mounted using Prolong anti-fade reagent. Fluorescent images were observed and analysed under a laser-scanning confocal microscope.
Preparation of nuclear extracts
Cell stimulation was terminated by the addition of ice-cold PBS, and a modified procedure based on the method of Diaz-Guerra et al.11 and Schreiber et al.29 was used. Cells were harvested, washed with PBS, resuspended in 200 μl hypotonic lysis buffer (10 mm HEPES, pH 7·9; 10 mm KCl and 1·5 mm MgCl2) supplemented with a protease inhibitor cocktail, and incubated for 15 min at 4°. Cells were lysed by the addition of 25 μl 2·5% nonidet P-40. After 10 min, the tubes were gently vortexed for 15 seconds and nuclei were collected by centrifugation at 8000 g for 15 min. The supernatants were stored at −80° (cytosolic extracts) and the pellets were resuspended in extraction buffer (10 mm HEPES, pH 7·9; 100 mm NaCl; 1·5 mm MgCl2; 0·1 mm ethylenediaminetetraacetic acid and 0·1 mm dithiothreitol) supplemented with protease inhibitor cocktail. After gently shaking for 30 min at 4°, samples were centrifuged at 13 000 g for 15 min. The protein levels in the samples were then quantified using the colorimetric method with bicinchoninic acid solution. All the steps of cell fractionation were carried out at 4°.
Western blot analysis
Equivalent amounts of protein were loaded on a 12% sodium dodecyl sulphate–polyacrylamide gel for electrophoresis. The gels were transferred to nitrocellulose membranes using an electroblotting apparatus (Bio-Rad, Richmond, CA) and reacted with the appropriate primary antibody according to standard methods. Bound immunocomplexes were visualized on X-ray film by ECL reagents (Amersham Pharmacia Biotech, Piscataway, NJ). β-Actin was used as an internal control to monitor equal protein sample loading and, to avoid contamination of the nucleus and the cytoplasm, antibodies against β-actin and histone were used for the nuclear and cytoplasmic proteins, respectively.
Statistics or reproducibility
Data in figures are the mean ± standard deviation (SD) of at least three different experiments performed in triplicate. Differences between means were compared using the Student's t-test and P values < 0·05 were considered significant.
Results
LPS stimulates up-regulation and nuclear translocation of Ref-1 in RAW 264.7 cells
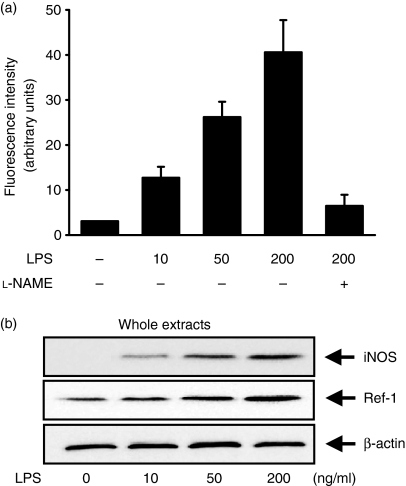
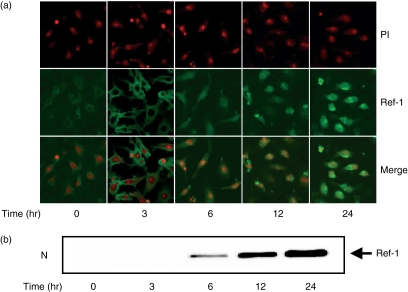
To assess the role of Ref-1 in macrophage activation, we first examined the level of Ref-1 expression in macrophage RAW 264.7 cells stimulated with LPS. Cells were stimulated with various concentrations of LPS, the most notable activator for the activation including tumour necrosis factor-α secretion and NO synthesis in macrophages, for 12 or 24 hr. The amount of intracellular NO secreted was determined from the measurement of fluorescence intensity of the specific NO indicator DAF2. As shown in Fig. 1(a), an LPS concentration-dependent increase of NO production was observed in macrophages stimulated with LPS. To determine whether the macrophage activation by LPS was correlated with Ref-1 expression, we used Western blotting to analyse the amount of Ref-1 induced. As shown in Fig. 1(b), LPS increased total cellular Ref-1 compared to the control in RAW 264.7 cells. Next, the subcellular distribution of Ref-1 was determined by two-colour analysis under confocal microscopy (Fig. 2a). In unstimulated cells Ref-1 was detected weakly in the cytoplasm (green fluorescence) and not in the nucleus, which was stained red by PI. At 3 hr after LPS treatment, there was no Ref-1 nuclear translocation in cells stimulated with LPS. At this time, the green fluorescence became more intense in the cell cytoplasm compared with the level of fluorescence in the control cells, which suggested that there was de novo protein synthesis. Stimulation with LPS induced Ref-1 translocation to the nucleus, as could be seen by the considerable increase in orange fluorescence indicative of colocalization in the nucleus 6 hr after treatment with LPS (Fig. 2a). Western blot analysis was performed on nuclear extracts from parallel experiments. As shown in Fig. 2(b), the amount of nuclear Ref-1 was increased markedly at 6 hr after treatment. These data demonstrate that LPS stimulates the up-regulation and nuclear translocation of Ref-1 in activated macrophages.
