Abstract
The goal of the present study was to determine an optimum exposure regimen for alterations in sleep induced by chronic alcohol treatments in rats. We used two different exposure routes (alcohol in water and alcohol in liquid diet at two different doses in each regimen (6% and 12% alcohol in water and 3% and 6% alcohol in liquid diet)). All treatments were for 6 weeks. We found the effects of the 6% and 12% in water and 3% in liquid diet to be very similar; all three produced increases in slow-wave sleep (SWS) only in the dark period with no changes in rapid-eye-movement sleep (REMS). On the other hand 6% alcohol in liquid diet caused much more dramatic changes, with alterations in both SWS and REMS in both the dark and light periods. These animals spent less time in SWS and REMS during the light period but more time in SWS and REMS in the dark period. Additionally, the variation of slow-wave amplitude (SWA) across day and night in this group of alcoholic animals is blunted with the loss of the peak of SWA at the beginning of light onset compared to the other groups. We conclude that future alcohol treatment regimens used to investigate the effects of alcohol on sleep in adult rats should use an exposure protocol of at least 6 weeks with 6% alcohol in liquid diet.
Keywords: Alcohol, Sleep, Alcoholism, Chronic alcohol, Sleep disturbance
1. Introduction
Disrupted sleep is a frequent complaint in human alcoholics. During periods of alcohol consumption sleep is fragmented with frequent wakenings and suppression of rapid-eye-movement sleep (REMS) (Brower, 2001; Mello and Mendelson, 1970). During early abstinence there is a rebound in REMS but sleep onset remains delayed and sleep efficiency (percent time in bed asleep) continues to be reduced (Aubin et al., 1993; Brower and Hall, 2001; Drummond et al., 1998; Gann et al., 2001; Gillin et al., 1990, 1994; Snyder and Karacan, 1985; Williams and Rundell, 1981). In addition, alcohol can also intensify other sleep disorders such as sleep apnea and periodic limb movements (Aubin et al., 1993; Brower and Hall, 2001; Gann et al., 2002; Landolt and Borbely, 2000). Many of these altered sleep patterns persist even during prolonged recovery from drinking (Aubin et al., 1993; Brower, 2001; Drummond et al., 1998; Landolt and Borbely, 2000; Williams and Rundell, 1981). Disrupted sleep patterns, especially increased REMS pressure during early abstinence and continued insomnia, is predictive to relapse into drinking behavior (Brower, 2003; Brower et al., 1998; Drummond et al., 1998; Gann et al., 2001; Gillin et al., 1994). Despite the well documented fact of disturbed sleep in alcoholics, and the strong relationship between poor sleep and relapse drinking, the basis for chronic alcohol induced alteration of sleep is poorly understood.
An animal model would be a powerful tool that could help us understand the cause of alcohol induced alterations in sleep. However, only a few studies have directly assessed this issue. Some studies have used indirect measures of sleep, such as patterns of running wheel activity or loss of righting reflex, that fail to reveal changes in sleep architecture. In studies that use EEG recordings to assess sleep, alcohol induced alterations in sleep architecture have been observed. For example, Ehlers and Slawecki have reported that rats exposed to 6 weeks of ethanol vapor had a reduction in the spectral power of the EEG in both low frequency and high frequency bands, but no changes in slow-wave sleep (SWS) latency or duration (Ehlers and Slawecki, 2000). They also found a suppression of the evoked potential and rebound enhancement of the EEG power during withdrawal (Ehlers and Chaplin, 1991). However, one issue in these studies was that EEG recordings were performed for only short periods of time (4 h), thus changes in daily sleep patterns could not be observed. Veatch (2006) and Mendelson et al. (1978) both found loss of total sleep time with ethanol exposure, and a rebound in REMS during withdrawal, but their exposure protocols were relatively brief (4 days or less) and they focused mostly on the withdrawal period rather than on the pathologies associated with chronic alcohol exposure. Two prior studies have focused specifically on the alcohol induced pathologies that follow chronic alcohol exposure for 2–3 weeks. In the study by Rouhani et al. (1990) alcohol was given by scheduled infusions via an indwelling gastric catheter. After 13 days they observed decreases in percent SWS and REMS and increase in wake only in the light period. In the study by Kubota et al. (2002) alcohol was given in a liquid diet (6% alcohol) for 3 weeks. They observed a suppression of SWS in the dark period and a blunting of the normal circadian variation of REMS, that is, the amount of REMS decreased in the light period but increased in the dark period. Finally, like the study by Ehlers and Slawecki (2000), they observed a suppression of the power spectra of the EEG in the high frequency bands (>5.0 Hz). While these studies document that alcohol can induce changes in sleep architecture in rodents, they represent only the beginning in understanding the relationship between alcohol consumption and altered sleep.
