Abstract
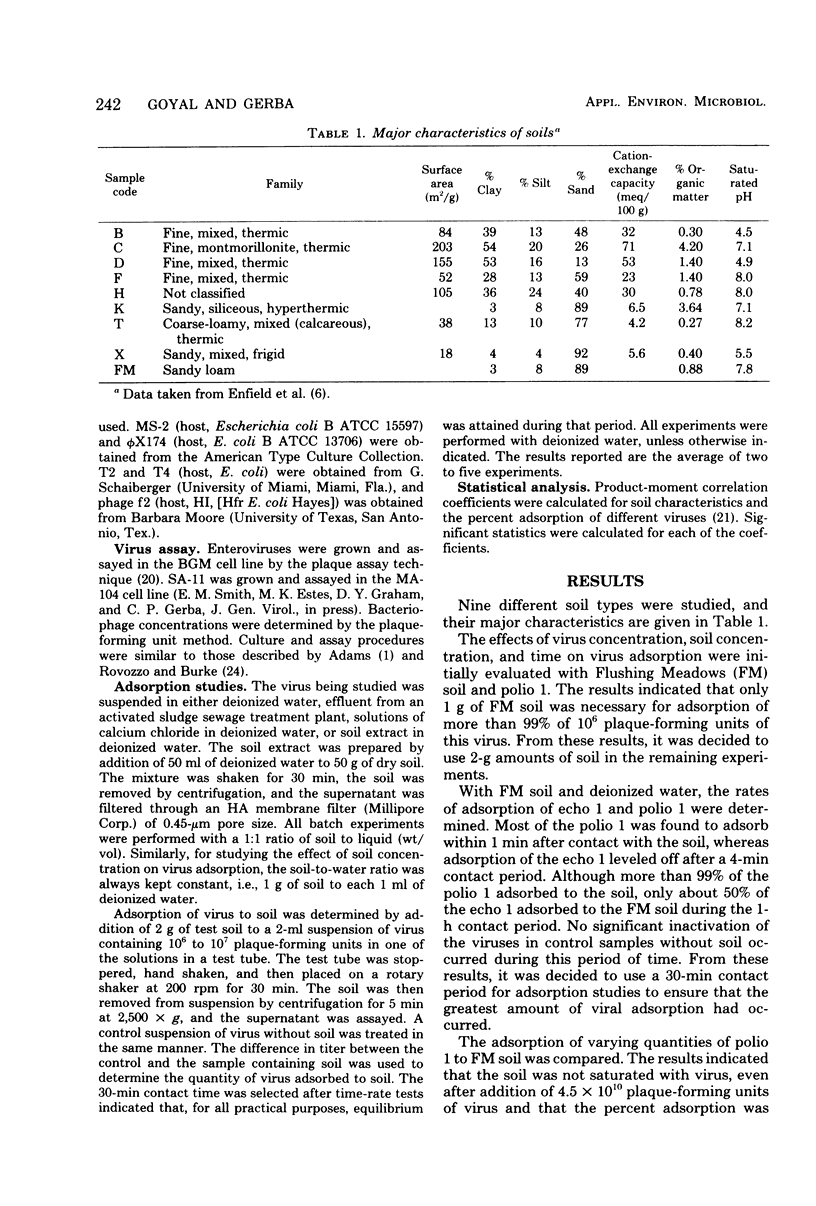
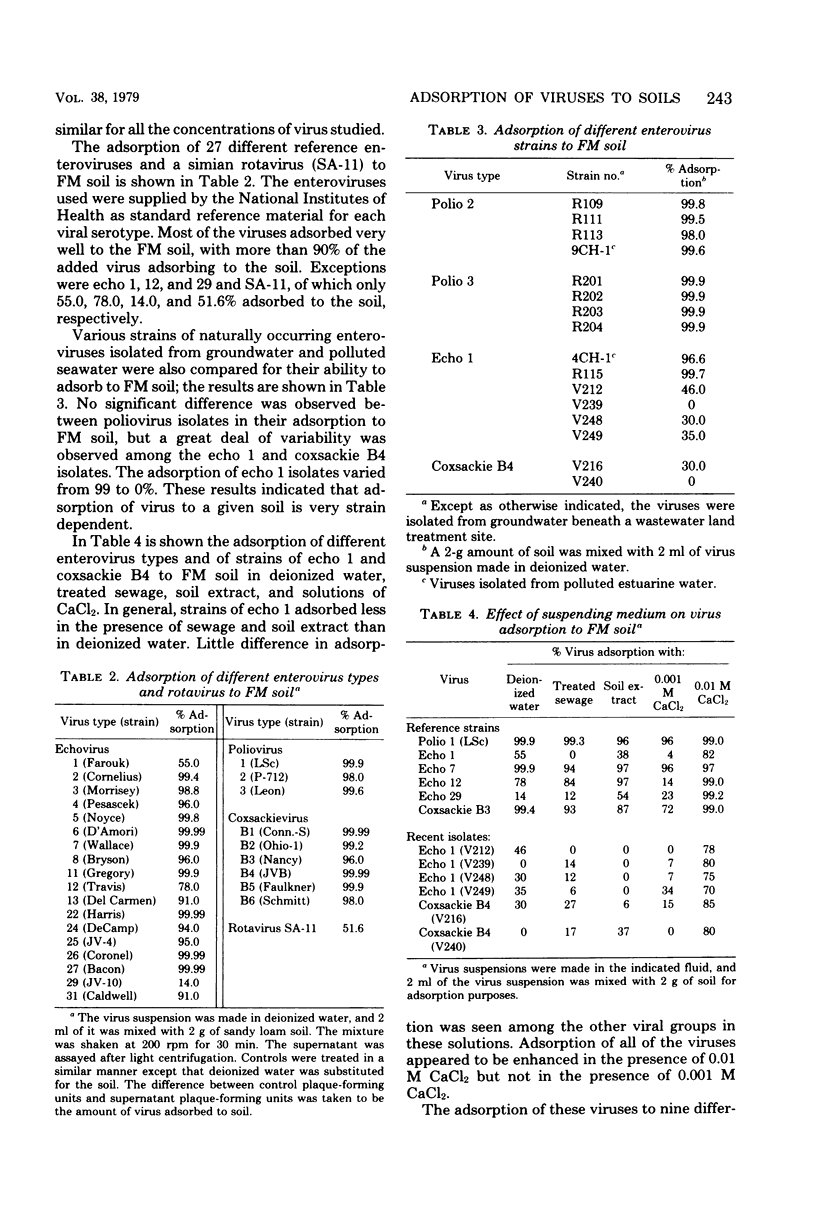
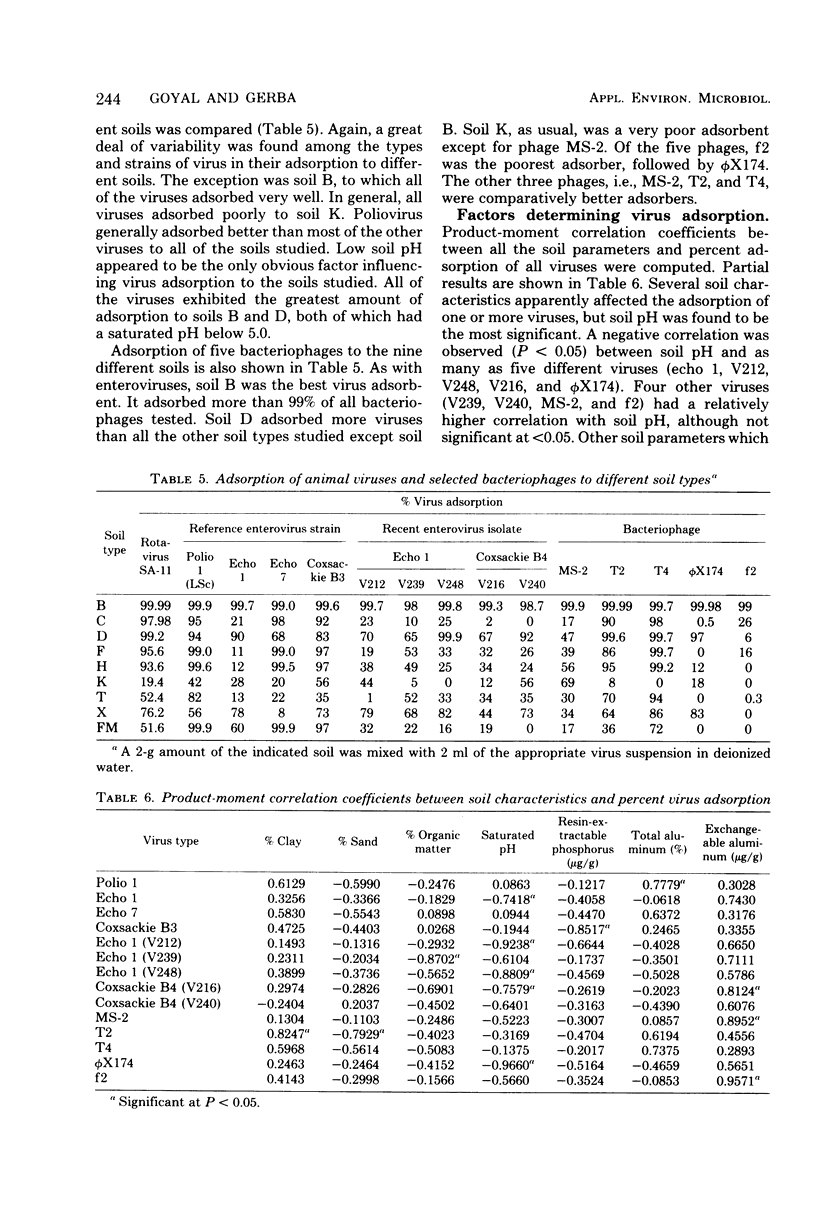
Virus adsorption to soils is considered to be the most important factor in removing viruses after land treatment of wastewater. Most of the studies on virus adsorption to soils have utilized poliovirus as the model system. In the present study, comparative adsorption of a number of different types and strains of human enteroviruses and bacteriophages to nine different soil types was studied. Under the experimental conditions of this study, greater than 90% of all viruses adsorbed to a sandy loam soil except echovirus types 1, 12, and 29 and a simian rotavirus (SA-11), which adsorbed to a considerably lower degree. A great deal of variability was observed between adsorption of different strains of echovirus type 1, indicating that viral adsorption to soils is highly strain dependent. Of the five phages studied, f2 and phi X174 adsorbed the least. In addition to being dependent on type and strain of virus, adsorption was found to be influenced also by type of soil. Thus, soils having a saturated pH of less than 5 were generally good adsorbers. From these results, it appears that no one enterovirus or coliphage can be used as the sole model for determining the adsorptive behavior of viruses to soils and that no single soil can be used as the model for determining viral adsorptive capacity of all soil types.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cramer W. N., Kawata K., Krusé C. W. Chlorination and iodination of poliovirus and f2. J Water Pollut Control Fed. 1976 Jan;48(1):61–76. [PubMed] [Google Scholar]
