Abstract
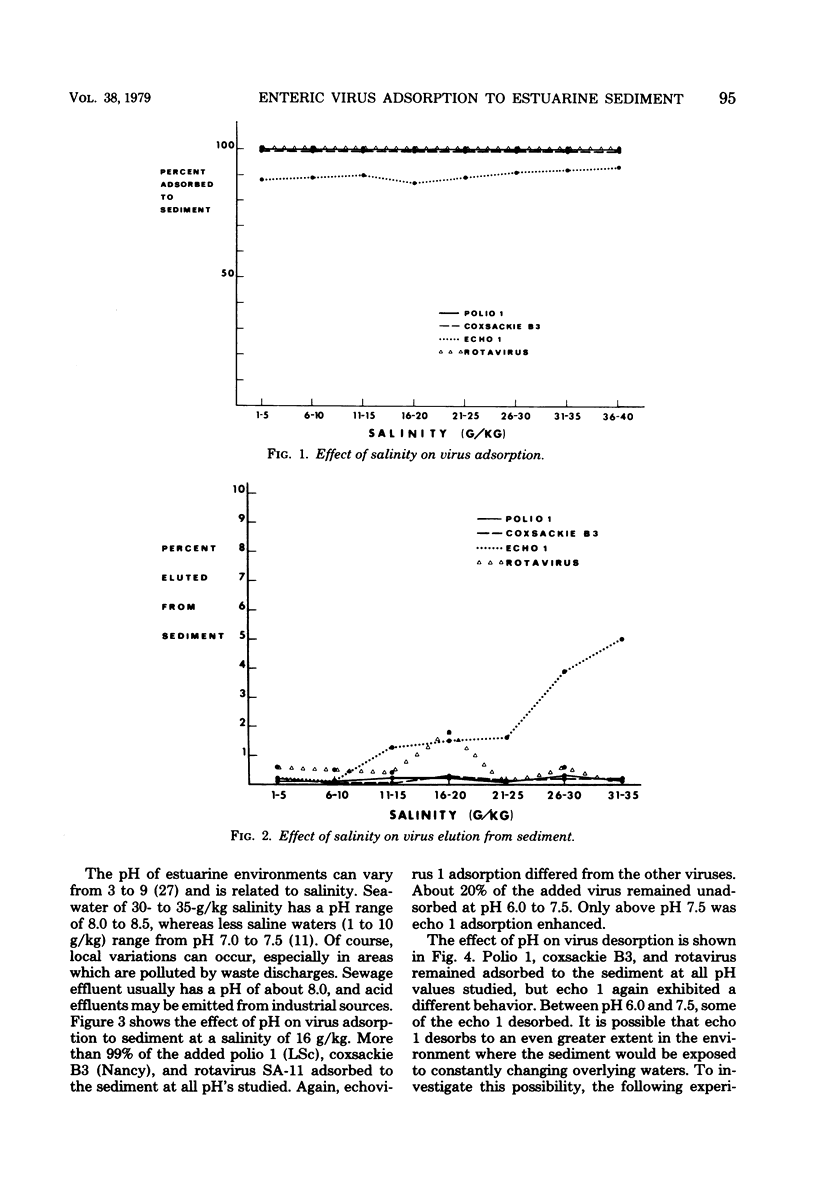
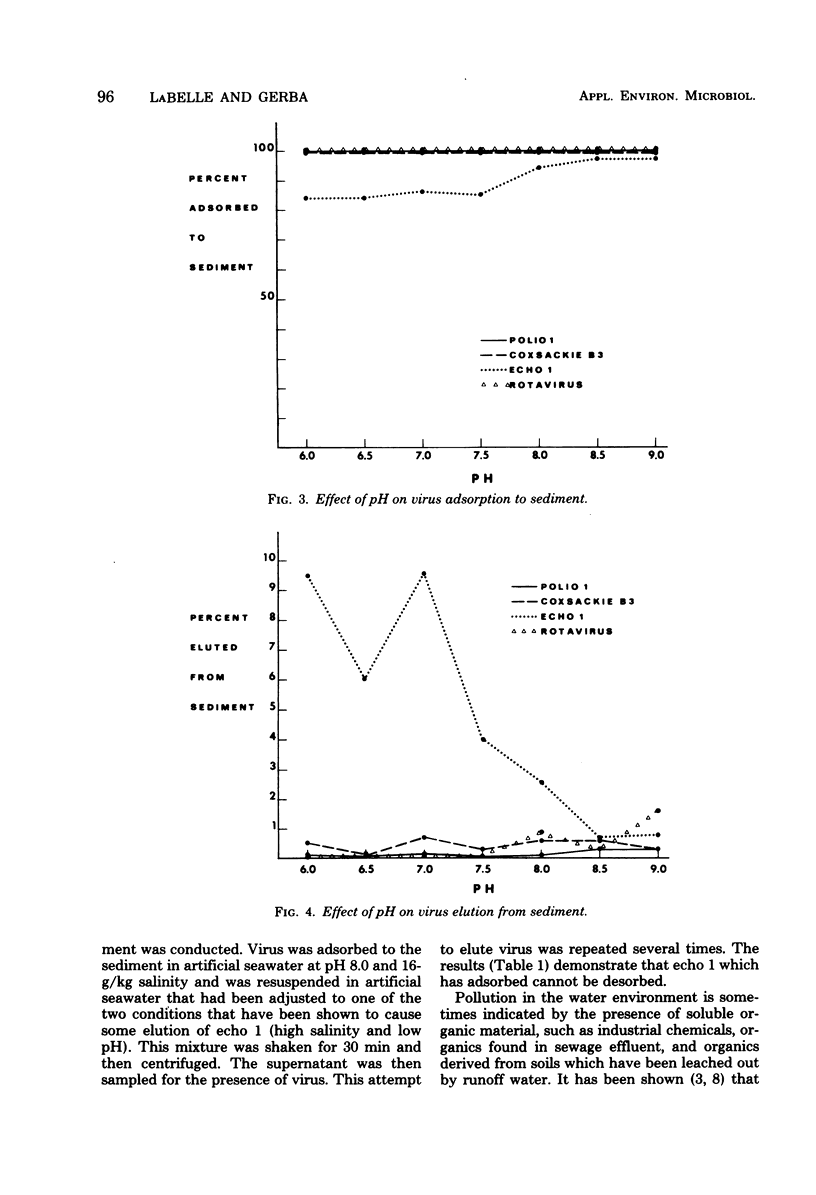
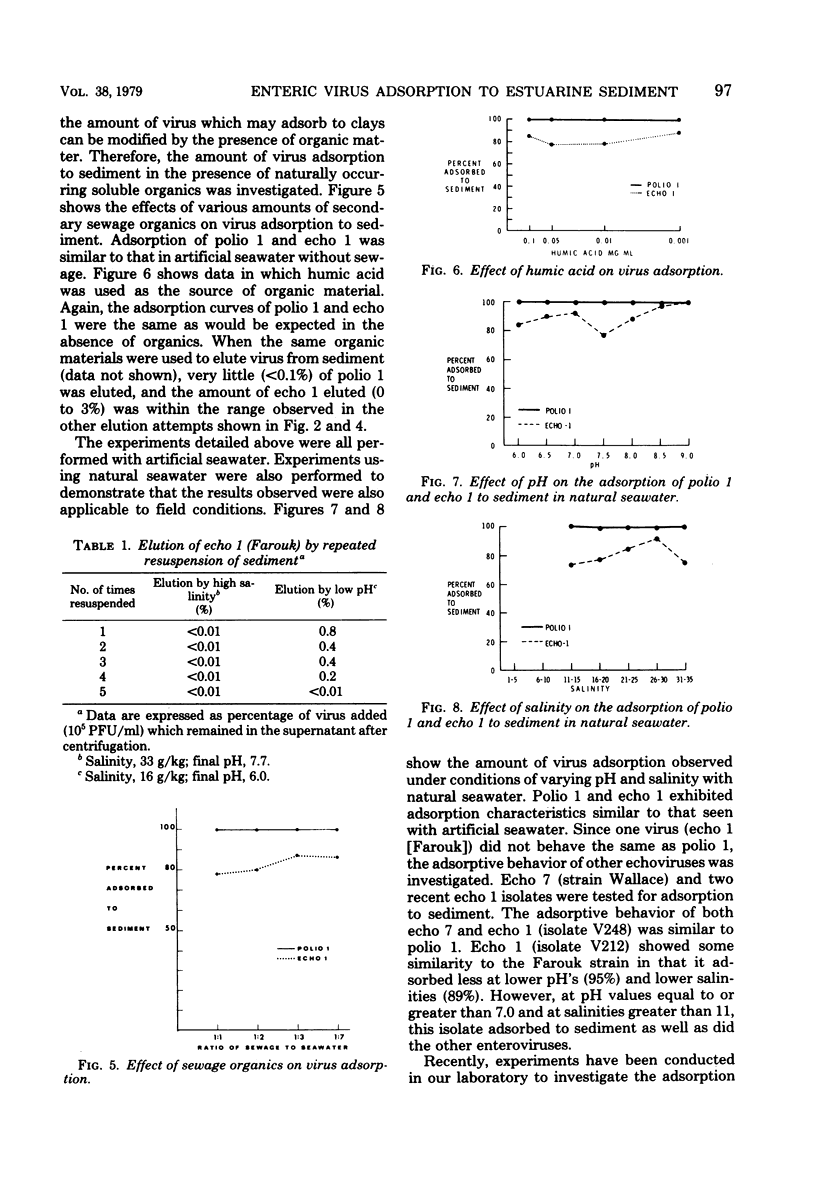
This study was designed to determine the degree of adsorption of enteric viruses to marine sediment and factors controlling this association. Adsorption and elution characteristics of several enteroviruses and one rotavirus to estuarine sediments were studied under varying conditions of pH, salinity, and presence of soluble organics. Greater than 99% of the added poliovirus type 1 (LSc), coxsackievirus type B3 (Nancy), echovirus type 7 (Wallace), and rotavirus (SA-11) adsorbed to sediment. Echovirus 1 (Farouk) and a recent isolate typed as coxsackievirus B4 adsorbed significantly less than poliovirus 1 under similar conditions of varying salinity and pH. The presence of soluble organic matter, in the form of secondary sewage effluent or humic acid, did not affect these patterns of adsorption. Only echovirus 1 (Farouk) desorbed when the pH or salinity was altered and then only to a small extent. Three recent isolates of echovirus 1 and echovirus 29 (strain JV-10) also demonstrated varying amounts of adsorption to sediment. These data