In the present study we modeled our alcohol exposure paradigm on the one used by Kubota et al. (2002), 6% alcohol in liquid diet. We decided to use this paradigm as opposed to alcohol vapor or scheduled alcohol infusions because if alcohol was inducing alterations in sleep patterns, a behavior with a very strong circadian rhythm, allowing the animal some choice as to when to or not to consume alcohol may have a significant impact on the alterations of sleep patterns observed. We directly addressed two questions in this study. The first was whether 3% alcohol in liquid diet for 6 weeks would be as effective in inducing alcohol induced changes in sleep patterns as 6% alcohol in liquid diet for 6 weeks. The second was whether alcohol delivered in water rather than food would produce sleep pathologies that differed from those when the alcohol was given in food. We found that both the lower dose (3% in liquid diet) and alcohol delivered in water were capable of inducing sleep pathologies similar to those observed by Kubota et al. (2002). However, we also found that the 6% alcohol in liquid diet for 6 weeks produced even more profound alterations in sleep than any of the other exposure regimens and that the alterations induced by this regimen most mimicked the effects observed in human alcoholics. Because of this finding the 6% alcohol in liquid diet for 6 weeks should be at least the minimal exposure regimen in future studies in this area of research.
2. Results
2.1. Alcohol consumption
At the end of the treatment regimens the average daily alcohol consumption was calculated for each protocol. For the animals treated with alcohol in liquid diet the 6% alcohol animals consumed 11.1±0.2 g alcohol/kg body weight/day whereas the 3% alcohol animals consumed 5.5±0.1 g alcohol/kg body weight/day. We have previously reported that the 6% in liquid diet produces blood alcohol levels of 0.11 to 0.13% at the end of the dark period (Kubota et al., 2002). It was our intention that the amount of alcohol consumed in the alcohol in water diets would be similar to that consumed in the liquid diet, and we had noted that the rats drank about half the volume of water compared with the liquid diet (unpublished observation), so we selected 6% and 12% alcohol in water for these treatments. Unfortunately, when alcohol was added to the water, the amount of water consumed by the rats was less than what we had anticipated and the average daily alcohol consumption for these rats were 3.9±0.2 g alcohol/kg body weight/day for the 6% in water group and 7.3±0.2 g alcohol/kg body weight/day for the 12% in water group. We did not want to push the concentration of alcohol in water any higher than 12% because in other experiments we have performed, we found that when rats consume 15% alcohol in water with ad lib access, they further reduce their water consumption, and start to show signs of dehydration as measured by rapid free water consumption when a source of tap water is placed into the cages (unpublished observation).
2.2. Weight gain during alcohol treatments
While all the rats used in the protocols were young adult males, because of the limited number of recording chambers, the start of the diets were staggered, and thus the average starting weights of the different groups tended to be slightly different. In addition, we have previously found that when rats are first started on alcohol in liquid diet, they eat less the first few days of the protocol until they acclimate to the presence of alcohol in their diets. Finally, the rate of weight gain in rats is somewhat dependent on their starting weight in that as they get bigger, their weight gain tends to become slow. Thus to make comparisons of weight gains for the different treatment groups we examined the rate of gain for each group when the animals had been into their individual protocols at least a week (range 7 to 25 days), and the group average weight was 350±10 g. Weight gains were then calculated over a period of ~3 weeks (17 to 24 days). The one exception to this was the 6% alcohol in water group that was slightly larger than the other animals at the start of their regimen, so the starting point for their average daily gain was when the group average was 400 g. The values for the average daily gain are shown in Fig. 1. As can be seen, the group that had ab lib access to pelleted food and water group grew the fastest. While the other groups were not statistically different from each other, there was a trend for the 3% in liquid diet group (and their pair-fed controls) to gain at a slightly higher rate than the other groups. Finally, one would expect the 6% in water group to gain somewhat faster than what we observe since this was the group with the lowest alcohol intake, but they were virtually identical to the other treatment groups. This is perhaps due to their slightly larger size at the beginning of the measurement.
Fig. 1.
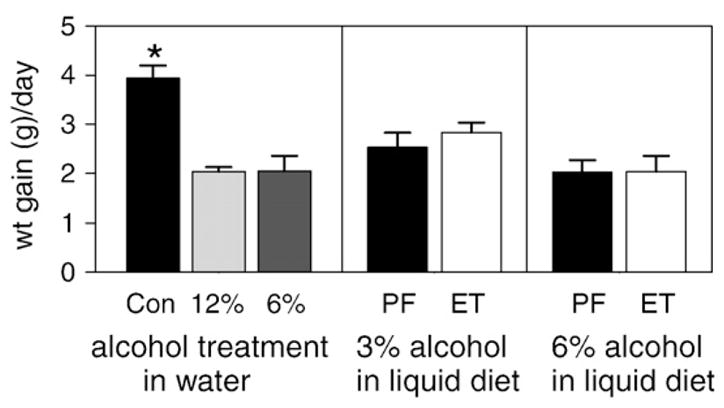
Average weight gain (in g) per day from rats in the various treatment groups. Weight gains were monitored after at least 1 week in treatment over a period of 3 weeks. Values are averages ± SE (N=6 to 8 rats in each group). The bar labeled Con is the ad lib food and water control, the bars labeled 12% and 6% are from the rats treated with 12% and 6% alcohol in water, and the bars labeled PF and ET are from the pair-fed and alcohol in liquid diet treated groups, respectively. The asterisk indicates the group significantly different from all other groups (p<0.01, ANOVA).