Figure 1.
LPS increases NO production and Ref-1 protein levels in the macrophage cell line RAW 264.7. (a) Fluorescence intensity by NO indicator. Cells were stimulated for 12 hr with the indicated concentrations of LPS. Then, the cells were washed and incubated for another 1 hr in KRP buffer containing DAF2 in the presence or absence of l-NAME (1 mm). In supernatants, NO production was measured with a fluorescence microplate reader. (b) After stimulation with LPS for 24 hr, the cell lysates were analysed by Western blotting using antibodies for iNOS and Ref-1. β-Actin was used as an internal control to monitor equal protein loading. For (a), values are presented as means ± SD of three independent cell preparations. Differences between means were compared using Student's t-test (P < 0·05).
Figure 2.
LPS activates Ref-1 nuclear translocation in macrophages. (a) Cells were stimulated with LPS (200 ng/ml) at the indicated times. The cells were fixed, then reacted with Ref-1 antibody and PI for analysis by confocal microscopy. The upper panels show nuclear staining with PI (red), the middle panels show immunoreactivity of anti-Ref-1 antibody (green), the bottom panels show superposition of PI and anti-Ref-1 immunoreactivity (orange). (b) Cells stimulated with LPS (200 ng/ml) were harvested at each time-point and nuclear protein was isolated for Western blot analysis.
Ref-1 acts as a key regulator for iNOS expression in LPS-stimulated macrophages
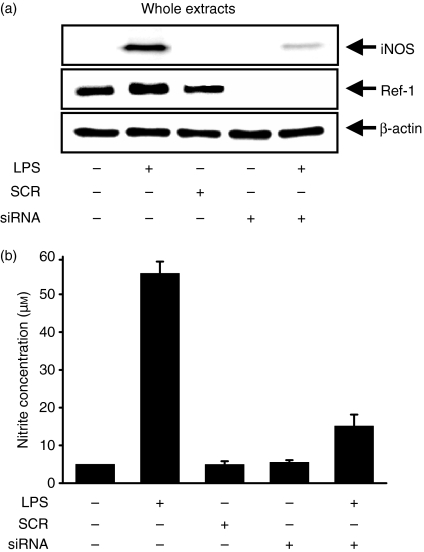
The role of Ref-1 in the regulation of NO synthesis in LPS-stimulated macrophages was investigated by knockdown of Ref-1. Surprisingly, siRNA to Ref-1 significantly inhibited iNOS expression (Fig. 3a) and reduced the amount of NO, as measured by the method of Griess after conversion to nitrite (Fig. 3b), in LPS-stimulated macrophages. Ref-1 is therefore an important regulator of iNOS expression and NO synthesis in RAW 264.7 cells stimulated with LPS.
Figure 3.
