Abstract
The fully ripened fruit of Katsura-uri Japanese pickling melon (Cucumis melo var. conomon) has rarely been used for food because the midripened fruit is utilized for making pickles, but the fully ripened fruit is no longer valuable for pickles due to the fruit body being too soft. We have considered the utilization of the fully ripened Katsura-uri fruit that may be used for nonpickling products, particularly if the fully ripened fruit demonstrated health benefits such as anticarcinogenic properties. The phytochemical extract from the fully ripened fruit of Katsura-uri Japanese pickling melon was purified via a bioassay-guided fractionation scheme, which was based on the induction of differentiation in a RCM-1 human colon cancer cell line. On the criteria of two differentiation markers (duct formation and alkaline phosphatase activity), the most potent fraction contained a compound identified as 3-methylthiopropionic acid ethyl ester, based on GC retention time, EI-MS, 1H NMR, and 13C NMR spectra. Previously, the role of 3-methylthiopropionic acid ethyl ester was considered as an odor producing compound in many fruits, but this study indicates potential medical benefits of this compound.
Keywords: Differentation, color, cancer, melon, Cucumis melo
INTRODUCTION
Katsura-uri Japanese pickling melon (Cucumis melo var. conomon) is an heirloom vegetable in Kyoto, Japan (Figure 1). The fruit is 40–90 cm in length and 2–5 kg in weight (4–12 times larger than the conventional type in Japan) and not sweet in any of its ripening stages, and the midripened fruit is primarily used as pickles in traditional Japanese cuisine. However, the fully ripened fruit of Katsura-uri has rarely been used in Japanese eating habits, because the midripened fruit is utilized for making pickles, but the fully ripened fruit is no longer valuable for pickles due to the flesh being too soft.
Figure 1.
Morphological shape of Katsura-uri fruit. The whole body of Katsura-uri Japanese pickling melon (A), and the longitudinal cross section (B). Bar inset = 10 cm.
Alternatively, we have considered the utilization of the fully ripened Katsura-uri fruit with the unique properties of not being sweet and the possession of a strong melon-like odor that may be used for nonpickling products, particularly if the fully ripened fruit has demonstrated health benefits. One potential health benefit is in the prevention of cancer. In our previous study, the fully ripened Katsura-uri Japanese pickling melon exhibited bioantimutagenic activity assessed by UV-induced mutation assays using Escherichia coli B/r WP2, at levels ten times higher than that from midripened Katsura-uri or the conventional type, Shiro-uri (1). Some of the bioantimutagenic compounds identified from vegetables in this assay were S-methyl methanethiosulfonate from cauliflower (2) and 4-methylthio-3-butenyl isothiocyanate from daikon (Japanese white radish) (3). These compounds also exhibited anticarcinogenic activities in animal experiments (4–8). Thus these results warranted further investigation into the anticarcinogenic properties of Katsura-uri Japanese pickling melon.
Colon cancer is one of the most widely distributed cancers in the world. Prevention and early detection of the premalignant lesion or early stage tumor is of vital importance for the successful eradication of colon cancer, because most cases have few to no symptoms, such as pain, and the tumor is often diagnosed in the later stages of the disease (9, 10). Under the present circumstances, an appropriate therapeutic strategy, other than prevention, has been strongly required in colon cancer. The conventional cancer therapy is surgery followed by the administration of anticancer drugs to kill any remaining tumorigenic cells (11–13). Unfortunately many patients suffer from the side effects of these cytotoxic drugs. Therefore, alternative chemotherapies, such as the induction of differentiation and apoptosis of cancer cells, are believed to be potentially better approaches.
Some anticarcinogenic compounds from natural sources, such as vitamin D analogues (14, 15), butyric acid (16–18), maslinic acid (19), and kaempferol (20), are also known to induce the differentiation in well-differentiated human colon cancer cells. Here, we focus on an identification of differentiation inducer in the RCM-1 human colon cancer cell line derived from a colon cancer tissue diagnosed as a well-differentiated rectum adenocarcinoma.
In this study, we used silica gel column and silica gel thin layer chromatography procedures to purify the differentiation inducers from an n-hexane extract of fully ripened Katsura-uri Japanese pickling melon via a bioassay-guided fractionation scheme. The bioassay system used two markers of differentiation (duct formation and alkaline phosphatase activity) in RCM-1 cells. This bioassay-guided fraction scheme allowed us to identify the active ingredient, which was 3-methylthiopropionic acid ethyl ester (MTPE).
MATERIALS AND METHODS
Chemicals
3-Methylthiopropionic acid ethyl ester (98.0% pure grade, CAS no. 13327-56-5) was purchased from Avocado Research Chemicals Ltd. (Lancashire, England).
Plant Samples
Katsura-uri (Japanese pickling melon, Cucumis melo var. conomon) was harvested after it was midripened and fully ripened in August of each year from 2005 to 2006 in an open field culture system at the Kyoto Prefectural Agricultural Research Institute (Kameoka City: longitude 135°34′E, latitude 35°01′N, altitude of 110 m, annual mean air temperature of 14.6 °C, annual precipitation of 1590 mm), Japan. Katsura-uri fruits were cooled on harvest date in a refrigerator (4 °C), and overnight freighted to Kyoto Prefectural University for immediate analysis within 2 days after being held at 4 °C. Cucumber (Cucumis sativus), muskmelon (Cucumis melo var. riticulatus), Makuwa-uri (Cucumis melo var. makuwa), Shiro-uri (Cucumis melo var. conomon), and wax gourd (Benincasa hispida) shown in Table 1 were purchased from local supermarkets in Kyoto City from July to November 2007.