- Duboise S. M., Moore B. E., Sagik B. P. Poliovirus survival and movement in a sandy forest soil. Appl Environ Microbiol. 1976 Apr;31(4):536–543. doi: 10.1128/aem.31.4.536-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from estuarine water. Appl Environ Microbiol. 1977 May;33(5):1192–1196. doi: 10.1128/aem.33.5.1192-1196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Farrah S. R., Goyal S. M., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl Environ Microbiol. 1978 Mar;35(3):540–548. doi: 10.1128/aem.35.3.540-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Lance J. C. Poliovirus removal from primary and secondary sewage effluent by soil filtration. Appl Environ Microbiol. 1978 Aug;36(2):247–251. doi: 10.1128/aem.36.2.247-251.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. G., Gerba C. P., Rice R. C., Bouwer H., Wallis C., Melnick J. L. Virus and bacteria removal from wastewater by land treatment. Appl Environ Microbiol. 1976 Sep;32(3):333–338. doi: 10.1128/aem.32.3.333-338.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P., Melnick J. L. Virus movement in soil columns flooded with secondary sewage effluent. Appl Environ Microbiol. 1976 Oct;32(4):520–526. doi: 10.1128/aem.32.4.520-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Rennick V., Hampil B., Schmidt N. J., Ho H. H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull World Health Organ. 1973;48(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- O'CONNOR J. R., MORRIS J. A. Recovery of Texas-1 type Coxsackie virus from blood of wild rabbit and from sewage contaminating rabbit's feeding ground. Am J Hyg. 1955 May;61(3):314–320. doi: 10.1093/oxfordjournals.aje.a119755. [DOI] [PubMed] [Google Scholar]
- PECZENIK A., DUTTWEILER D. W., MOSER R. H. An apparently water-borne outbreak of infectious hepatitis. Am J Public Health Nations Health. 1956 Aug;46(8):1008–1017. doi: 10.2105/ajph.46.8.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub S. A., Sorber C. A. Virus and bacteria removal from wastewater by rapid infiltration through soil. Appl Environ Microbiol. 1977 Mar;33(3):609–619. doi: 10.1128/aem.33.3.609-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. M., Landry E. F., Baranosky L. J., Beckwith C. A., Dahl M. C., Delihas N. C. Survey of human virus occurrence in wastewater-recharged groundwater on Long Island. Appl Environ Microbiol. 1978 Jul;36(1):47–51. doi: 10.1128/aem.36.1.47-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W., Pierce L. V. Demonstration of virus in groundwater after effluent discharge onto soil. Appl Microbiol. 1975 Jun;29(6):751–757. doi: 10.1128/am.29.6.751-757.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