2.3. Comparisons of sleep among the control groups
We compared sleep parameters among the two pair-fed control groups and the control for the water treated groups (Fig. 2). While there were no differences found between the ad lib water and food control and the pair-fed to the 3% alcohol in liquid diet group in any parameter, the pair-fed to the 6% alcohol in liquid diet group had statistically significant more SWS and less wake than the other two control groups in the light period (compare solid bars in unshaded panels; p<0.007 for wake and p<0.008 for SWS). This group also had more SWS and less wake in the dark period that nearly reached statistical significance (compare solid bars in shaded panels; p=0.12 for wake and p=0.056 for SWS). There were no differences in amount of REMS between any of the control groups in either the light or dark period (Fig. 2).
Fig. 2.
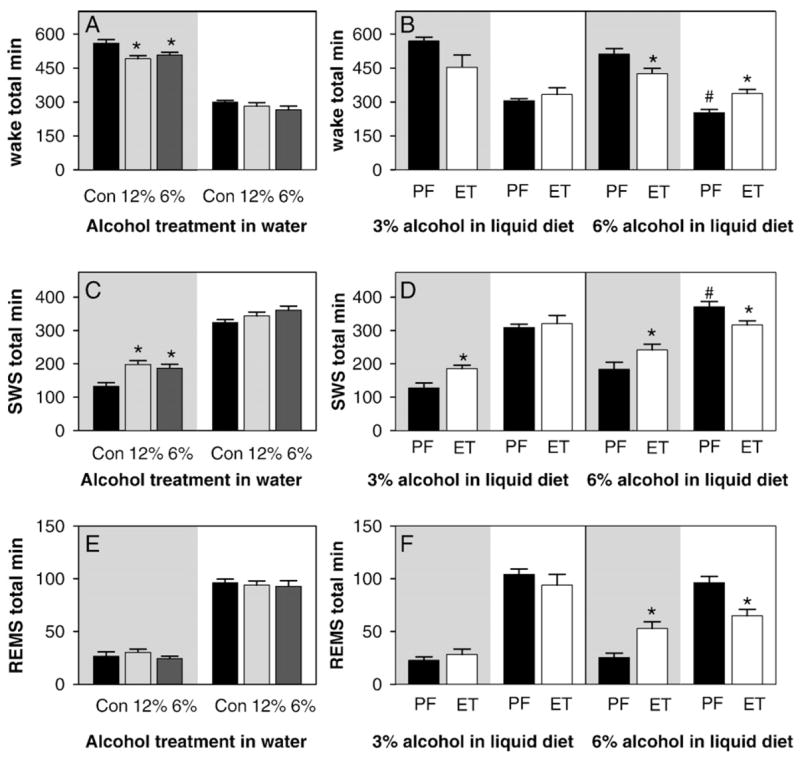
Total time spent in different vigilance states (wake, SWS — slow-wave sleep; REMS — rapid-eye-movement sleep). In this and other figures the shaded panel denotes the dark period and the unshaded panel denotes the light period. All values are expressed as total min within the period. Light period values, recorded for only 10 h, have been multiplied by 1.2 so that they can be directly compared to dark period values (recorded for 12 h). The results from the animals treated with alcohol in water are shown in the left hand panels (A, C, and E). The solid black bars (labeled Con) are from the ad lib control animals, the light gray bars and dark gray bars are from the 12% alcohol and 6% alcohol in water groups, respectively. Asterisks indicate that the value is significantly different from control (p<0.05 by ANOVA with Holm–Sidak post hoc comparisons). The results from the animals treated with alcohol in liquid diet are shown in the right hand panels (B, D, and F). Asterisks indicate the values significantly different from the adjacent PF value (p<0.05, non-paired t-test). Pound signs indicate the values significantly different from the control recordings from other groups (compare solid bars within shaded or within unshaded panels, p<0.05, ANOVA).
2.4. Effects of alcohol treatment on sleep
Treatment with alcohol in water (12% or 6%) caused a decrease in the time spent in wake and an increase in the time spent in SWS only in the dark period (Figs. 2A and C). The time spent in REMS was not altered (Fig. 2E). When 3% alcohol in liquid diet was given to the rats, only the time spent in SWS in the dark period was statistically significantly altered (Fig. 2D, left panel). There was a non-significant decrease in the time spent in wake in the dark period that almost reached statistical significance (Fig. 2B, left panel, p=0.097). As with the alcohol treatments in water, REMS was not affected (Fig. 2F, left panel).
While the altered patterns of sleep produced by the 6% and 12% alcohol in water, and 3% alcohol in liquid diet were very similar to one another, the effects of 6% alcohol in liquid diet were much more dramatic in altering sleep patterns. All three vigilance states were altered by the alcohol treatment (Figs. 2B, D, F, right hand panels). Interestingly, while wake was suppressed and SWS and REMS were increased in the dark period, the changes in the light period were in the opposite direction.
The duration and the number of episodes of SWS and REMS of animals treated with alcohol in water are reported in Table 1. While the overall time spent in SWS is increased in the dark period for both these groups, at this sub-level of analysis the only parameter that was statistically significantly altered was an increase in episode duration in the 12% alcohol in water group, although the same parameter for the 6% alcohol in water almost reach statistical significance (p=0.039 but critical level in the multiple comparison test was 0.025). The number of SWS episodes also tended longer in the alcohol treated groups but the changes were not statistically significant (p=0.111 between groups). However, when these two parameters are multiplied together to produce total SWS time (Fig. 2C) the increase in SWS in the dark period is clearly significant.