Table 1.
Concentration of MTPE in the Cucurbitaceous Familya
| variety | MTPE (μg/100 g) |
|---|---|
| Cucumis melo var. conomon | |
| Katsura-uri (fully ripened) | 3.8 (2.9–4.3) |
| Katsura-uri (midripened) | Ldlb |
| Shiro-uri | Ldlb |
| Cucumis melo var. makuwa | |
| Makuwa-uri | 0.6 (Ldl-1.8)c |
| Cucumis melo var. riticulatus | |
| muskmelon (red-fleshed) | 2.5 (1.0–3.6) |
| muskmelon (green-fleshed) | 1.1 (Ldl-1.7)c |
| Cucumis stativus | |
| cucumber | Ldlb |
| Benincasa hispida | |
| wax gourd | Ldlb |
Each value represents mean and range in parentheses of 2 to 13 different samples of the same variety: 10 of muskmelon (red-fleshed); 8 of muskmelon (green-fleshed); 4 of Katsura-uri (fully-ripened); 3 of Makuwa-uri; 2 of Katsura-uri (mid-ripened), Shiro-uri, cucumber and wax gourd.
Ldl means less than detection limit.
The average was calculated as the value of Ldl as zero.
Cell Lines and Culture
The RCM-1 colon cancer cell line was diagnosed as a well-differentiated rectum adenocarcinoma derived from a 73-year-old female human (21), and was obtained from Dr. H. Kataoka of the University of Miyazaki (Miyazaki, Japan). The RCM-1 cell line is characterized as a partially differentiated type that is suitable to purify “differentiation inducers” for the following reasons: (1) RCM-1 cells spontaneously differentiate as determined by the formation of ducts resembling villiform structures and ALPase activity, which is achieved after the cells reach confluency in plastic culture plates, and (2) chemical differentiation inducers, such as kaempferol, further enhance the number and size of ducts, and ALPase activity from the background levels of spontaneously differentiated RCM-1 cells (22). Thus, the real-time morphological observation of duct formation enables a simple assay for a rapid assessment of cell differentiation properties of potential chemopreventive compounds, thus being an ideal assay system to effectively assess the most active anticarcinogenic compounds in plant extracts.
Treatment of Cells with Sample
RCM-1 cells (3 × 106) were each plated into 35-mm plastic culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) with 2 mL of 45% RPMI1640 medium with 45% Ham’s F12 medium (GIBCO-Invitrogen corporation, Grand Island, NY) and 10% fetal bovine serum (10% FBS-RPMI+F12). The cells were then treated with samples extracted from Katsura-uri (DMSO-vehicle concentration = 0.1%) or MTPE and kaempferol (acetonitrile-vehicle concentration = 1.0%) in 2 mL of 10% FBS-RPMI+F12 for 4 days. The reason we chose 4 days for the incubation of the test compound is based on our previous experiments where an n-hexane extract of Katsura-uri showed maximal enhancement of duct formation at day 4. We always used the sample dose that exhibited no cytotoxicity.
Duct Formation Bioassay
When RCM-1 cells (3 × 106) were each plated into 35-mm plastic culture plates, RCM-1 cells spontaneously and partially differentiated after reaching confluency in plastic culture plates and began to exhibit contact-insensitive growth, followed by forming some ducts 2 days after reaching confluency. However, the differentiation of the RCM-1 cells is partial, and the duct formation is increased with kaempferol, which is an inducer of differentiation accompanied with ALPase activity (a common differentiation marker of colon cancer cells) (Table 2). When RCM-1 cells were cultivated with samples for 4 days, the duct number and size were measured from digital photographs at 50× with a Zeiss Axiovert 25 microscope equipped with a CCD camera. Duct formation activity was calculated as the fraction of the solvent control. To identify an active fraction we used three different criteria. The first criterion determined the lowest dose needed to induce a 50% increase of the number of ducts over the control. The second criterion determined the lowest dose needed to induce a 50% increase of the total area occupied by duct structures over the control. The third criterion determined the lowest dose needed to induce a 50% increase of the diameter of the maximum size of the duct over the control. A fraction was determined active if it met at least one of these three criteria.
Table 2.
Stimulation of ALPase Activity by MTPE in RCM-1 Cellsa
| dose (mM) | ALPase activity (mU/mg protein) | |
|---|---|---|
| cells before confluency | 0 | 6.0 ( 2.2a |
| cells after confluency with authentic MTPE | 0 | 17.3 ± 1.2b |
| 0.25 | 22.3 ± 6.4c | |
| 0.5 | 24.1 ± 1.1c | |
| 1.0 | 28.4 ± 3.2d | |
| 2.0 | 42.4 ± 7.1e | |
| cells after confluency with kaempferol | (20 μM) | 28.2 ± 5.1 |
RCM-1 cells (3 × 106) were each seeded into 35-mm plastic culture plates and cultured overnight. The cells were treated for 4 days (1 day for “cells before confluency”) with vehicle (acetonitrile-vehicle concentration = 1.0%) or authentic MTPE and kaempferol. Each value represents an average of eight replicates (± SEM. Values followed by different letters (among a, b, c, d, and e) are significantly different (p < 0.05) by multiple comparison test of Fisher’s PLSD.