Ref-1 depletion by siRNA prevents LPS-induced NO synthesis in macrophages. (a) Cells were transfected with siRNA for the silencing of the Ref-1 gene, and were stimulated with 200 ng/ml LPS for 24 hr. The cell lysates were analysed by Western blotting using antibodies for iNOS and Ref-1. β-Actin was used as an internal control to monitor equal protein loading. (b) Supernatants were collected, and the amount of nitrite released by macrophages was measured by the method of Griess. Values are presented as means ± SD of three independent cell preparations. Differences between means were compared using the Student's t-test (P < 0·05).
Ref-1 mediates translocation of NF-κB to the nucleus in LPS-stimulated macrophages
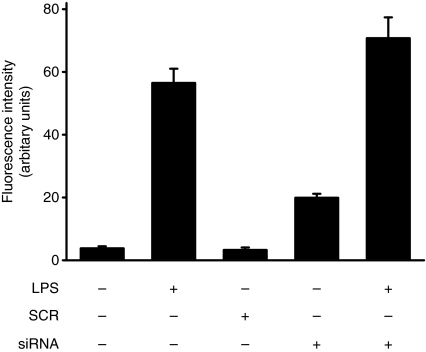
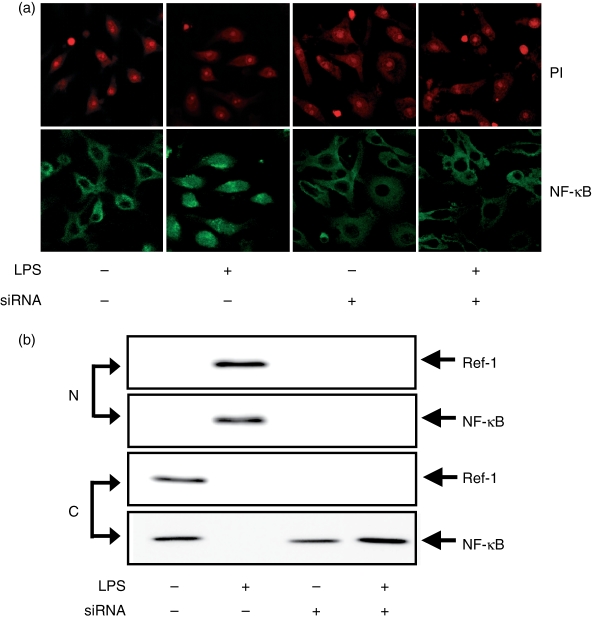
Generally, LPS-stimulated macrophages generate ROS via the activation of a NADPH oxidase, and ROS plays an important role in NF-κB activation. To determine whether the deficiency of Ref-1 prevents NF-κB nuclear translocation in macrophages stimulated with LPS, immunofluorescence staining was conducted. As shown in Fig. 4, silencing of Ref-1 resulted in a slight increase of intracellular ROS levels in both macrophages treated with LPS and untreated cells. However, the silencing of Ref-1 did not stimulate nuclear localization of NF-κB even in LPS-treated cells (Fig. 5a,b). Ref-1 is therefore a critical factor in the process of the nuclear translocation required for NF-κB activation in LPS-stimulated macrophages.
Figure 4.
Ref-1 depletion by siRNA increases ROS generation in macrophages. Cells transfected with Ref-1 siRNA were stimulated with 200 ng/ml LPS for 1 hr and then cells were incubated with 10 μm H2DCF-DA for an additional 10 min. Cells were washed twice with PBS, and the intracellular levels of ROS were analysed by confocal microscope. Absolute DCF fluorescence intensities were determined from the same numbers of cells in a randomly selected area. Values are means ± SD of experiments (n ≥ 3). Differences between means were compared using the Student's t-test (P < 0·05).
Figure 5.
Ref-1 depletion by siRNA prevents NF-κB nuclear translocation in LPS-stimulated macrophages. (a) Cells transfected with Ref-1 siRNA were stimulated for 24 hr as indicated in Fig. 3. The cells were fixed, reacted with NF-κB p65 antibody (green) and PI (red) for analysis by confocal microscopy. (b) Nuclear (N) and cytoplasmic (C) proteins were isolated and used for Western blot analysis.