Table 1.
SWS and REMS episode durations and number of episodes when alcohol was administered in water
| Parameter | Episode duration (s)
|
Number of episodes
|
||
|---|---|---|---|---|
| Dark period | Light period | Dark period | Light period | |
| SWS | ||||
| Control: | 61.9±3.9 | 95.3±5.0 | 135.0±4.3 | 165.8±4.8 |
| 12% alcohol: | 76.9±2.5* | 115.6±6.0 | 155.2±9.3 | 150.8±8.9 |
| 6% alcohol: | 73.8±4.6 | 110.4±10.4 | 153.0±6.2 | 169.9±11.4 |
| REMS | ||||
| Control: | 67.8±3.8 | 88.7±3.9 | 26.5±4.8 | 56.2±3.2 |
| 12% alcohol: | 58.0±3.3 | 84.9±3.2 | 31.0±1.7 | 55.8±3.2 |
| 6% alcohol: | 58.7±5.0 | 94.0±5.7 | 25.7±2.4 | 50.1±3.2 |
The episode duration of SWS or REMS is the average duration of individual SWS or REMS episodes within the dark or light periods, while the number of episodes is the average of the total number of episodes for each individual animal in the respective periods. The dark period episode numbers are over 12 h whereas the light period episode numbers are for 10 h. Values are averages ± SE; N=6 for ad lib controls, N =6 for animals treated with 12% alcohol, and N=7 for animals treated with 6% alcohol. Asterisks: significantly different from the ad lib control
p<0.05; one way ANOVA.
As with the alcohol treatments in water, the sub-level analysis of episode duration and frequency for the 3% alcohol in liquid diet (Table 2) revealed no statistically significant alterations, although there were trends in both increase episode duration (p=0.077) and increase episode frequency (p=0.413), that while by themselves were not statistically significant, produced a statistically significant increase in total SWS in this group (Fig. 2D, left hand panel). On the other hand, animals treated with 6% alcohol in liquid diet had multiple alterations in the sub-level analysis (Table 2). These animals had a dramatic increase in SWS episode duration that mostly accounts for the prolonged SWS in the dark period. The loss of SWS in the light period was due primarily to a decrease in the frequency of SWS episodes, rather than a change in duration. The same is true of REMS episodes. The increase in total REMS in the dark period is due to a dramatic increase in REMS episode duration, whereas the loss of REMS in the light period is due to a dramatic decrease in the number of REMS episodes.
Table 2.
SWS and REMS episode durations and number of episode when alcohol was administered in liquid diet
| Parameter | Episode duration (s)
|
Number of episodes
|
||
|---|---|---|---|---|
| Dark period | Light period | Dark period | Light period | |
| 3% alcohol (liquid diet) | ||||
| SWS | ||||
| Pair-fed: | 63.7±6.3 | 90.0±4.4 | 123.0±13.5 | 222.6±56.8 |
| Alcoholic | 83.5±7.4 | 92.8±14.7 | 136.7±9.3 | 184.0±14.2 |
| REMS | ||||
| Pair-fed: | 68.9±7.2 | 83.7±4.8 | 20.8±3.9 | 63.2±4.8 |
| Alcoholic | 67.8±6.1 | 85.2±4.7 | 25.3±4.2 | 55.8±6.9 |
| 6% alcohol (liquid diet) | ||||
| SWS | ||||
| Pair-fed: | 70.1±9.3 | 108.0±10.5 | 154.8±13.9 | 173.2±9.4 |
| Alcoholic: | 99.8±5.2* | 113.3±9.4 | 144.1±8.5 | 142.9±6.5* |
| REMS | ||||
| Pair-fed: | 57.6±6.1 | 86.0±4.5 | 27.0±3.5 | 58.3±5.8 |
| Alcoholic: | 85.7±6.2* | 81.2±5.6 | 36.8±3.4 | 41.1±4.7* |
The episode duration of SWS or REMS is the average duration of individual SWS or REMS episodes within the light or dark periods, while the number of episodes is the average of the total number of episodes for each individual animal in the respective periods. The dark period episode numbers are over 12 h whereas light period episode numbers are for 10 h. Values are averages ± SE; N=6 for 3% alcohol treated animals, N=5 for pair-fed to 3% alcohol treated animals, N=8 for 6% alcohol treated animals, and N=6 for pair-fed to 6% alcohol treated animals. Asterisks: significantly different from the pair-fed control
p<0.05; non-paired t-test.
An additional parameter that we examined was the slow-wave amplitude (SWA) across the day. SWA represent the delta power (0.5 to 4 Hz) during SWS and is generally interpreted as an indicator of the depth of the SWS period. To examine SWA across the day we averaged the amplitude of all delta wave frequency components within SWS episodes within each 2-h block of time across the day and then determined an average value for the entire day by averaging these 2-h block values together. The SWA of each individual block is then expressed as a percent of this average value. In Fig. 3 the SWA of the first 2 h of dark, the first 2 h of light, and the last 2 h of light (hours 8–10 of the light period) are shown for each treatment group. As is normal, the SWA for almost all treatment groups is highest at the onset of sleep (i.e., the first 2 h of the light period). The one exception was the animals treated with 6% alcohol in liquid diet. These animals had an abolition of the peak of SWA at sleep onset (Fig. 3C, middle panel). This loss of circadian variation in SWA is similar to the blunting of circadian variation of other sleep parameters produced by the 6% alcohol in liquid diet regimen.