Duct Formation Bioassay-Guided Fractionation of Katsura-uri
Katsura-uri fruit (3.1 kg) was homogenized and extracted twice with methanol (3.0 L) at room temperature for 1 h. The filtered extract was mixed and evaporated under 40 °C to ~500 mL of an aqueous crude extract solution with a rotary evaporator. The fractionation procedure for each fraction is summarized in Figure 3. The methanol extract was divided into fractions A–D by solvent extraction with n-hexane, chloroform, and ethyl acetate successively. The n-hexane, chloroform and ethyl acetate layers were evaporated to dryness under 40 °C and the aqueous layer was lyophilized. All fractions were weighed and examined for duct formation. Fraction A (n-hexane extract, 220 mg) showed an ability to enhance duct formation. It was then divided into fractions E–I by silica gel (6 g) column chromatography (Merck; Silica gel 60, 35–70 mesh; ϕ 0.8 × 24 cm), using 60 mL of each eluent: n-hexane; 2.5%, 5%, 10%, 50% acetone in n-hexane. Fraction F (83.3 mg), which showed an ability to enhance duct formation, was spotted on preparative-TLC plate (Merck; silica gel 60 F254, 20 × 20 cm), developed with the solvent of n-hexane–acetone (95:5). The spot of the compound was detected visually by a method of sulfuric acid-mist with heat, using the edge of the TLC plate. The broadbands of Rf value 1–0.8 in fraction J, 0.8–0.6 in fraction K, 0.6–0.4 in fraction L, 0.4–0.2 in fraction M, 0.2–0 in fraction N were collected. Fraction K (8.4 mg), which showed an ability to enhance duct formation, was spotted on preparative-TLC plate (Merck; silica gel 60 F254, 20 × 20 cm), developed with the solvent of n-hexane–acetone (95:5). The spot of the compound was detected visually by the method of sulfuric acid-mist with heat, using the edge of the TLC plate. The broadbands of Rf value 0.8–0.7 in fraction O and 0.7–0.4 in fraction P were collected. The ability to enhance duct formation was shown in Fraction P (2.0 mg) but not in Fraction O.
Figure 3.
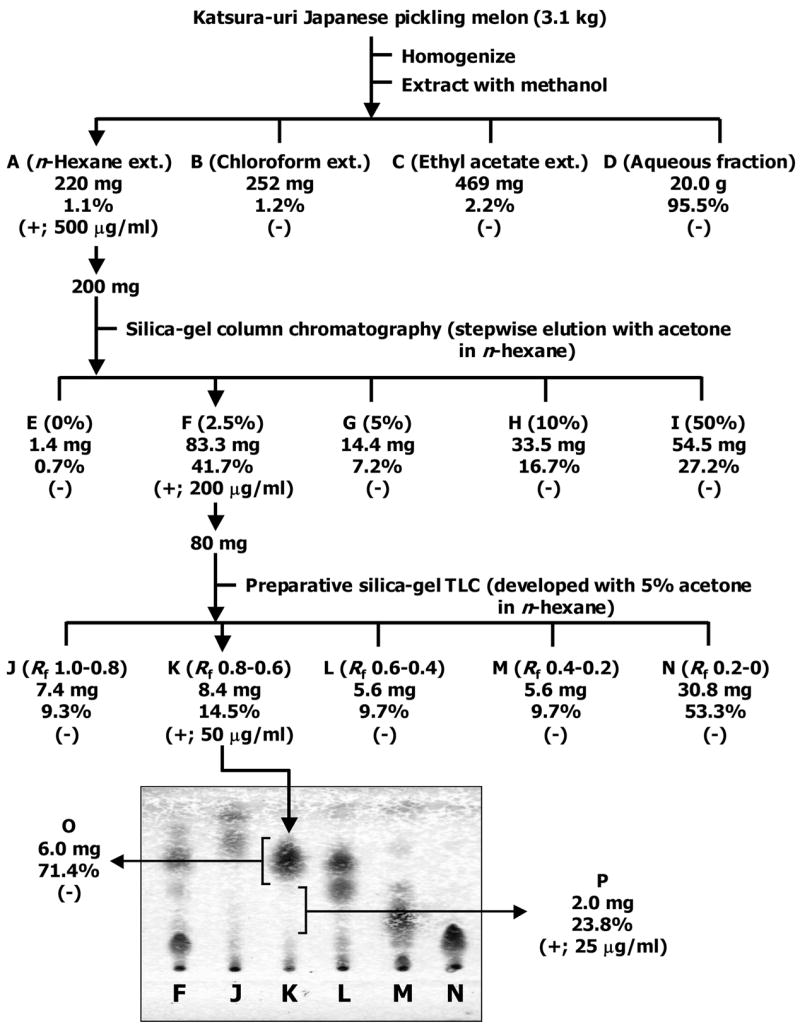
Duct formation bioassay-guided fractionation of Katsura-uri fruit. The yield (weight, %) and activity (+ or −) was indicated directly under each fraction labeled A–P. The spots on TLC were detected visually by a method of sulfuric acid-mist with heat after development with the solvent of n-hexane–acetone (95:5).
Gas Chromatography–Mass Spectrometry (GC-MS), 1H NMR, and 13C NMR Spectrometry
GC-MS analyses were performed on a model JEOL JMS-AMSUN200 mass spectrometer, coupled on a Hewlett-Packard 6890 gas chromatograph. The capillary column was a DB-5 (25 m × 0.2 mm, 0.33 μm film thickness; J&W Scientific, Folsom, CA). The column oven temperature was held at 60 °C for 5 min and then was increased to 250 at 5 deg/min. 1H NMR and 13C NMR spectra were obtained on a JEOL GX-270W, using CDCL3 as the solvent with TMS as internal standard.