NADPH oxidase activity is responsible for LPS-induced Ref-1 nuclear translocation and iNOS expression
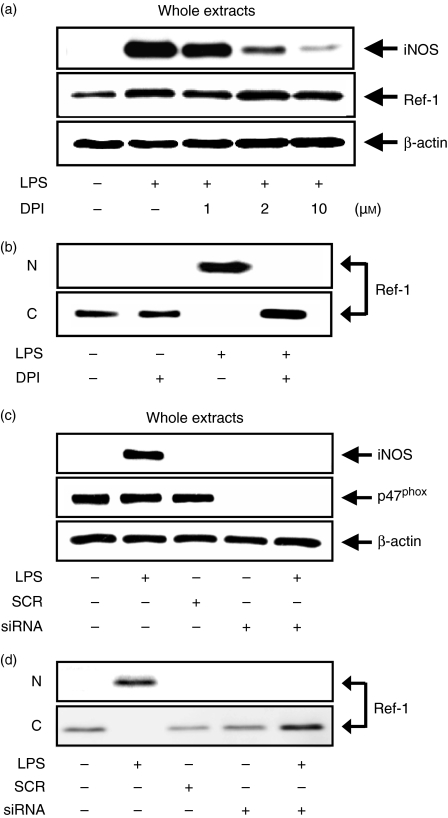
Next, we assessed the role of NADPH oxidase in regulating the nuclear translocation of Ref-1 in macrophages stimulated with LPS. LPS-stimulated macrophages initiate NO synthesis via the activation of the NADPH oxidase involved in ROS generation and the modulation of enzymes that are downstream of NADPH oxidase. Treatment with the NADPH oxidase inhibitor DPI significantly reduced the intracellular levels of ROS in RAW 264.7 cells stimulated with LPS (data not shown). DPI suppressed iNOS expression in a dose-dependent manner, as expected, but not Ref-1 (Fig. 6a). Interestingly, DPI inhibited the nuclear translocation of Ref-1 that was stimulated by LPS (Fig. 6b). We also used p47phox siRNA for the inhibition of NADPH oxidase activity and efficiently silenced the p47phox gene. In the p47phox-deficient macrophages, LPS did not induce ROS generation (data not shown). Along with these results, the silencing of the p47phox gene also significantly inhibited iNOS expression (Fig. 6c) and blocked the translocation of Ref-1 from cytosol to nucleus (Fig. 6d) in LPS-stimulated macrophages. These data suggest that Ref-1 nuclear translocation and iNOS expression in LPS-stimulated macrophages require the activation of NADPH oxidase.
Figure 6.
Blockage of NADPH oxidase activity inhibits Ref-1 nuclear translocation and iNOS expression in LPS-stimulated macrophages. (a) Cells were pretreated with the indicated concentrations of DPI for 30 min, and then stimulated with 200 ng/ml LPS for another 24 hr. The cell lysates were analysed by Western blotting using antibodies for iNOS and Ref-1. (b) Nuclear (N) and cytoplasmic (C) proteins were isolated and used for Western blot analysis. (c) Cells were transfected with siRNA for the silencing of the p47phox gene. Then, cells were stimulated with 200 ng/ml LPS for 24 hr. The cell lysates were analysed by Western blotting using antibodies for iNOS and p47phox. (d) Nuclear (N) and cytoplasmic (C) proteins were isolated and used for Western blot analysis. β-Actin was used as an internal control to monitor equal protein loading. For (b) and (d), antibodies against β-actin and histone were used for contamination of the nuclear and cytoplasmic proteins, respectively.
