Abstract
Finely myelinated (type Aδ) and unmyelinated (type C) fibers are the major afferent inputs to spinothalamic tract neurons mediating sensory and reflex responses to noxious and thermal stimuli. These two fiber types differ in their sensory and biophysical properties, raising questions about the interaction of their supraspinal responses. Therefore, we investigated the interaction of cortical responses to stimuli that preferentially excite these fibers in human subjects using evoked potential recordings in a paired conditioning stimulation (CS) and test stimulation (TS) paradigm. There were two experiments, one with Aδ as CS and C as TS (Aδ-C) and another with these stimuli reversed (C-Aδ). We used intra-epidermal electrical pulses applied to the dorsal left hand at 2 and 1 × pinprick threshold (pp) for the preferential stimulation of Aδ fibers and 37 – 50°C contact heat pulses applied to the left or right thenar and left hypothenar eminences for the preferential stimulation of C fibers. We found that the cortical response to preferential Aδ or C fiber stimulation was attenuated whenever either cortical response preceded the other. Standardized values of peak and integrated amplitudes were < 1 in all paring conditions and in all subjects in both experiments. The suppressive effect varied in magnitude with the intensity of the conditioning stimulus in both Aδ-C and C-Aδ experiments. Furthermore, intra-segmental interaction was differentially effective for Aδ conditioning, (peak amplitude, p < 0.008; ANOVA). Our experiments provide the first neurophysiological evidence for a somatotopically distributed, mutually suppressive interaction between cortical responses to preferentially activated Aδ and C afferents in humans. This suppressive interaction of cortical responses suggests contrasting and possibly mutually exclusive sensori-motor functions mediated through the Aδ and C fiber afferent channels.
Keywords: somatosensory, inhibition, human, evoked potentials, cerebral cortex, pain, temperature, spinothalamic tract
As a population, spinothalamic neurons receive direct synaptic input from a variety of somatic and visceral afferent fibers. The cutaneous afferent inputs include large diameter myelinated (Aβ) fibers that are activated only by tactile stimuli and small diameter, finely myelinated (Aδ) and unmyelinated (C) fibers innervating receptors responding to innocuous and noxious mechanical and thermal stimulation. Some spinothalamic cells receive converging input from all of these fiber types, but others, such as neurons in laminae 1 and 2 of the spinal dorsal horn, respond only to thermal or noxious stimuli that excite Aδ or C fibers (for review, see (Cervero and Iggo, 1980),(Willis, 2004) and (Craig, 2003)).
Some Aδ and C fibers respond to tactile stimuli (Traub and Mendell, 1988; Vallbo et al., 1993), but a unique characteristic of both Aδ and C fibers is that they innervate somatic thermoreceptors and nociceptors (Burgess and Perl, 1967; Bessou and Perl, 1969; Beitel and Dubner, 1976; Perl, 1984; Traub and Mendell, 1988). Anatomical and physiological studies have consistently demonstrated the convergence of these inputs onto spinothalamic tract neurons in the substantia gelatinosa and deeper laminae of the spinal and medullary dorsal horn (Christensen and Perl, 1970; Chung et al., 1979; Light and Perl, 1979a; Gobel et al., 1981; Cruz et al., 1987; Rethelyi et al., 1989; Yoshimura and Jessell, 1989). Most studies have revealed a predominantly excitatory input from both fiber types with the relatively short duration postsynaptic response to stimulation of Aδ fibers preceding the C fiber response (Willis et al., 1974; Menetrey et al., 1977; Chung et al., 1979; Yoshimura and Jessell, 1989); this is consistent with the facilitatory effect of cutaneous thermal stimulation on human nocifensive reflexes (Plaghki et al., 1998).