Concentration of MTPE
The pieces of Katsura-uri and fruit samples were cut into equal parts and 80 to 100 g were put into an automatic homogenizer (CQM-V2, Toshiba) with 0.7–1 mL of 6 M HCl to avoid degradation of MTPE by any intact esterase. The homogenate (50 g) was filtered and the residue was extracted twice with 4 mL of distilled water. The water extracts were combined and diluted to 50 mL to prepare an aqueous crude sample solution and centrifuged (1500g). The supernatant (45 mL) was absorbed to the C18 cartridge column (Waters, Sep-pak, catalogue number WAT020515) and washed with 10 mL of water and 10 mL of 10% methanol, and then the sample was eluted with 5.5 mL of 70% methanol and collected to subject it to the MTPE analysis. The solution was diluted with 3 mL of water, and then extracted four times, each with 1 mL of n-hexane. The n-hexane extracts were combined and concentrated to 0.4 mL, and then MTPE was measured by reverse-phase HPLC-UV, using an HPLC (Shimadzu, Kyoto, Japan) model LC-20AT with a SPD-20A UV detector and a C-R8A integrator. A YMC ODS-H80 (ϕ 4.6 × 150 mm) column was used for MTPE analyses based on reverse-phase chromatography. A 10-μL aliquot of sample was injected and isocratically eluted by 27% acetonitrile in 0.1% trifluoroacetate mobile phase for MTPE. The UV absorbance at 215 nm was used to detect MTPE, which eluted from the column at 13.5 min. The concentration of MTPE was calculated from a linear equation derived from a standard curve of peak areas vs. known MTPE concentration. The detection limit for MTPE was calculated as 0.27 μg/100 g of fresh fruit.
Cytostaticity Assay of MTPE
RCM-1 cells (1.5 × 104) were each plated into 35-mm plastic culture plates (8 plates per treatment). Twenty-four hours after plating, cells were treated with MTPE in 2 mL of 10% FBS-RPMI+F12 for 4 days, and were counted by using a hemacytometer, after trypsinization with 0.25% trypsin and 1 mM EDTA. At day 4, the cells were still in the log phase of growth and not confluent.
Alkaline Phosphatase (ALPase) Activity
Cells were washed with phosphate buffered saline (PBS) twice, and lysed in a buffer containing 0.1 M carbonate, 2 mM MgCl; pH 9.5. The lysate was sonicated, and the protein was quantified by using a DC assay kit (Bio-Rad Corp., Richmond, CA). The lysate (20 μL) and 0.3 mL of ALPase substrate solution (0.3 mg of p-nitrophenyl phosphate/0.2 M Tris buffer; Sigma, St. Louis, MO) were thoroughly mixed in a 1.5-mL plastic tube. The tube was incubated at 37 °C for 15 min, and 300 μL of 0.2 M NaOH was added to terminate the substrate–enzyme reaction. The ALPase activity was calculated by reading sample absorption at 405 nm from a linear standard equation derived from the absorption readings of consecutive known ALPase activities. We monitored ALPase activity from 1 to 7 days after MTPE treatment with different doses (0.016–2 mM). At day 4, these cells are the most sensitive to the differentiating effects of MTPE (data not shown), thus we used cells cultured for 4 days in the presence of MTPE to assess the active component of the fractionated fruit extract.
Statistical Analysis
Statistical comparisons were made by using Fisher’s PLSD method after analysis of variance (ANOVA). The results were considered significantly different with p < 0.05.
RESULTS
Purification of the Duct Formation Enhancement Compound
We purified a compound from Katsura-uri fruit that enhanced duct formation in a well-differentiated human colon cancer cell line, which spontaneously formed some villi-like ducts. An overall purification scheme for the compound from the fruit of Katsura-uri is shown in Figure 3. The activity of duct formation and the weight of the fractions are also shown in Figure 3. The active fraction was defined as the fraction exhibiting a 50% increase in duct number or area as compared to the untreated cells. On the basis of this criterion, n-hexane extract of Katsura-uri fruit enhanced the number of ducts in RCM-1 cells at 500 μg/mL (Figure 3). The chloroform, ethyl acetate, and aqueous fractions did not show the activity in the induction of differentiation (Figure 3), thus further fractionation focused only on the n-hexane extract. The next fractionation step used silica gel column chromatography resulting in the highest activity in Fraction F (2.5% acetone in n-hexane elution) in which a dose of 200 μg/mL induced duct formation (Figure 3). Fraction F (83.3 mg) was further purified by using preparative silica gel thin layer chromatography (TLC). The active fraction (50 μg/mL) that exhibited a 150% increase in duct number as compared to the untreated cells was found in a band labeled Fraction K with an Rf value of 0.8–0.6 and a yield of 8.4 mg. Fraction K was purified further by using preparative TLC, yielding an active band (25 μg/mL) labeled Fraction P with an Rf value of 0.7–0.4 and with a yield of 2.0 mg. In the series of purification steps, the active ingredient in Katsura-uri fruit was successfully purified into Fraction P.