ROS is not sufficient for the nuclear translocation of Ref-1 in macrophages
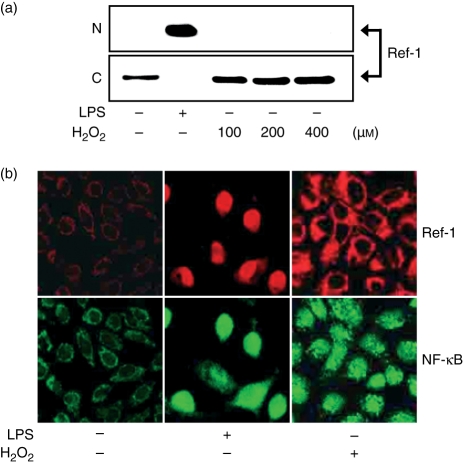
Only ROS generated by NADPH oxidase was sufficient for nuclear translocation of Ref-1 in LPS-stimulated macrophages. Antioxidant NAC was utilized to inhibit the generation of LPS-stimulated ROS. Pretreatment of the cells with 5 or 10 mm NAC for 30 min before LPS stimulation did not inhibit the LPS-induced nuclear translocation of Ref-1 (data not shown). We also investigated the effect of H2O2 on Ref-1 nuclear translocation in macrophages. It is well documented that H2O2, an agent commonly produced during the oxidative stress generated in phagocytic cells, activates the DNA binding of the transcription factor NF-κB in a variety of cells including macrophages. Cells were stimulated for 24 hr with LPS or increasing concentrations of H2O2, and nuclear and cytoplasmic extracts were analysed by Western blotting. As shown in Fig. 7(a,b), H2O2 itself induced Ref-1 expression in macrophages. However, H2O2 at the concentrations tested did not stimulate nuclear translocation of Ref-1 (Fig. 7b). These data suggest that ROS alone generated by activation of NADPH oxidase is insufficient for Ref-1 nuclear translocation in LPS-stimulated macrophages, possibly reflecting that Ref-1 nuclear translocation in LPS-stimulated macrophages requires the activation of other signalling molecules downstream by the activation of NADPH oxidase.
Figure 7.
H2O2 does not activate Ref-1 nuclear translocation in macrophages. (a) Nuclear and cytoplasmic proteins were isolated in cells stimulated for 24 hr with 200 ng/ml LPS or the indicated concentrations of H2O2 and analysed by Western blotting using antibodies for Ref-1. Equivalent protein loading was confirmed by Ponceau S staining. (b) Cells were stimulated for 24 hr with 200 ng/ml LPS or 400 μm H2O2. The cells were fixed and reacted with antibody for Ref-1 and NF-κB p65 for analysis by confocal microscopy. The upper panels show immunoreactivity of anti-Ref-1 antibody (red), the bottom panels show immunoreactivity of anti-NF-κB p65 antibody (green).
Discussion
Reports to date have demonstrated that iNOS expression is induced by the endotoxin LPS and by cytokines such as interferon-γ (IFN-γ) and interleukin-2. Two upstream DNA regions of the iNOS promoter, the RI and RII domains, are required for the maximal promoter activation by LPS, and the RII domain mediates promoter trans-activation of IFN-γ. Both of these domains comprise multiple sequences homologous to those of the cis elements involved in transcriptional activation. Many data point to activation of NF-κB, a redox-sensitive transcription factor, as a critical role for iNOS gene expression in several cell types, including murine macrophages.8,11,30,31 The activation of NF-κB is induced by ROS produced from macrophages following stimulation with LPS and depends on the degradation of the corresponding IκBα and IκBβ, which maintain the inactivity of the NF-κB complex in the cytosol.32 The activation of NF-κB can also be induced by direct H2O2 exposure. However, H2O2 exposure cannot enhance iNOS expression and NO production without the addition of another stimulator such as IFN-γ.33,34
Recent studies have demonstrated that redox potential-based mechanisms play a critical role in the regulation of transcription factors.7,12,35 Redox regulation of transcription factors is largely based on the action of the Ref-1 protein. It was suggested that activation of transcription factors by Ref-1 occurs through reduction of cysteine residues in the DNA-binding domain,36 but the molecular mechanism involved in macrophage activation, including iNOS expression, remains to be elucidated. In this paper, we demonstrate that Ref-1 is induced and translocated from the cytoplasm into the nucleus in response to LPS stimulation, and the deficiency of Ref-1 fails to induce iNOS expression as well as NF-κB nuclear translocation by stimulation. Our present study provides the first evidence that Ref-1 is an essential factor for iNOS expression and NO synthesis in RAW 264.7 cells.