There is evidence, however, that the interactions between the central processes initiated by Aδ and C fiber stimulation are complex, as suggested by the synaptic anatomy of the substantia gelatinosa (Light and Perl, 1979b; Cruz et al., 1987; Rethelyi et al., 1989). Electrophysiological investigations reveal both inhibitory and excitatory postsynaptic responses of nociresponsive dorsal horn neurons, including inhibitory interactions mediated through the activation of Aδ and C fibers (Christensen and Perl, 1970; Chung et al., 1984a; Chung et al., 1984b; Traub and Mendell, 1988; Yoshimura and Jessell, 1989; Tsuruoka et al., 1990; Schneider and Perl, 1994; Shimizu et al., 1995; Sandkuhler et al., 1997; Liu et al., 1998). Subsequent human neurophysiological and psychophysical studies revealed the well-known suppressive effect of tactile and vibrotactile stimulation on various forms of pain and pain-related evoked responses (Melzack and Wall, 1965; Zoppi et al., 1991; Marchand et al., 1991; Kakigi and Shibasaki, 1992; Akyuz et al., 1995; Svensson et al., 1999; Watanabe et al., 1999; Hoshiyama and Kakigi, 2000; Nahra and Plaghki, 2003) but the opposite interaction has been demonstrated also (Apkarian et al., 1992; Apkarian et al., 1994; Tran et al., 2003). Earlier human neurophysiological studies provided evidence that the activation of Aδ-fibers suppresses the cortical response mediated by C-fibers (Bromm and Treede, 1987a; Bromm and Treede, 1987b). Indeed, human cortical responses mediated exclusively by C-fibers can be elicited by only a few methods that selectively activate C-fibers without simultaneously activating Aδ-fibers (Magerl et al., 1999) (for review see (Plaghki and Mouraux, 2003)). Early psychophysical studies showed that heat stimulation suppresses Aδ fiber-mediated first pain sensation, but this was considered to be caused by suppression of Aδ heat nociceptors (Price et al., 1977). However, subsequent psychophysical investigations revealed that innocuous warm stimuli can markedly attenuate heat pain sensation by central mechanisms (Casey et al., 1993) and that homotopic electrocutaneous stimulation of Aδ and C fibers can markedly reduce heat and mechanical pain for nearly an hour (Nilsson and Schouenborg, 1999; Nilsson et al., 2003). The cumulative evidence thus suggests that human neurophysiological studies should show inhibitory interactions between supraspinal responses to Aδ and C fiber stimulation but this has not been investigated systematically.
To investigate the central, supraspinal interaction of Aδ and C fibers and to obtain estimates of the magnitude and site of this interaction, we used recently developed methods for the preferential stimulation of each fiber type (Inui et al., 2002; Granovsky et al., 2005) combined with a paired conditioning stimulation (CS) and test stimulation (TS) paradigm to measure the peak and integrated amplitude of cerebral potentials evoked uniquely by Aδ and C fiber stimulation.
Experimental procedures
Subjects
Ten paid healthy volunteers participated in this study. There were two experiments (see below); four subjects participated in both experiments. In experiment 1, the seven subjects (3 males and 4 females) were 24.7 ± 7.8 (mean ± SD) years old; in experiment 2, the seven subjects (3 males and 4 females) were 24.4 ± 8.0 (mean ± SD) years old. The local institutional review board of the Ann Arbor Veteran’s Affairs Medical Center approved the study protocol. Each subject signed a consent form after receiving a complete explanation of the purpose and design of the study.
Preferential Aδ-fiber stimulation
We used electrical epidermal stimulation, similar to the method described previously (Inui et al., 2002). We placed a push-pin needle electrode (cathode) on the dorsum of the left hand between the first and second metacarpal bones. The needle electrode is mounted in the center of a 4 × 4 × 3 cm polyoxymethylene block, which is strapped to the hand so that the needle tip penetrates into the epidermis. A plastic stop device on top of the needle limits epidermal penetration to 0.4 mm. To eliminate any risk for blood-borne infections the stainless steel needle electrodes are steam sterilized between subjects (132°C, 4 min). A dry-gel surface electrode (anode, 20 mm in diameter) is placed on the hand dorsum at around mid-point of the fourth metacarpal bone. We applied constant current square wave pulses of 1 ms duration at intensities just sufficient to evoke a pinprick sensation (pp) at the lowest intensity and at twice that level (2pp) for higher intensities (see below).