Fig. 3.
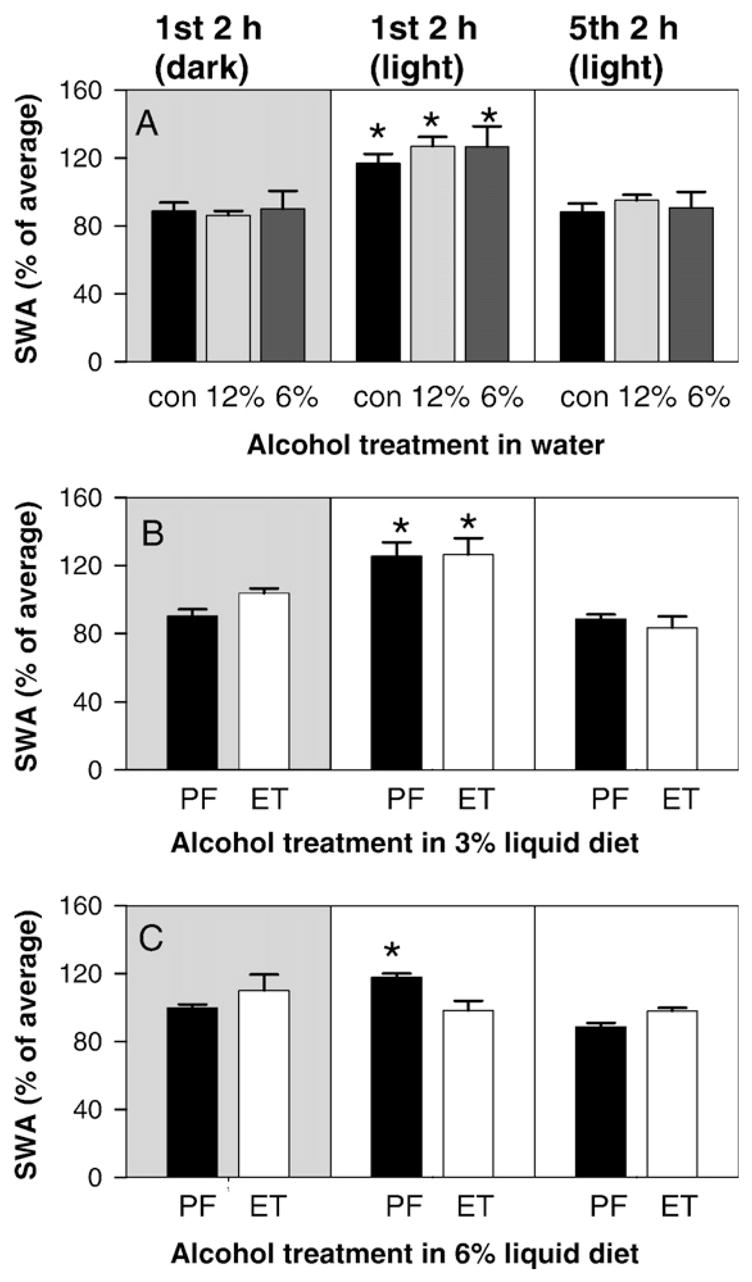
Variation of slow-wave amplitude (SWA) in alcohol in water treated animals (top panel), in 3% alcohol in liquid diet group (middle panel), and 6% alcohol in liquid diet group (bottom panel). The shaded left most panels show SWA of the 1st 2 h of the dark period. The unshaded middle panels show SWA in the 1st 2 h of the light period, and the right most unshaded panels show SWA in the last 2 recorded hours of the light period (hours 8–10 of the light period). SWA amplitude is compared within a group across time. The peak of the SWA occurs during the first 2-h block after lights on (first long sleep period) in all animals except the animals treated with 6% alcohol in liquid diet. Comparisons within treatments were made by one way ANOVA (asterisks indicate p<0.05 compared to other time periods).
3. Discussion
In the present study we examined the effects of different chronic alcohol treatment regimens on sleep in rats. We found that alcohol given in water (6% or 12%) was capable of altering sleep parameters similar to that observed with 3% alcohol given in a liquid diet. Given that the measured amount of alcohol consumed in these various protocols were similar (3.9, 5.5, and 7.3 g alcohol/kg body weight/day), it would suggest that administration of alcohol in liquid diet or water makes no difference in the final effect of alcohol. However, the most significant finding was that the group treated with 6% alcohol in liquid diet had much more dramatic alterations in sleep patterns than any of the other groups. Further, the alterations seen in our group treated with 6% alcohol in liquid diet for 6 weeks were also much more dramatic than what our group has previously shown to occur with the 6% alcohol diet for 3 weeks (Kubota et al., 2002). The major difference between the 6% alcohol in liquid diet and 3% alcohol in liquid diet is the exposure amount (11.1 g/kg/day vs. 5.5 g/kg/day). These findings demonstrate that exposures for periods shorter than 6 weeks, and at levels less than those achieved by 6% alcohol in liquid diet, do not fully elaborate all the changes that can occur with chronic alcohol exposure on sleep mechanisms.