Ref-1 is described in the literature as a nuclear protein, and would therefore not be predicted to regulate cytoplasmic factors. However, it is also observed in the cytoplasm of a variety of cell types, including endothelial cells, hepatocytes and thyroid cells.25,28,37 Furthermore, differentiation of circulating monocytes to tissue macrophages is associated with a shift of Ref-1 expression from the nucleus to the cytoplasm with a concomitant decrease in phorbol ester-stimulated ROS generation,24,38 which implicates a role for cytoplasmic Ref-1 in the regulation of the macrophage oxidative burst. The observation that Ref-1 modulates the activity of a Rac1-regulated oxidase may also have relevance to immune cell function. Rac proteins regulate the activity of the NADPH oxidase that is responsible for the ROS generation in macrophages and neutrophils.39,40 Previous reports have shown that forced cytoplasmic overexpression of Ref-1 suppresses hypoxia/reoxygenation, LPS-induced oxidative stress, NF-κB activation, NO synthesis and apoptosis.28,41 This is achieved through modulation of NADPH oxidase-mediated ROS generation by higher cytoplasmic Ref-1 expression.
From the possible physiological relevance of our results, we can speculate that Ref-1 participates in regulation of iNOS gene expression in LPS-stimulated cells, favouring the co-operation of other stimuli to induce nuclear translocation of NF-κB. Our data show that LPS stimulates up-regulation and nuclear translocation of Ref-1 in murine macrophages, and NADPH oxidase activity is responsible for LPS-induced Ref-1 nuclear translocation and iNOS expression. When the cells were pretreated with DPI or p47phox siRNA for inhibition of NADPH oxidase activity, LPS did not stimulate the nuclear translocation of Ref-1. In addition, we wished to analyse whether ROS is sufficient for the nuclear translocation of Ref-1 in murine macrophages. Other investigators have demonstrated that nuclear translocation of Ref-1 is activated by oxidant stress in other cell types.18,42,43 Also, exogenous H2O2 has been reported to be a potent stimulus for translocation from cytoplasm to nucleus of Ref-1 in B lymphoid cells, Raji, and ARO cells.44,45 These findings do not fit with work from our laboratory, which demonstrates that the direct use of H2O2 stimulated the translocation to the nucleus of NF-κB in macrophages, but not Ref-1. In addition, antioxidant NAC did not inhibit the LPS-stimulated nuclear translocation of Ref-1. Our data suggest that ROS alone generated by activation of NADPH oxidase is insufficient for Ref-1 nuclear translocation in LPS-stimulated macrophages, possibly reflecting that Ref-1 nuclear translocation in macrophages requires the activation of other downstream signalling molecules by the activation of NADPH oxidase.
This report also focuses on the regulation by Ref-1 of NF-κB nuclear translocation as a mechanism for its effect on iNOS expression in LPS-stimulated macrophages. Recent work demonstrated that loss of Ref-1 significantly attenuates NF-κB signalling in the intact vasculature of Ref-1-deficient mice,46 indicating that Ref-1 is essential for maximal activation of NF-κB following treatment with LPS. Although the mutation of redox-sensitive cysteine 64 (cysteine 65 in human) or the deletion of the N-terminal 116 amino acid domain decreases NF-κB transcriptional activation,36,47 the functional role of Ref-1 in NF-κB nuclear translocation after stimulation is not known. We have shown here that silencing the Ref-1 gene inhibits iNOS expression through the inhibition of NF-κB nuclear translocation in LPS-stimulated macrophages. These accumulated lines of evidence provide a functional connection between the iNOS expression mediated by NF-κB and the generation of intracellular ROS via the activation of NADPH oxidase in the activation of macrophages.
In conclusion, we have demonstrated for the first time that Ref-1 is a critical intrinsic factor in LPS-stimulated iNOS expression via regulating nuclear translocation of NF-κB. Ref-1 is a clear candidate for the role of shuttle factor between the cytoplasm and the nucleus, being able to modify the cytoplasmic/nuclear redox potential of the cell. Indeed, the regulation of Ref-1 may play an essential role in regulating the development of activation and death in macrophages. Thus, for further studies it is worth considering that cytoplasmic Ref-1 may also, directly or indirectly, affect other steps in the NF-κB activation sequence, such as the activity of IκB kinases, binding affinity to IκB, or binding of other redox-sensitive factors such as thioredoxin, glutaredoxin and glutathione.