Preferential C-fiber stimulation
To activate C fibers preferentially, we used a contact heat evoked potential stimulator (CHEPS) on glabrous skin.(Granovsky et al., 2005) The CHEPS has a thermode with a skin contacting area of 572.5 mm2 (Medoc Ltd, Ramat Yishai, Israel). The thermode is comprised of an external heating thermofoil (Minco Products, Inc., Minneapolis, MN) covered with a 25 micron layer of thermoconductive plastic (Kapton®). Two thermocouples are embedded 10 microns within this conductive coating, which contacts the skin directly, thus providing an indirect estimate of the skin temperature at the thermode surface. The thermofoil permitted a heating rate of up to 70°C/s (without skin contact) and an underlying thermistor-controlled Peltier device maintained baseline temperature and allowed a heating and cooling rate of ~40°C/s in contact with the skin. Cooling began immediately following attainment of the target heat pulse temperature, which was set by the investigator using software provided by the manufacturer. In this study, the baseline temperature was 35°C and the target temperature was 37°C and 50°C. The heat stimulus was a rapidly increasing and decaying heat wave pulse with a full-width-half-maximum duration of approximately 350 ms.
Evoked potential (EP) recordings
We acquired data with the Neuroscan 4.3 system (Compumedics USA, El Paso, TX, USA) running continuously during each recording session; we used this system to store and analyze data off-line. The exploring electrode was placed at Cz according to the 10–20 International System, and was referred to linked earlobes (A1 + A2). The ground electrode was placed on the forehead between Fpz and Fz. Impedance was maintained below 5kΩ The EP was recorded at a filter frequency of 0.1–100 Hz and a sampling rate of 500 Hz. The recording epoch spanned 2100 ms including 100 ms pre- and 2000 ms post-stimulus. Time zero was indicated by the onset of the CS, which was signaled to the recording system at the onset of the electrical (Aδ) stimulation and at the offset of a TTL pulse initiating the heat (C) stimulation. Electrooculography was simultaneously recorded for artifact rejection.
Experimental design
We used the conditioning stimulus (CS) - test stimulus (TS) paradigm in this study. There were two experiments, one with Aδ as CS and C as TS and another one with the conditions reversed. The inter-stimulus intervals of 200 ms with Aδ as CS and 1000 ms with C as CS were based on a pilot study showing that these interstimulus intervals assured that the CS potentials would arrive at the cortex at least 500 ms before the TS signals. This cortical response interval permitted accurate measurements of the response to the TS without interference from the response evoked by the CS and avoided attentional factors (see Discussion).
In both experiments, the intradermal electrical Aδ stimulation was applied at the dorsum of the left hand; this placement was maintained throughout the experiment to maintain a reliable sensation produced at that electrode position. The C fiber stimulation was applied by moving the contact thermode to the left thenar, hypothenar, and right thenar eminence, corresponding to intra-segmental, inter-segmental and contralateral C fiber stimulation relative to Aδ stimulation. Thus, we tested 3 interactions for each experiment: intrasegmental (Intra), intersegmental (Inter) and contralateral (Con). The intensity of the conditioning and testing stimuli varied depending on the interaction under study (see below). To avoid changes in peripheral sensitivity, we used stimulus intensities that were just sufficient to evoke cerebral potentials consistently.
Table 1 shows an example of the data acquisition schedule for a typical subject in experiment 1 and experiment 2. We delivered 80 stimulation trials for each conditioning configuration (Aδ-C and C-Aδ), 40 during Intra and 20 each during Inter and Con testing. To avoid habituation and subject fatigue in both experiment 1 and experiment 2, we examined intrasegmental (Intra) conditioning with two intensities of Aδ stimulation (pp and 2pp) in one session on one day (20 + 20 stimuli), and Inter and Con conditions with Aδ stimulation at pp or 2pp intensity in another session (40 stimuli) on a different day. Subjects had a 3–5 min rest between runs of 20 stimulation trials. Sequences of sessions and conditions within each session were pseudorandom and counter-balanced among subjects. Subjects sat in an armchair in a quiet room with an ambient temperature of approximately 24°C throughout each experimental session.
TABLE 1.
SAMPLE PROTOCOL SCHEDULE FOR A PARTICIPANT
| Aδ - C | C - Aδ | |||||||
|---|---|---|---|---|---|---|---|---|
| day/run1 | #stim2 | Conditioning | Test | day/run1 | #stim2 | Conditioning | Test | |
| 1/1 | 20 | intra pp | C50 | 3/1 | 20 | C50 | intra pp | |
| 10 | 0 (control) | C50 | 20 | C37 | intra pp | |||
| 1/2 | 20 | intra 2pp | C50 | 3/2 | 20 | C50 | intra 2pp | |
| 10 | 0 (control) | C50 | 20 | C37 | intra 2pp | |||
| 2/1 | 20 | inter 2pp | C50 | 4/1 | 20 | C50 | inter pp | |
| 10 | 0 (control) | C50 | 20 | C37 | inter pp | |||
| 2/2 | 20 | contra 2pp | C50 | 4/2 | 20 | C50 | contra pp | |
| 10 | 0 (control) | C50 | 20 | C37 | contra pp | |||
Each run was repeated once to confirm reproducibility of responses.