Identification of the Duct Formation Enhancement Compound in Fraction P
Fraction P contained one compound on the total ion chromatogram from the low-resolution–gas chromatography–electron ionization (LR-GC-EI) mass spectroscopy analysis. The compound, appearing at tR 12.50 min, showed the ion peak at an m/z 148 (M)+, and prominent fragment ions with masses of 133, 119, 103, 87, 74, 61, and 47. In addition, the isotope peaks at 149 (M + 1)+ and 150 (M + 2)+ were observed for the mass 148 (M)+ fragment, which indicates that this is a sulfur-containing compound. 1H NMR (270 MHz, CDCL3) spectra were assigned: δ 1.27 (3H, t, J8,7 = 7.15, H-8), δ 2.12 (3H, s, H-1), δ 2.60 (2H, br t, J4,3 = 6.84, H-4), δ 2.75 (2H, br t, J3,4 = 6.84, H-3), δ 4.16 (2H, q, J7,8 = 7.15, H-7). 13C NMR (67.8 MHz, CDCl3) spectra were assigned: δ 14.09 (C-8), δ 15.53 (C-1), δ 29.15 (C-3), δ 34.54 (C-4), δ 60.59 (C-7), δ 171.71 (C-5). The molecular weight and 1H NMR and 13C NMR spectra analyses indicated that the isolated compound is 3-methylthiopropionic acid ethyl ester (MTPE). The chemical structure of MTPE is shown in Figure 2. To validate our tentative identification, we used authentic MTPE, which had the same retention times and m/z ratio of the ion peak [tR 12.50 min, m/z 148 (M)+] as the isolated compound. The 1H NMR and 13C NMR spectra also corresponded with those of authentic MTPE. Thus, the compound isolated in fraction P that enhanced duct formation was successfully identified to be that of MTPE.
Figure 2.
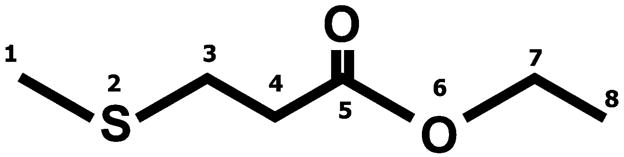
Chemical structure of 3-methylthiopropionic acid ethyl ester.
Concentration of MTPE
The levels of MTPE varied as a result of differences from midripened to fully ripened Katsura-uri (C. melo var. conomon), ranging from zero (less than detection limit) to 3.8 μg/100 g of fresh weight (Table 1). These results indicate that Katsura-uri begins to produce MTPE between the midripening to fully ripening stage of fruit development. Shiro-uri, a conventional variety for Katsura-uri, did not contain MTPE. Differences in MTPE levels were observed between varieties of Katsura-uri, where the wild cultivar of melon, Makuwa-uri (C. melo var. makuwa), contained lower levels of MTPE (0.6 μg/100 g) than the red-fleshed muskmelon varieties (C. melo var. riticulatus), which contained 2.5 μg/100 g. Green-fleshed varieties contained considerably lower levels of MTPE (1.1 μg/100 g). In other species that belong to the same plant family, Cucurbitaceae, as Katsura-uri, such as cucumber (C. stativus) and wax gourd (B. hispida) did not contain detectable levels of MTPE, indicating that this compound is more specific to plant species than to the plant family.
The Ability of MTPE To Enhance the Formation of Ducts
Authentic MTPE (0.25–2 mM) did not show any cytotoxic effect on RCM-1 cells, as determined by the lack of cell detachment from the culture plates. MTPE also did not show any cytostatic effect on RCM-1 cells, as determined by cell growth. After authentic MTPE (0.25–2 mM) treatment for 4 days, the growth rate of RCM-1 cells did not decline. MTPE is consequently considered to be neither cytotoxic nor cytostatic for RCM-1 cells up to a concentration of 2 mM (Figure 4).
Figure 4.
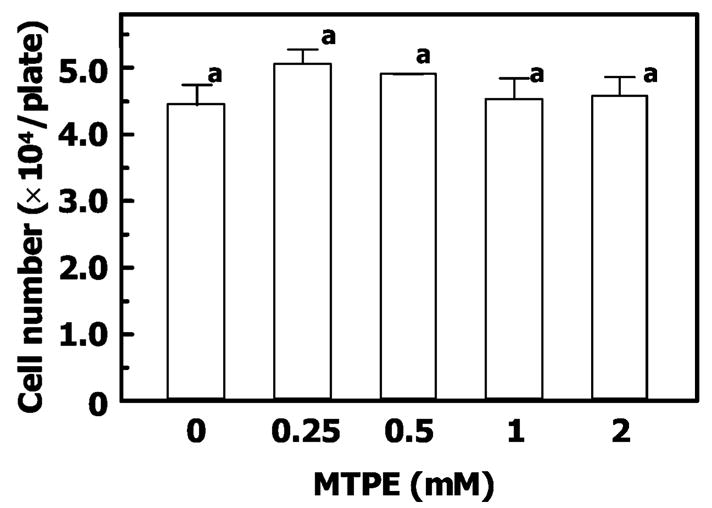
Cytostaticity of MTPE in RCM-1 cells. RCM-1 cells (1.5 × 104) were each seeded into 35-mm plastic culture plates for cytostatic effects of MTPE. The cells were treated for 4 days with vehicle or authentic MTPE, and then the number of cells was determined. Each value represents an average of eight replicates (± standard deviation. Values followed by the same letter (a) are not significantly different (p < 0.05) by multiple comparison test of Fisher’s PLSD.
RCM-1 cells spontaneously formed a small number of duct-like structures (Figure 5A). Treatment of RCM-1 cells for 4 days with authentic MTPE between the doses of 0.25 and 2 mM progressively increased the percent area occupied by duct structures relative to the control, and also induced an increase in the number and the maximum diameter of the ducts in each culture plate (Figure 5B–F).