The lesser alcohol exposure regimens did not necessarily produce alterations in sleep patterns that were fundamentally different than what we observed with 6% alcohol in liquid diet, rather the trends that were apparent in the lesser exposure regimens appear to not have fully elaborated. For example, all regimens increased SWS in the dark period, but only the 6% in liquid diet caused a reciprocal decrease in SWS in the light period. Likewise, all exposure regimens decreased wake in the dark period, but only the 6% in liquid diet increased wake in the light period. The nature of these changes agrees well with previous reports. Kubota et al. (2002) also observed an increase in SWS in the dark period after 3 weeks of 6% alcohol in liquid diet, similar to all the protocols we used for 6 weeks. Interestingly, they observed the same suppression of REMS in the light period with a reciprocal increase of REMS in the dark period that we observed. This would suggest that perhaps REMS is more sensitive to the amount of alcohol (observed in both studies when 6% in liquid diet was used for either 3 weeks or 6 weeks) but that changes in SWS require longer treatments (decreased SWS in the light period was only observed after 6 weeks of 6% alcohol in liquid diet). In further agreement that REMS is more sensitive to the amount of alcohol given, Rouhani et al. (1990) reported a decrease of REMS in the light period after 2 weeks when alcohol (10 g/kg/day) was given by gastric infusion. This is an exposure level very similar to our 6% in liquid diet. Rouhani et al. also observed increases in wake with loss of SWS, but only in the light period, again similar to our observations with 6% alcohol in liquid diet. On the other hand, Rouhani et al. did not observe any changes in sleep parameters in the dark period. Perhaps the failure of Rouhani et al. to observe changes in the dark is due to the nature of the schedule infusion of alcohol (equal volumes given at 4–5 h intervals across the day to maintain a constant exposure level across the day) rather than allowing the rats to consume alcohol more in tune with their normal circadian patterns (the vast majority of drinking in our protocols (estimated at >80%) was during the dark period (unpublished observation). More insight into the nature of the changes in sleep patterns induced by 6% alcohol in liquid diet can be obtained by a more detailed examination of the results from these animals. We are currently performing these additional analyses on this set of animals to be published in a subsequent report.
These observations suggest that allowing the animals to consume alcohol on a more natural schedule may be important in observing changes in a behavior that has a strong circadian entrainment. While controlled gastric infusion and alcohol vapor may be very effective means to get rats to be dependent on alcohol in a relatively short period of time, these may not be ideal protocols for studies of alcohol in sleep. Indeed, our protocols suffer from the mixing of alcohol with nutritive intake or water intake. Thus the motivation to consume alcohol is mixed with other motivational drives, and may result in a somewhat unnatural pattern of alcohol consumption. This issue remains to be tested in future studies.
Another possible weakness in our study was that we did not get as close of a match as we had wanted between levels of alcohol consumption and the two delivery vehicles. This weakness will always be a potential confounding influence when the rats are allowed to self-regulate their consumption levels. However, because the alcohol consumed in the two alcohol in water protocols bracketed the alcohol consumed in the 3% alcohol in liquid diet protocol, and for the most part the effects on the sleep patterns were similar, we are somewhat confident that we can conclude water or food delivery does not have a major impact on the nature of the changes, it is just easier to get more alcohol in the animals when the alcohol is mixed with food. On the other hand, perhaps if the exposure protocols were to run for longer than 6 weeks, we might be able to tease apart differences in the nature of the alcohol effect on sleep when given in water or food. This issue remains for future study.
A final issue with our study is that we did observe significant differences in rate of weight gain between our groups, and we did observe increased SWS in our pair-fed controls to the 6% alcohol in liquid diet as compared to the other two control groups (ad lib control and pair-fed to 3% in liquid diet). The fact that the ad lib control and pair-fed to 3% alcohol in liquid diet had no differences in their sleep while they gained weight at significantly different rates, suggests rate of weight gain in and of itself does not account for any of the effects we observed. A possible reason for the increased total SWS in the pair-fed controls to the 6% alcohol in liquid diet could be that others have found that food restriction, specifically when access to food is restricted to just the dark period, does increase SWS in the dark period (Alvarenga et al., 2005). While the degree of decreased food consumption in our study is nowhere near that in the Alvarenga et al. study (2005), the changes we observed in this control group relative to the others were also more modest than that observed by Alvarenga et al. In spite of this change in the control group behavior, the alcohol treatment led to an even further increase in SWS in the light period. Because the 6% alcohol in liquid diet and their pair-fed controls consumed the same amount of food, we are confident that the change in SWS between these groups can be contributed to alcohol treatment and not a decrement in food consumption relative to the animals that had ad lib access to food and water.