Includes conditioning and test stimuli.
Experiment 1: Aδ-fiber CS and C-fiber TS (Aδ-C)
To detect the effect of Aδ conditioning stimulus intensity, we used two intensities of Aδ stimulation, pp and 2pp, when C stimuli were applied to the thenar (intra-segmentally: Intra-pp and Intra-2pp). In anticipation of weaker extrasegmental effects, and to minimize subject fatigue, we used only an intensity of 2pp for Aδ stimulation when we applied C stimulation inter-segmentally (hypothenar, Inter) or contralaterally (Con). The temperature of C stimulation was 50°C for all conditions because this stimulus produced potentials that were sufficiently large and consistent for accurate measurements.
In each run of 20 stimulus trials, ten trials were C-only as control without Aδ stimulation and ten trials were with paired stimuli (CS-TS of Aδ-C). The Aδ-CS was applied 200 ms before C-TS. The C-only and Aδ-C trials were given randomly, and the inter-trial intervals were randomized between 10 and 15 s. The mean pp stimulus intensity of Aδ stimulation was 0.20 ± 0.09 mA (mean ± SD). Thus, all electrical stimuli were well below the very high electrical threshold for the excitation of C fibers by direct nerve root stimulation (Ikeda et al., 2000; Ling et al., 2003) or by intracutaneous stimulation in rats (Falinower et al., 1994) or humans (Schmidt et al., 2002); the stimulation parameters were also well outside the range that has been used to evoke C-fiber-mediated itch (Ikoma et al., 2005).
Experiment 2: C-fiber CS and Aδ-fiber TS (C-Aδ)
To detect the effect of the intensity of both conditioning and testing stimuli, we used two intensities of C-fiber conditioning stimulation (37 and 50°C) and two intensities of Aδ- fiber stimulation (pp and 2pp) during the Intra interaction condition. This design allowed us to test the intensity effects of the conditioning stimulation and avoid the extra stimuli that would be required if heat stimuli were applied alone as a control. In anticipation of weaker conditioning effects, we used only pp Aδ test stimulation for Inter and Con interactions; these potentials were sufficiently large and consistent for accurate measurements. Subjects received two runs of 20 trials each for each condition. In each run, ten trials were C37-Aδ with the low target temperature at 37°C for comparison with ten trials of paired stimulation at 50°C (C50-Aδ). The C-CS was applied 1000 ms before Aδ-TS. The C37-Aδ and C50-Aδ trials were given randomly, and the inter-trial intervals were randomized between 10 and 15 s. The mean pp stimulus intensity was 0.18 ± 0.06 mA (mean ± SD).
Verbal Sensation Rating (SR)
To assure attention to the stimuli, there was a verbal warning signal 3–5 s before each trial. Subjects were asked to rate the perceived intensity of CS and TS stimuli 3 s after the trial. For both the electrical and heat stimuli, the ratings were based on a 0–10 scale, the extremes of which were “no sensation” at 0 and “intolerable pain” at 10. A level of 4 was the designated pain threshold. For example, if the subject said “4, 3” that meant he felt two stimuli, and the rating of CS was 4 and TS was 3.