Figure 5.
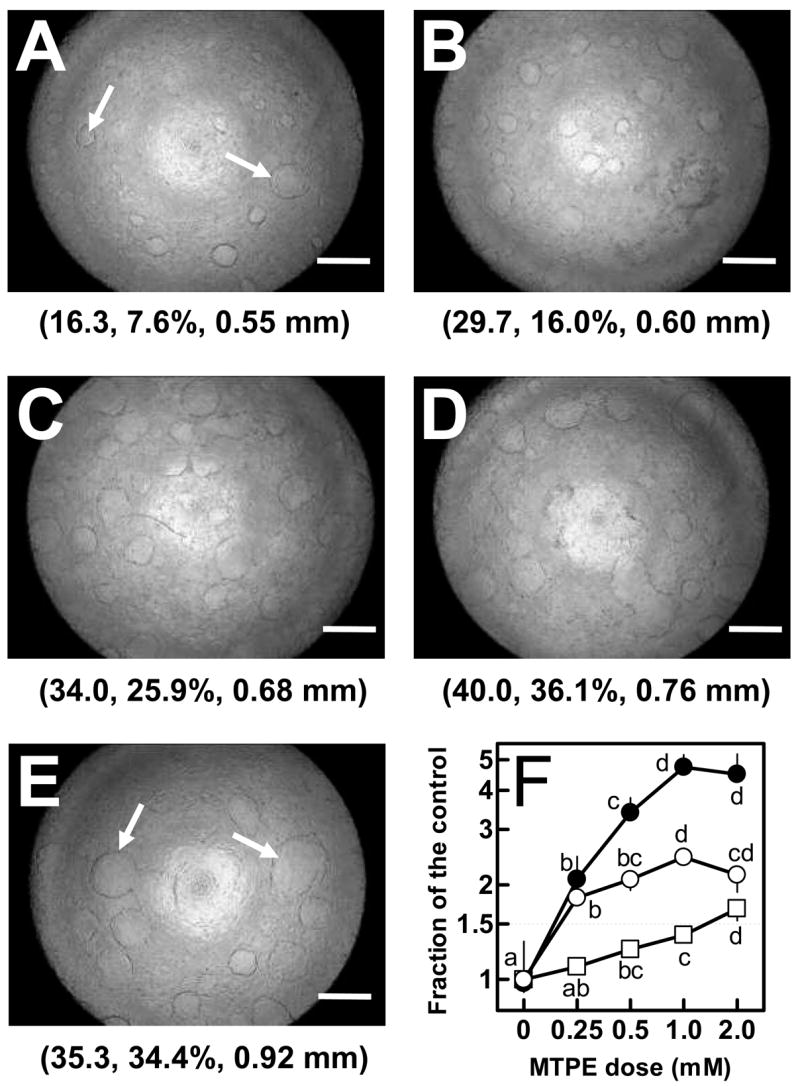
Enhancement of the duct formation of MTPE in RCM-1 cells. The differentiation potential of MTPE was determined by seeding 3 × 106 RCM-1 cells into 35-mm plastic culture plates and then treated for 4 days with vehicle (A), and authentic MTPE (B: 0.25 mM; C: 0.5 mM; D: 1 mM; E: 2 mM), and then the formation of ducts were visually determined by using phase contrast microscopy and the resulting images were used for quantifying three parameters of these ducts. The values of the three parameters are indicated in parentheses, and sequentially represent the number of ducts, percent of the total area occupied by duct structures, and the diameter of the maximum size of duct in each culture plate. Each value represents an average of three replicates (± standard deviation. The data were plotted as a fraction of the control for each parameter (F: ○, the number of ducts; ●, percent of the total area occupied by duct structures; □, the diameter of the maximum size of duct). Bar inset = 0.75 mm. Arrows show an example of typical duct formation. Values followed by different letters (among a, b, c, and d) are significantly different (p < 0.05) by multiple comparison test of Fisher’s PLSD.
Effect of MTPE on the Activity of ALPase in RCM-1 Cells
The constitutive activity of ALPase in RCM-1 cells before reaching confluency was 6.0 mU/mg of protein (Table 2). A general increase of ALPase activity (17.3 mU/mg of protein) occurred after reaching confluency, which is when the ducts began forming. Kaempferol (20 μM), which is a chemical known to induce differentiation, enhanced the activity of ALPase to 28.2 mU. After the treatment of RCM-1 cells with authentic MTPE for 4 days, the ALPase activity increased to 22.3 and 42.4 mU at 0.25 and 2 mM MTPE, respectively. These results indicate that MTPE possess potential anticarcinogenic properties by inducing differentiation in well-differentiated colon cancer cells.
DISCUSSION
A crude extract from Japanese pickling melon, Katsura-uri, exhibited anticarcinogenic properties by inducing differentiation in a well-differentiated human colon cancer cell system, RCM-1. To identify the active ingredient in this extract we used a bioassay guided purification scheme that relied on the induction of differentiation of RCM-1 cells as determined by duct formation and alkaline phosphatase activity. The fractionation scheme began with various solvent extractions followed by silica gel column chromatography and then silica gel thin-layer chromatography. Using this fractionation scheme, we successfully purified the active ingredient, which was identified as 3-methylthiopropionic acid ethyl ester, based on GC retention time, EI-MS, and 1H NMR and 13C NMR spectra. These results were further confirmed when authentic MTPE gave the same retention times and mass and NMR spectra as the isolated compound. The dose range of authentic MTPE that induced differentiation in RCM-1 cells was between 0.25 to 2 mM. This is similar to the dose range (1 to 10 mM) that butyrate induced differentiation in other well-differentiated human colon cancer cell lines (Caco-2 and HT-29) (16, 17). Butyrate is a commonly used benchmark compound in cell differentiation experiments.