The connection between disturbed sleep patterns in sober alcoholics and propensity to relapse drinking remains unknown. It could be related to the decrement in performance that accompanies poor sleep contributes to psychological and/or social pressures to begin drinking again, or that recovered alcoholics with insomnia actually return to drinking as a form of self-medication (Brower, 2003), but scant support exists for either of these hypotheses. A number of investigators have begun to incorporate strategies for improving sleep in abstinent alcoholics with the hope that this will decrease relapse drinking (Allen et al., 1977; Arnedt et al., 2007; Gann et al., 2004; Hornyak et al., 2004; Karam-Hage and Brower, 2000; Staner et al., 2006; Zarcone and Hoddes, 1975). Obviously, improvements in this potential therapeutic intervention could be made if a better understanding of alcohol induced sleep pathologies was available. If an animal model is to be useful in uncovering the mechanistic basis for sleep pathologies associated with alcohol consumption, then the animal model should demonstrate similar pathologies to that observed in humans. With this perspective it is interesting to note that in the animal studies of chronic alcohol consumption, especially at the higher exposure levels, there is a loss of REMS and SWS in the rest period. This is similar to the loss of REMS and insomnia complaints of chronic alcoholics (Brower, 2003). It is also worth noting that in the present study we found that an alcohol exposure of at least 6 weeks was required to observe a more fully elaborated spectrum of sleep alterations. Given that human alcoholics may be abusing alcohol for decades prior to starting abstinence; it is possible that even 6 weeks of exposure in an animal model is too short to fully appreciate the effects that alcohol has to disrupt sleep. Thus we conclude that studies in this area should use a minimum exposure of at least 6 weeks in duration at a level of alcohol that is equivalent to 6% alcohol in liquid diet (or 11.5 g alcohol/kg body weight/day), and that future studies are warranted that further explore the relationship between amount of alcohol consumed and the length of time of exposure to the nature of the sleep pathologies produced.
4. Experimental procedures
4.1. Alcohol treatments
Forty-eight young adult (2–5 months of age) male Sprague–Dawley rats (Taconic, Germantown, NY) were used. The experiments were performed under protocols approved by the Institutional Animal Care and Use Committee at Washington State University. Animals were housed individually throughout the experiment. Alcohol treated rats were given grain alcohol (Everclear) v/v in liquid diet (Bio-Serv, Frenchtown, NJ) or in tap water. For the alcohol in liquid diet experiments each pair-fed rat was weight matched to a treated rat and given alcohol-free liquid diet of equal caloric content (calories from sucrose) as that consumed by the weight-matched alcoholic rat. All rats in the protocol where alcohol was given in liquid diet had ad lib access to water. Animals treated with alcohol in water had forced drinking but had ad lib access to pelleted food throughout the treatments. The control animals for the alcohol in water animals had ad lib access to pelleted food and water. The duration of alcohol exposure in all the protocols was for 6 weeks. The body weight of the rats was noted every three to four days, until the end of the experiment. Consumption of liquid diet or water in the alcohol in water groups was noted daily during the last hour of the light period.
4.2. Instrumentation for sleep recordings
All rats were instrumented in the fifth week of alcohol treatment. We followed standard instrumentation procedures as previously reported (Kubota et al., 2002). Briefly, under ketamine (87 mg/kg) and xylazine (13 mg/kg) anesthesia, 3 EEG electrodes (Plastics One, Roanoke, VA) were implanted, one in the left frontal (5 mm A and 2 mm L, from bregma), one over the right parietal (−5 mm A and 6 mm L, from bregma) and the ground electrode over the left occipital cortex (−11 mm and 4 mm L, from bregma). One EMG electrode was implanted in the dorsal neck muscle. The rats were allowed 1 week to recover from the instrumentation before sleep recordings commenced. The rats were maintained on the alcohol treatment during this period until the end of the recording.
4.3. Sleep recordings
To minimize any disturbances to the rats during the period of data collection, sleep recordings were made in enclosed environmental chambers (4 rats per chamber, each chamber ~6 ft ×2 ft ×6 ft; width × depth × height) with constant room air flow through the chamber. The interior of the chamber was illuminated by a 15 W light (12 h: 12 h light: dark cycle) and maintained at 22±2 °C. We began to collect EEG and EMG for analysis of sleep patterns after three days of acclimatization to the chambers. We recorded for two consecutive days. Recordings were interrupted for 1 h each day (last hour of the light period) for animal care. In the present study we report findings from recordings on the last recording day.
4.4. Collection and analysis of EEG and EMG signals
The EEG and EMG signals were collected through a wire harness tethered to an overhead multi-channel commutator (Plastics One, Roanoke, VA). The signals were amplified by polygraph amplifiers (Grass-Telefactor, Inc., West Warwick, RI) and digitized at 128 Hz through a computer software program (SleepSign for Animals, Kissei America Inc.). The EEG/EMG recordings were analyzed in 8-s epochs and each epoch was assigned to a particular vigilance state: wake, SWS, or REMS by standard criteria embedded in the software program. Each continuous period in the same vigilance state is referred to as an episode, and we placed a minimum requirement of three consecutive epochs before a change in vigilance state of episodes was noted. Thus minimum episode durations were 24 s in length. All scoring was visually inspected to ensure correct assignment of vigilance states. We scored recordings for 22 h of each 24 h period because of the daily disruption for animal care (12 h in the dark period and 10 h in the light period). In addition to assigning a vigilance state, the software program enabled us to analyze the EEG waveforms by fast Fourier transform within each epoch so that we could examine the power of the component frequencies within the EEG and determine slow-wave amplitude (SWA) from the power in the delta band (0.5–4 Hz) during SWS.