Data analysis
The two runs in each condition confirmed the reproducibility of the responses. For quantitative analysis, we averaged twenty artifact-free trials of 2 runs in each condition. The peak latency and amplitude, and integrated amplitude (total microvolt values for the data points) of EP were determined from these averaged waveforms. First, to determine the effect of conditioning (TS only vs. CS-TS), site and intensity we performed two two-way repeated measures analysis of variance (ANOVA) of the peak and integrated amplitude and the sensory rating (SR) in each experiment. In experiment 1 (Aδ-C) the factors of the two two-way ANOVAs were conditioning and TS site (intra, inter, contra), and conditioning and CS intensity (pp, 2pp). In experiment 2 (C-Aδ) the factors were conditioning intensity (C37 vs. C50) and CS site (intra, inter, contra) and conditioning intensity and TS intensity (pp vs. 2pp). To determine the effect of conditioning (TS only vs. CS-TS) in experiment 2, we used a two-way ANOVA (without repeated measures) to compare group means of the amplitudes of the unconditioned Aδ responses in experiment 1 with those of the conditioned Aδ responses in experiment 2. Second, to investigate further the attenuating effect across conditions, we expressed the peak and integrated amplitude values as a ratio, a standardized value, which was obtained by dividing each test value by the control value for each individual in each experiment in the same condition. We performed a one-way analysis of variance of these standardized values to determine the effect of conditioning across conditions and applied the Tukey HSD test for post-hoc comparisons. In all cases, P-values of less than 0.05 were considered significant. We used SPSS 11.5 and 14.0 software for statistical analyses.
Results
Aδ and C evoked potentials
In experiment 1, we identified the positive phase (P2) of Aδ- and C-mediated responses in all recordings except for P2 of the C response in the Intra-2pp condition in one subject (Table 2, footnote c). We identified the negative N2 potentials of C-only responses in only 8 out of 28 recordings (4 conditions × 7 subjects) and only one subject showed N2 potentials in all four conditions. Because the negative N2 potentials were not consistent, we analyzed only the P2 component of C responses. In experiment 2, we identified the P2 potentials of C responses in all C50-Aδ tests, but in only 9 out of 28 recordings (4 conditions × 7 subjects) during C37-Aδ testing. The P2 potentials of Aδ responses were defined in all recordings except for two recordings of the Intra-pp condition (Table 2, footnote d). The negative N2 potentials of Aδ responses were recognized in only 11 out of 28 recordings (4 conditions × 7 subjects) of C37-Aδ, and only one subject showed N2 potentials in all four conditions. Because the negative N2 potentials were not consistent, we analyzed only the P2 component of Aδ responses.
Table 2.
Mean and standard deviation of the peak and integrated amplitudes of P2 of Aδ (Aδ-P2) and P2 of C (C-P2) responses, and their mean standardized values in experiment 1 (Aδ-C) and 2 (C-Aδ).
| Aδ-Ca | Intra-2pp | Intra-pp | Inter-2pp | Con-2pp |
|---|---|---|---|---|
| Peak amplitude (µV) | ||||
| Aδ-P2 peak of Aδ-C | 33.1 ± 14.5 | 25.2 ± 10.3 | 33.4 ± 9.0 | 28.2 ± 7.9 |
| C-P2 peak of Aδ-C | 10.1 ± 4.6c | 15.4 ± 6.6 | 9.9 ± 4.9 | 9.3 ± 4.9 |
| C-P2 peak of C only | 28.8 ± 8.4 | 21.8 ± 8.5 | 20.8 ± 12.9 | 17.4 ± 4.6 |
| Mean stand. values of C-P2 | 0.27 ± 0.18 | 0.71 ± 0.08 | 0.52 ± 0.11 | 0.51 ± 0.15 |
| Integrated amplitude (µV) | ||||
| C-P2 of Aδ-C | 298 ± 242c | 500 ± 191 | 517 ± 297 | 482 ± 192 |
| C-P2 of C only | 1258 ± 565 | 1194 ± 495 | 1244 ± 726 | 1220 ± 489 |
| Mean stand. values of C-P2 | 0.22 ± 0.19 | 0.47 ± 0.21 | 0.45 ± 0.16 | 0.41 ± 0.16 |
| C-Aδb | Intra-2pp | Intra-pp | Inter-pp | Con-pp |
| Peak amplitude (µV) | ||||
| C-P2 37C peak of C-Aδ | 14.3 ± 7.3 | 14.3 ± 4.6 | 12.6 ± 2.5 | 8.5 ± 1.5 |
| C-P2 50C peak of C-Aδ | 17.9 ± 8.7 | 18.0 ± 8.1 | 15.5 ± 5.9 | 13.4 ± 4.3 |
| Aδ-P2 peak of 37C-Aδ | 16.4 ± 9.4 | 11.2 ± 6.9d | 15.1 ± 6.9 | 14.0 ± 6.8 |
| Aδ-P2 peak of 50C-Aδ | 11.3 ± 8.4 | 6.9 ± 5.8d | 9.0 ± 7.5 | 8.0 ± 4.7 |
| Mean stand. values of Aδ-P2 | 0.66 ± 0.19 | 0.42 ± 0.34 | 0.56 ± 0.34 | 0.57 ± 0.13 |
| Integrated amplitude (µV) | ||||
| Aδ-P2 of 37C-Aδ | 827 ± 414 | 535 ± 151d | 1136 ± 511 | 870 ± 412 |
| Aδ-P2 of 50C-Aδ | 491 ± 358 | 288 ± 177d | 373 ± 248 | 327 ± 241 |
| Mean stand. values of Aδ-P2 | 0.61 ± 0.24 | 0.35 ± 0.35 | 0.30 ± 0.29 | 0.43 ± 0.26 |
CS-TS interval adjusted from 200 ms
adjusted from 1000 ms
from 6 subjects
from 5 subjects.