Acknowledgments
This work was supported by grant no. KRF-2004-005-E00007 from the Korea Research Foundation.
Glossary
Abbreviations
- DAF2-DA
diaminofluorescein-2 diacetate
- DPI
diphenyleneiodonium
- ECL
enhanced chemiluminescence
- H2DCF-DA
2′-7′-dichlorodihydrofluorescein diacetate
- iNOS
inducible NO synthase
- KRP
Krebs–Ringer phosphate
- NF-κB
nuclear factor-κB
- LPS
lipopolysaccharide
- NAC
N-acetyl cysteine
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PI
propidium iodide
- Ref-1
redox factor-1
- ROS
reactive oxygen species
- SCR
scrambled RNA
- siRNA
small interfering RNA
References
- 1.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 3.Sies H, Mehlhorn R. Mutagenicity of nitroxide-free radicals. Arch Biochem Biophys. 1986;251:393–6. doi: 10.1016/0003-9861(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 4.Ji Y, Akerboom TP, Sies H, Thomas JA. S-nitrosylation and S-glutathiolation of protein sulfhydryls by S-nitroso glutathione. Arch Biochem Biophys. 1999;362:67–78. doi: 10.1006/abbi.1998.1013. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–23. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan FM, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1996;8:872–7. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 8.Xie QW, Kashiwabar Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 9.Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon-γ and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–4. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-γ and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–84. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Guerra MJ, Velasco MM, Martin-Sanz P, Bosca L. Evidence for common mechanisms in the transcriptional control of type II nitric oxide synthase in isolated hepatocytes: requirement of NF-κB activation after stimulation with bacterial cell wall products and phorbol esters. J Biol Chem. 1996;271:30114–20. doi: 10.1074/jbc.271.47.30114. [DOI] [PubMed] [Google Scholar]
- 12.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase molecular interactions of oxidase proteins. J Leukoc Biol. 1996;60:677–91. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 14.Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci USA. 1991;88:11450–4. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robson CN, Hickson ID. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991;19:5519–23. doi: 10.1093/nar/19.20.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seki S, Akiyama K, Watanabe S, Hatsushika M, Ikeda S, Tsutsui K. cDNA and deduced amino acid sequence of a mouse DNA repair enzyme (APEX nuclease) with significant homology to Escherichia coli exonuclease III. J Biol Chem. 1991;266:20797–802. [PubMed] [Google Scholar]
- 17.Rothwell DG, Barzilay G, Gorman M, Morera S, Freemont P, Hickson ID. The structure and function of the HAP/Ref-1 protein. Oncol Res. 1997;9:275–80. [PubMed] [Google Scholar]
- 18.Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci USA. 1998;95:5061–6. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–70. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 21.Mitomo K, Nakayama K, Fujimoto K, Sun X, Seki S, Yamamoto K. Two different cellular redox systems regulate the DNA-binding activity of the p50 subunit of NF-kappa B in vitro. Gene. 1994;145:197–203. doi: 10.1016/0378-1119(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Yao KS, Xanthoudakis S, Curran T, O’Dwyer P. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol Cell Biol. 1994;14:5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosch S, Fritz G, Kaina B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 1998;58:4410–6. [PubMed] [Google Scholar]
- 24.Kakolyris S, Kaklamanis L, Giatromanolaki A, et al. Expression and subcellular localization of human AP endonuclease 1 (HAP1/Ref-1) protein: a basis for its role in human disease. Histopathology. 1998;33:561–9. doi: 10.1046/j.1365-2559.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 25.Duguid JR, Eble JN, Wilson TM, Kelley MR. Differential cellular and subcellular expression of the human multifunctional apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme. Cancer Res. 1995;55:6097–102. [PubMed] [Google Scholar]