Most chemicals presently known to induce differentiation in colon cancer cell types are typically at cytostatic concentrations (14–20). For instance, induction of differentiation by 1α-hydroxy- and 1β-(hydroxymethyl)-vitamin D inhibited cyclin D1-dependent cell proliferation (15), and induction of differentiation by butyrate resulted in the inhibition of protein kinase C, JNK, and MEK-ERK signal transduction pathways (16, 17), and induction of differentiation by kaempferol inhibited ERK and STAT3 (20). We have determined that the cytostatic dose of MTPE in RCM-1 cells was over 4 mM, thus MTPE uniquely induces differentiation (0.25 to 2 mM) at noncytostatic levels. However, MTPE did not induce any duct formation at 4 mM in RCM-1 cells. These results are similar to kaempferol, which is a known inducer of differentiation, which also did not induce duct formations at cytostatic doses (23). We consequently hypothesized that the RCM-1 cells will show duct formation without cyctostatic-induced cell growth arrest by differentiation inducers.
MTPE, a common component in melons, was first identified as a volatile constituent of muskmelon (C. melo var. reticulatus) fruit (24). Following the report, different cultivars of melons such as cantaloupe (C. melo var. cantalupensis), golden crispy (C. melo cv. golden crispy), and Queen Anne’s pocket melon (C. melo var. dudaim) were found to be a rich source of MTPE (25–27). MTPE is also found in the fruits of other plant families, such as prickly pear (Opuntia ficus) and Asian pear (Pyrus serotina). Presently, the distribution of MTPE is considered to be mainly in melons, and to a lesser extent in some fragrant fruits (28, 29). MTPE is formed as a fragrant ester of fruits that correlate with ripening, and for many years the function of this compound was considered to be primarily for the production of these odors in the ripening process of melon type fruits. However, the potential medical benefits of this fragrant ester have not been realized. The results of this study indicating that MTPE can induce differentiation of the RCM-1 well-differentiated human colon cancer cells is the first report of anticarcinogenic properties for this compound. Our findings indicate that the chemopreventative properties of a fruit, such as Katsura-uri, Makuwa-uri, and muskmelon, can depend on phytochemicals, i.e., MTPE, which are neither the vitamin nor fiber-based components. In addition, wild melons contain more MTPE than the midshelf and long-shelf life cultivars indicating differences in the ripening behaviors (30), and can thus influence the anticarcinogenic potential of melons.
Lastly, since MTPE identified in this study represents a principal differentiation activity found in a fully ripened Katsura-uri Japanese pickling melon and other melon cultivars, future experiments determining the underlying mechanisms of how this compound induces differentiation will help us understand its anticarcinogenic properties.
Acknowledgments
We thank Mr. Kinji Ueda of Kanematsu greengrocery in Nishiki Market (Kyoto, Japan) for supplying gratis some plant samples, muskmelon and Makuwa-uri.
This research was partially supported by NIEHS grant no. R01 ES013268-01A2 to Upham and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
ABBREVIATIONS USED
- MTPE
3-methylthiopropionic acid ethyl ester
- ALPase
alkaline phosphatase
LITERATURE CITED
- 1.Nakamura Y, Suganuma E, Kuyama N, Sato K, Ohtsuki K. Comparative bio-antimutagenicity of common vegetables and traditional vegetables in Kyoto. Biosci Biotechnol Biochem. 1998;62:1161–1165. doi: 10.1271/bbb.62.1161. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura Y, Matsuo T, Shimoi K, Nakamura Y, Tomita I. S-Methyl methanethiosulfonate, bio-antimutagen in the homogenates of Cruciferae and Liliaceae vegetables. Biosci Biotechnol Biochem. 1996;60:1439–443. doi: 10.1271/bbb.60.1439. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura Y, Iwahashi T, Tanaka A, Koutani J, Matsuo T, Okamoto S, Sato K, Ohtsuki K. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish) J Agric Food Chem. 2001;49:5755–5560. doi: 10.1021/jf0108415. [DOI] [PubMed] [Google Scholar]
- 4.Kawamori T, Tanaka T, Ohnishi M, Hirose Y, Nakamura Y, Satoh K, Hara A, Mori H. Chemopreventionofazoxymethane-induced colon carcinogenesis by dietary feeding of S-methyl methane thiosulfonate in male F344 rats. Cancer Res. 1995;55:4053–4058. [PubMed] [Google Scholar]
- 5.Sugie S, Okamoto K, Ohnishi M, Makita H, Kawamori T, Watanabe T, Tanaka T, Nakamura Y, Nakamura Y, Tomita I, Mori H. Suppressive effects of S-methyl methanethiosulfonate on promotion stage of diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis model. Jpn J Cancer Res. 1997;88:5–11. doi: 10.1111/j.1349-7006.1997.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Y, Kawai K, Furukawa H, Matsuo T, Shimoi K, Tomita I, Nakamura Y. Suppressing effects of S-methyl methanethiosulfonate and diphenyl disulfide on mitomycin C induced somatic mutation and recombination in Drosophila melanogaster and micronuclei in mice. Mutat Res. 1997;385:41–46. doi: 10.1016/s0921-8777(97)00033-5. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Nakamura Y, Nakamura Y. Suppression of aflatoxin B1- or methyl methanesulfonate-induced chromosome aberrations in rat bone marrow cells after treatment with S-methyl methanethiosulfonate. Mutat Res. 1997;393:307–316. doi: 10.1016/s1383-5718(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa A, Kitamura Y, Kuroiwa Y, Kanki K, Ishii Y, Umemura T, Nakamura Y, Sato K, Ohtsuki K, Hirose M. Chemopreventive effects of naturally occurring isothiocyanates on pancreatic carcinogenesis in hamsters. The International Society of Cancer Prevention Symposium; May 20, 2005; Kyoto, Japan. [Google Scholar]