We estimated response latencies of both Aδ and C mediated responses in the same subjects and under the same conditions when the electrical stimulator was used to trigger the Neuroscan recorder. The mean latency of the peak P2 component of the unconditioned Aδ mediated response (the CS) ranged from 289.9 (20.6 s.d) ms during 2pp stimulation to 308.3 (19.1 s.d.) during pp stimulation. With the electrical CS stimulator off, we estimated the mean latency of the peak P2 component of the unconditioned C mediated response. We used the C mediated response to 50°C for latency estimation because it was the most reliable (see above) and found that the mean latencies ranged from 632.9 (52.2 s.d.) to 688.6 (134.1 s.d.) ms. The Aδ mediated response latency is consistent with the average latency of 302.8 ms estimated by (Inui et al 2002) for epidermal electrical stimulation of the hand. The C mediated response is slightly longer than the average C mediated response estimated by Granovsky et al (2005) (611 ms) from CHEP thenar stimulation; however, group differences in arm length are likely to contribute to these latency differences because the participants in the Granovsky study were selected to maximize the range of arm lengths.
The means and standard deviations of the peak and integrated amplitudes of P2 of Aδ (Aδ-P2) and P2 of C (C-P2) responses, and their mean standardized values in experiment 1 (Aδ-C) and 2 (C-Aδ) are summarized in Table 2.
Effect of Aδ on C cerebral responses
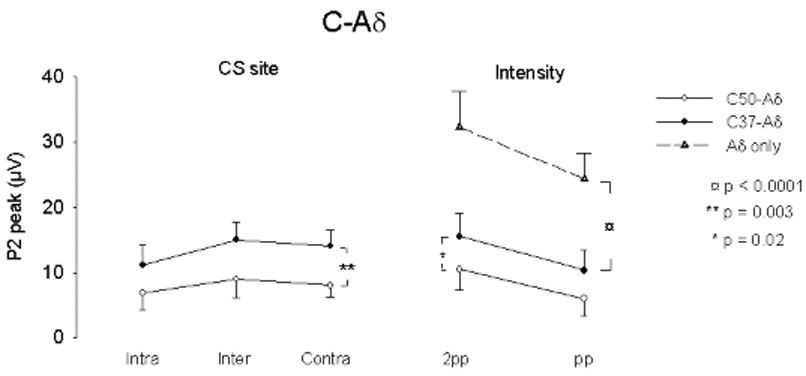
Under all conditioning configurations, the electrical stimulation of Aδ fibers attenuates the response to the stimulation of heat-sensitive C fibers (Fig. 1 and Fig. 2). Table 3 gives the statistical summary of the two-way ANOVA analyses, showing a significant main effect of conditioning across site (p< 0.0001) and intensity (p < 0.0001) on peak evoked potential amplitude; the main effects are confirmed for the integrated amplitude (not shown; intensity, p< 0.004; site, p< 0.006). There is a strong interaction of conditioning and site (p = 0.008) with evidence for a greater effect of Intra and Contra conditioning (p< 0.001) compared to Inter conditioning (p = 0.045). Likewise, there is a strong interaction of conditioning and stimulus intensity (p< 0.0001) with evidence for a greater effect of the stronger conditioning stimulus. Figure 2 is a graphical presentation of these results.