- 26.Kojima H, Sakurai K, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem Pharm Bull (Tokyo) 1998;46:373–5. doi: 10.1248/cpb.46.373. [DOI] [PubMed] [Google Scholar]
- 27.Park YC, Jun CD, Kang HS, Kim HD, Kim HM, Chung HT. Role of intracellular calcium as a priming signal for the induction of nitric oxide synthesis in murine peritoneal macrophages. Immunology. 1996;87:296–302. doi: 10.1046/j.1365-2567.1996.456544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox-factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–25. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts”, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor BS, De Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–56. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 31.Hecker M, Preiss C, Klemm P, Busse R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophges: role of nuclear factor kappa B and interferon regulatory factor 1. Br J Pharmacol. 1996;118:2178–84. doi: 10.1111/j.1476-5381.1996.tb15660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–6. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 33.Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappaB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–15. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-κB activation: role of H2O2 and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide. 2001;5:504–13. doi: 10.1006/niox.2001.0367. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–74. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 36.Walker LJ, Robson CN, Black E, Gillespie D, Hickson ID. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993;13:5370–6. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tell G, Crivellato E, Pines A, et al. Mitochondrial localization of APE/Ref-1 in thyroid cells. Mutat Res. 2001;485:143–52. doi: 10.1016/s0921-8777(00)00068-9. [DOI] [PubMed] [Google Scholar]
- 38.Monick MM, Carter AB, Hunninghake GW. Human alveolar macrophages are markedly deficient in REF-1 and AP-1 DNA binding activity. J Biol Chem. 1999;274:18075–80. doi: 10.1074/jbc.274.25.18075. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Ono Y, Hirabayashi Y. Rapid and specific reactive oxygen species generation via NADPH oxidase activation during Fas-mediated apoptosis. FEBS Lett. 1998;425:209–12. doi: 10.1016/s0014-5793(98)00228-2. [DOI] [PubMed] [Google Scholar]
- 40.Lundqvist-Gustafsson H, Bengtsson T. Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J Leukoc Biol. 1999;65:196–204. doi: 10.1002/jlb.65.2.196. [DOI] [PubMed] [Google Scholar]
- 41.Yoo YH, Lim YJ, Park SE, Kim JM, Park YC. Overexpression of redox factor-1 negatively regulates NO synthesis and apoptosis in LPS-stimulated RAW 264.7 macrophages. FEBS Lett. 2004;556:39–42. doi: 10.1016/s0014-5793(03)01361-9. [DOI] [PubMed] [Google Scholar]
- 42.Tell G, Pellizzari L, Pucillo C, Puglisi F, Cesselli D, Kelley MR, Di Loreto C, Damante G. TSH controls Ref-1 nuclear translocation in thyroid cells. J Mol Endocrinol. 2000;24:383–90. doi: 10.1677/jme.0.0240383. [DOI] [PubMed] [Google Scholar]
- 43.Gillardon F, Bottiger B, Hossmann KA. Expression of nuclear redox factor ref-1 in the rat hippocampus following global ischemia induced by cardiac arrest. Brain Res Mol Brain Res. 1997;52:194–200. doi: 10.1016/s0169-328x(97)00237-4. [DOI] [PubMed] [Google Scholar]
- 44.Tell G, Zecca ZA, Pellizzari L, Spessotto P, Colombatti A, Kelley MR, Damante G, Pucillo C. An ‘environment to nucleus’ signaling system operates in B lymphocytes: redox status modulates BSAP/Pax-5 activation through Ref-1 nuclear translocation. Nucleic Acids Res. 2000;28:1099–105. doi: 10.1093/nar/28.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pines A, Perrone L, Bivi N, et al. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–94. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan Z, Basi D, Li Q, Mariash A, Xia YF, Geng JG, Kao E, Hall JL. Loss of redox factor 1 decreases NF-κB activity and increases susceptibility of endothelial cells to apoptosis. Arterioscler Thromb Vas Biol. 2005;25:96–101. doi: 10.1161/01.ATV.0000150418.14698.75. [DOI] [PubMed] [Google Scholar]
- 47.Hall JL, Wang X, Adamson V, Zhao Y, Gibbons GH. Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-κB-independent and -dependent pathways. Circ Res. 2001;88:1247–53. doi: 10.1161/hh1201.091796. [DOI] [PubMed] [Google Scholar]