- 9.Ferrández A, DiSario JA. Colorectal cancer: screening and surveillance for high-risk individuals. Expert Rev Anticancer Ther. 2003;3:851–862. doi: 10.1586/14737140.3.6.851. [DOI] [PubMed] [Google Scholar]
- 10.Telang NT, Li G, Katdare M. Prevention of early-onset familial/hereditary colon cancer: new models and mechanistic biomarkers (review) Int J Oncol. 2006;28:1523–1529. [PubMed] [Google Scholar]
- 11.Umar A, Viner JL, Hawk ET. The future of colon cancer prevention. Ann NY Acad Sci. 2001;952:88–108. doi: 10.1111/j.1749-6632.2001.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacob BP, Salky B. Laparoscopic colectomy for colon adenocarcinoma: an 11-year retrospective review with 5-year survival rates. Surg Endosc. 2005;19:643–639. doi: 10.1007/s00464-004-8921-y. [DOI] [PubMed] [Google Scholar]
- 13.Kahnamoui K, Cadeddu M, Farrokhyar F, Anvari M. Laparoscopic surgery for colon cancer: a systematic review. J Can Chir. 2007;50:48–57. [PMC free article] [PubMed] [Google Scholar]
- 14.Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–238. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- 15.Hofer H, Ho G, Peterlik M, Uskokovic MR, Lee JK, White MC, Posner GH, Cross HS. Biological effects of 1α-hydroxy- and 1β-(hydroxymethyl)-vitamin D compounds relevant for potential colorectal cancer therapy. J Pharmacol Exp Ther. 1999;291:450–455. [PubMed] [Google Scholar]
- 16.Fu H, Shi YQ, Mo SJ. Effect of short-chain fatty acids on the proliferation and differentiation of the human colonic adenocarcinoma cell line Caco-2. Chin J Dig Dis. 2004;5:115–117. doi: 10.1111/j.1443-9573.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 17.Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005;50:490–498. doi: 10.1007/s10620-005-2463-6. [DOI] [PubMed] [Google Scholar]
- 18.Comalada M, Bailon E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Galvez JJ. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. Cancer Res Clin Oncol. 2006;132:487–497. doi: 10.1007/s00432-006-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes FJ, Centelles JJ, Lupianez JA, Cascante M. (2α, 3β)-2 3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006;580:6302–6310. doi: 10.1016/j.febslet.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Chang CC, Mori T, Sato K, Ohtsuki K, Upham BL, Trosko JE. Augmentation of differentiation, gap junction function by kaempferol in partially-differentiated colon cancer cells. Carcinogenesis. 2005;26:665–671. doi: 10.1093/carcin/bgi003. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka H, Nabeshima K, Komada N, Koono M. New human colorectal carcinoma cell lines that secrete proteinase inhibitors in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:157–165. doi: 10.1007/BF02899077. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Nakayama Y, Park E, Sato K, Upham BL, Chang C-C, Trosko JE. Development of an assay system for simple and easy determination of cell differentiation in well-differentiated human colon cancer cells. Society of Toxicology Annual Meeting; March 29, 2007; Charlotte, NC. [Google Scholar]
- 23.Nakayama Y, Nakamura Y, Sato K, Chang C-C, Upham BL, Trosko JE. Induction of differentiation by kaempferol in colon cancer cells with different differentiation profiles. Society of Toxicology Annual Meeting; March 6, 2006; San Diego, CA. [Google Scholar]
- 24.Kemp T, Stoltz L, Knavel D. Volatile components of muskmelon fruit. J Agric Food Chem. 1972;20:196–198. [Google Scholar]
- 25.Yabumoto K, Jennings W. Volatile constituents of cantaloupe, Cucumis melo, and their biogenesis. J Food Sci. 1977;42:32–37. [Google Scholar]
- 26.Wyllie G, Leach D. Aroma volatiles of Cucumis melo cv. golden crispy. J Agric Food Chem. 1990;38:2042–2044. [Google Scholar]
- 27.Aubert C, Pitrat M. Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J Agric Food Chem. 2006;56:8177–8182. doi: 10.1021/jf061415s. [DOI] [PubMed] [Google Scholar]
- 28.Flath R, Takahashi J. Volatile constituents of prickly pear (Opuntia ficus indica Mill de Castilla variety) J Agric Food Chem. 1978;26:835–837. [Google Scholar]
- 29.Takeoka G, Buttery R, Flath R. Volatile constituents of Asian pear (Pyrus serotina) J Agric Food Chem. 1992;40:1925–1929. [Google Scholar]
- 30.Aubert C, Bourger NJ. Investigation of volatiles in Charentais cantaloupe melons (Cucumis melo var cantalupensis). Characterization of aroma constituents in some cultivars. J Agric Food Chem. 2004;52:4522–4528. doi: 10.1021/jf049777s. [DOI] [PubMed] [Google Scholar]