indicate that enteric viruses can become readily associated with sediment in the estuarine environment and that this association may play a major role in their hydrotransportation and survival.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton H., Pereira M. S. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet. 1977 Apr 9;1(8015):780–781. doi: 10.1016/s0140-6736(77)92960-9. [DOI] [PubMed] [Google Scholar]
- Gerba C. P., McLeod J. S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl Environ Microbiol. 1976 Jul;32(1):114–120. doi: 10.1128/aem.32.1.114-120.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Schaiberger G. E. Effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975 Jan;47(1):93–103. [PubMed] [Google Scholar]
- Gerba C. P., Smith E. M., Melnick J. L. Development of a quantitative method for detecting enteroviruses in estuarine sediments. Appl Environ Microbiol. 1977 Aug;34(2):158–163. doi: 10.1128/aem.34.2.158-163.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J. Release of sediment-bound fecal coliforms by dredging. Appl Microbiol. 1975 Jan;29(1):109–111. doi: 10.1128/am.29.1.109-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M., Wallis C., Melnick J. L. Concentration and purification of enteroviruses by membrane chromatography. Appl Environ Microbiol. 1976 Nov;32(5):689–693. doi: 10.1128/aem.32.5.689-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak P. A., Caraway C. T., Portnoy B. L. Oyster-associated hepatitis: lessons from the Louisiana experience. Am J Epidemiol. 1976 Feb;103(2):181–191. doi: 10.1093/oxfordjournals.aje.a112216. [DOI] [PubMed] [Google Scholar]
- Matossian A. M., Garabedian G. A. Virucidal action of sea water. Am J Epidemiol. 1967 Jan;85(1):1–8. doi: 10.1093/oxfordjournals.aje.a120666. [DOI] [PubMed] [Google Scholar]
- Matson E. A., Hornor S. G., Buck J. D. Pollution indicators and other microorganisms in river sediment. J Water Pollut Control Fed. 1978 Jan;50(1):13–19. [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirobokov V. P. Differentiation of coxsackieviruses based on the character of adsorption bentonite. Acta Virol. 1968 Mar;12(2):185–185. [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Henderson M., Melnick J. L. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973 Oct;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