4.5. Statistical analysis
In the course of the experiment the instrumentation on four animals failed before the first sleep recordings, two pair fed in 6% alcohol in the liquid diet and one pair fed in 3% alcohol in liquid diet and one animal in 6% alcohol in water group. All the animals in the remaining protocols were well and the instrumentation remained intact to the end of the measurements. Sleep was analyzed for 12 h in the dark period and the following 10 h in the light period. In our analysis for the total time spent in the light period in each vigilance state, we correct for the last 2 h, by multiplying the total time by a factor of 1.2. We used SigmaStat version 3.5 for the statistical analysis. For alcohol in liquid diets, we used a regular, two-tailed, t-test to compare results from alcohol treated animals to the pair-fed controls. For other comparisons with more than two groups (alcohol in water) we used analysis of variance (ANOVA) for with the Holm–Sidak post hoc multiple comparison test. In all cases p<0.05 was considered statistically significant.
Acknowledgments
This work was supported by National Institute of Alcohol Abuse and Alcoholism grant No. AA13248 awarded to S.M.S.
Abbreviations
- EEG
electroencephalogram
- EMG
electromyogram
- REMS
rapid-eye-movement sleep
- SWS
slow-wave sleep
- SWA
slow-wave amplitude
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Allen RP, Wagma AM, Funderburk FR. Slow wave sleep changes: alcohol tolerance and treatment implications. Adv Exp Med Biol. 1977;85A:629–640. doi: 10.1007/978-1-4899-5181-6_40. [DOI] [PubMed] [Google Scholar]
- Alvarenga TAF, Andersen ML, Papale LA, Antunes IB, Tufik F. Influence of long term food restriction on sleep in male rats. Brain Res. 2005;1057:49–56. doi: 10.1016/j.brainres.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26:41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Monfort JC, Benoit O, Goldenberg F, Roullet-Volmi MC, Barrucand D. Alcohol, sleep and biological rhythms. Neurophysiol Clin. 1993;23:61–70. doi: 10.1016/s0987-7053(05)80283-7. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effect on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;6:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Hall JM. Effects of age and alcoholism on sleep: a controlled study. J Stud Alcohol. 2001;62:335–343. doi: 10.15288/jsa.2001.62.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcohol relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Drummond SPA, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology. 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Hohagen F, van Calker D, Geiss D, Dieter R. Sleep and cholinergic rapid eye movement sleep induction test in patient with primary alcohol dependence. Psychiatry. 2001;50:383–390. doi: 10.1016/s0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Fasishi S, van Calker D, Vorderholzer U, Reimann D. Periodic limb movements during sleep in alcohol dependent patients. Arch Psychiatry Clin Neurosci. 2002;252:124–129. doi: 10.1007/s00406-002-0371-8. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Cloot O, Van Wasen H, Zinzgraf D, Hohagen F, Riemann D. Polysomnography during withdrawal with clomethiazole or placebo in alcohol dependent patient—a double-blind and a randomized study. Pharmacopsychiatry. 2004;37:228–235. doi: 10.1055/s-2004-832597. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Schuckit M. EEG sleep studies in “pure” primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol Psychiatry. 1990;27:477–488. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at the time of hospital admission predicts relapse in nondepressed patients with primary alcoholism with three month follow-up. Arch Gen Psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Haas P, Veit J, Gann H, Riemann D. Magnesium treatment of primary alcohol-dependent patients during subacute withdrawal: an open pilot study with polysomnography. Alcohol Clin Exp Res. 2004;28:1702–1709. doi: 10.1097/01.alc.0000145695.52747.be. [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown R, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep. Alcohol Clin Exp Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Borbely AA. Alcohol and sleep disorders. Ther Umsch. 2000;57:241–245. doi: 10.1024/0040-5930.57.4.241. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JK. Behavioral studies of sleep patterns in alcoholics during intoxication and withdrawal. J Pharmacol Exp Ther. 1970;175:94–112. [PubMed] [Google Scholar]
- Mendelson WB, Majchrowicz E, Mirmirani N, Dawson S, Gillin JC, Wyatt RJ. Sleep during chronic ethanol administration and withdrawal in rats. J Stud Alcohol. 1978;39:1213–1223. doi: 10.15288/jsa.1978.39.1213. [DOI] [PubMed] [Google Scholar]
- Rouhani S, Emmanouilidis E, Tran G, Durlach J, Payan C, Fermanian J, Manicom R, Soulairac A, Poenaru S. Circadian variation in vigilance states in the alcohol-dependent rat. Physiol Behav. 1990;48:637–640. doi: 10.1016/0031-9384(90)90203-g. [DOI] [PubMed] [Google Scholar]
- Snyder S, Karacan I. Sleep patterns of sober chronic alcoholics. Neuropsychobiology. 1985;13:97–100. doi: 10.1159/000118169. [DOI] [PubMed] [Google Scholar]
- Staner L, Boeijinga P, Danel T, Gendre I, Muzet M, Landron F, Luthringer R. Effects of acamprosate on sleep during alcohol withdrawal: a double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res. 2006;30:1492–1499. doi: 10.1111/j.1530-0277.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- Veatch LM. Disruption in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH., Jr Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Cin Exp Res. 1981;5:318–325. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Zarcone VP, Jr, Hoddes E. Effect of 5-hydroxytrytophan on fragmentation of REM sleep in alcoholics. Am J Psychiatry. 1975;132:74–76. doi: 10.1176/ajp.132.1.74. [DOI] [PubMed] [Google Scholar]


