Abstract
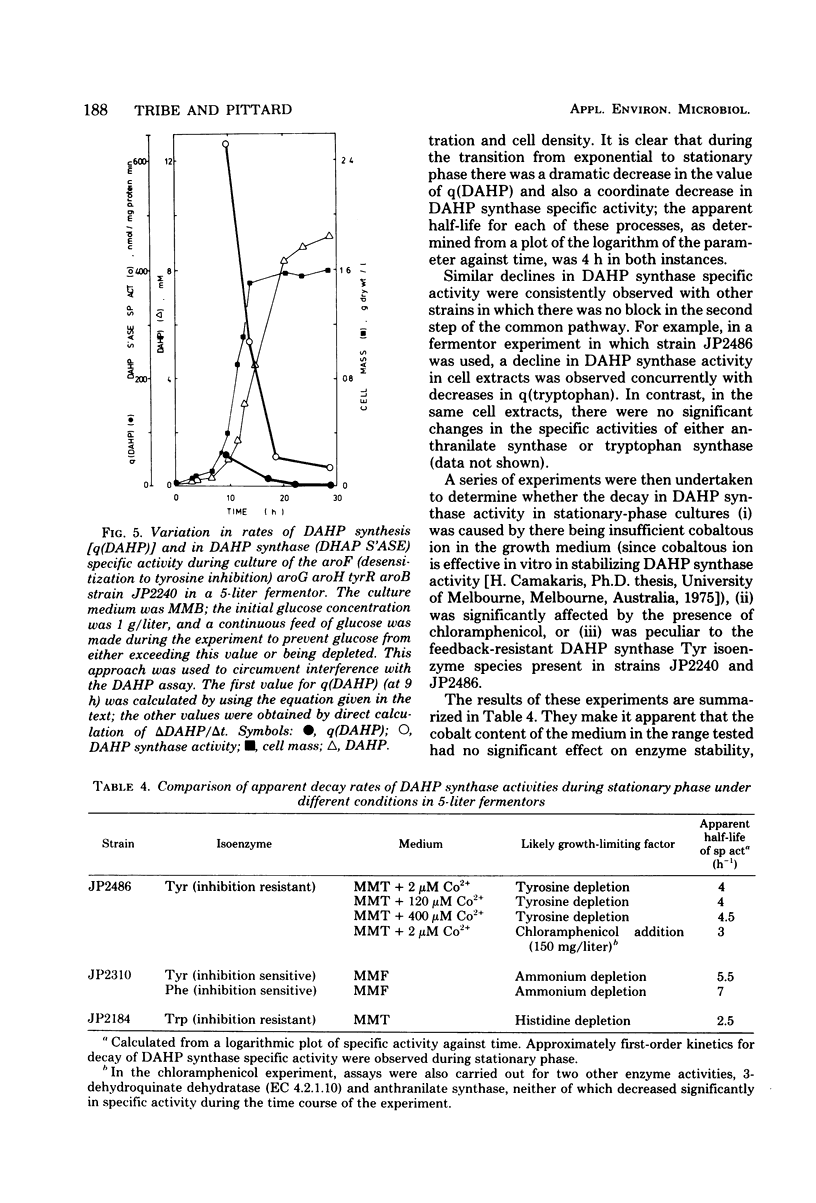
Conversion of glucose and ammonium salts into tryptophan by mutants of Escherichia coli was examined as part of a feasibility study on the manufacture of tryptophan. This involved construction, largely by transduction, or a variety of multiple-mutation strains with defined genotypes. By comparing the properties of these strains, we were able to define in biochemical terms several changes that significantly enhance process productivity, namely (i) release of the first enzyme of the common pathway of aromatic biosynthesis and the first enzyme of the tryptophan pathway (3-deoxy-D-arabino-heptulosonate 7-phosphate synthase and the anthranilate aggregate, respectively) from inhibition by end products, (ii) blockage of the diversion of chorismate to phenylalanine and tyrosine biosynthesis, and (iii) presence of highly elevated tryptophan pathway enzyme levels, such as result from interference with both repression and attenuation, combined with gene amplification. By using strains carrying appropriate mutations to effect all of these changes, high values of specific productivity were obtained in bath culture (approximately 80 mg/g [dry weight] per h). Furthermore, a pronounced decay in the level of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase activity was implicated as a cause of declining process producitivity during stationary phase, emphasizing the value of having derepressed levels of this enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera M., Morpurgo G., Ricci R. Tryptophan production by mutant strains of Escherichia coli K12. Experientia. 1967 Sep 15;23(9):724–725. doi: 10.1007/BF02154137. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Somerville R. L. Repression of aromatic amino acid biosynthesis in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):386–399. doi: 10.1128/jb.108.1.386-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camakaris H., Pittard J. Regulation of tyrosine and phenylalanine biosynthesis in Escherichia coli K-12: properties of the tyrR gene product. J Bacteriol. 1973 Sep;115(3):1135–1144. doi: 10.1128/jb.115.3.1135-1144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camakaris J., Pittard J. Repression of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) and enzymes of the tryptophan pathway in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):406–414. doi: 10.1128/jb.107.2.406-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. J., Nagano H., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Purification of the aggregate and regulatory properties of anthranilate synthetase. J Biol Chem. 1970 Mar 25;245(6):1416–1423. [PubMed] [Google Scholar]
- Im S. W., Davidson H., Pittard J. Phenylalanine and tyrosine biosynthesis in Escherichia coli K-12: mutants derepressed for 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (phe), 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A. J Bacteriol. 1971 Oct;108(1):400–409. doi: 10.1128/jb.108.1.400-409.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. G., Mateles R. I. Tryptophan- and indole-excreting prototrophic mutant of Escherichia coli. J Bacteriol. 1964 May;87(5):1051–1055. doi: 10.1128/jb.87.5.1051-1055.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Kuhn J. C., Somerville R. L. Feedback regulation in the anthranilate aggregate from wild type and mutant strains of Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):901–914. [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- Sahm H., Zähner H. Stoffwechselprodukte von Mikroorganismen. 90. Untersuchungen zur Tryptophan-Bildung bei Escherichia coli K 12. Arch Mikrobiol. 1971;76(3):223–251. [PubMed] [Google Scholar]
- Tribe D. E., Camakaris H., Pittard J. Constitutive and repressivle enzymes of the common pathway of aromatic biosynthesis in Escherichia coli K-12: regulation of enzyme synthesis at different growth rates. J Bacteriol. 1976 Sep;127(3):1085–1097. doi: 10.1128/jb.127.3.1085-1097.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Warren C. R. Surface Material of the Moon. Science. 1963 Apr 12;140(3563):188–190. doi: 10.1126/science.140.3563.188. [DOI] [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]