Fig. 1.

Waveforms of Aδ-C experiment in subject 1. Paired records show P2 waves (arrows) of evoked responses to unconditioned testing C (C-only, upper records) and conditioning Aδ fiber stimulation (Aδ-C, lower records). All four conditioning patterns are shown from top to bottom: intrasegmental at 2x pin prick threshold (Intra-2pp); intrasegmental at pin prick threshold (Intra-pp); intersegmental at 2x pin prick threshold (Inter-2pp); and contralateral at 2x pin prick threshold (Con-2pp). The peak and integrated amplitudes of C-P2 were smaller during Aδ-C conditioning than during the C-only condition.
Fig. 2.

Graphical summary of the analysis of the effect of Aδ conditioning on the mean (± s.e.m) C-mediated cortical response amplitude (C-P2). The C-mediated response (TS) is attenuated by the conditioning stimulus at all sites (left graph) but the intrasegmental (Intra) and contralateral (Contra) sites are most effective (see Table 3). The conditioning stimulus (CS) is effective at both intensities (2pp and pp) but most effective at the 2pp intensity (see Table 3).
TABLE 3.
Statistical summary of Aδ-C experiment.
| Effect on P2 peak amplitude of C | |||
|---|---|---|---|
| dF | F,t | p | |
| Main effects - TS site | |||
| Conditioning (C only vs. Aδ-C) | 1,5 | 89.4 | < 0.0001 |
| Site (intra, inter, extra) | 2,10 | 2.6 | 0.153 |
| Site (intra, inter, extra)2 | 1.4, 6.9 | 2.6 | 0.153 |
| Interactions | |||
| Conditioning (C only vs. Aδ-C) × Site | 2,10 | 8.2 | 0.008 |
| Conditioning (C only vs. Aδ-C) × Site2 | 1.6, 8.1 | 8.2 | 0.014 |
| Post hoc - Site1 | t | ||
| Conditioning intra | 5 | 11.6 | < 0.001 |
| Conditioning inter | 6 | 3.4 | 0.045 |
| Conditioning contra | 6 | 11.1 | < 0.001 |
| Main effects - CS intensity | F | ||
| Conditioning (C only vs. Aδ-C) | 1,5 | 154.6 | < 0.0001 |
| CS Intensity (2pp, pp) | 1,5 | 0.002 | 0.97 |
| Interactions | |||
| Conditioning(C only vs. Aδ-C) × CS Intensity | 1,5 | 71.8 | < 0.0001 |
| Post hoc - Intensity1 | t | ||
| Condition 2pp | 5 | 11.6 | < 0.001 |
| Condition pp | 6 | 6.0 | < 0.005 |
Bonferroni corrected
Greenhouse-Geisser corrected
dF - degrees of freedom, F - F statistic, t - t statistic, p - p-value (bold if < 0.05)
Normalized values of the peak amplitudes were < 1 in all conditions and subjects (Fig. 3). The ANOVA applied to these values showed that the peak amplitudes of C-P2 of Aδ-C are significantly smaller than those of C-P2 of C-only in all conditions (F4,28 = 31.9; p < 0.0001). There is an effect of stimulus intensity with Intra 2pp being more effective than Intra pp (p < 0.0001) and, on post-hoc testing (Tukey HSD) of stimulus site with Intra 2pp being more effective than either Inter 2pp (p = 0.014) or Contra 2pp (p = 0.012) and with no difference between the Inter and Contra conditioning (p = 1).
Fig. 3.

Suppressive effect of Aδ conditioning stimulation on C-mediated cerebral responses to 50°C stimulation across each of 4 conditions for each individual. Group mean (± s.e.m.) shown as solid line connecting the group means. The electronically averaged response amplitude obtained in each condition is standardized (normalized) by dividing the amplitude of the conditioned response by the amplitude of the control response for each individual. Therefore, there is only one value for each individual in each condition and no estimate of intra-individual variance; inter-individual variance estimates are shown in Table 2. Standardized values of peak amplitude were < 1 in all conditions for all subjects (p < 0.0001). There is an effect of stimulus intensity with Intra 2pp being more effective than Intra pp (**; p < 0.0001) and of stimulus site with Intra 2pp being more effective than either Inter 2pp (*; p = 0.014) or Contra 2pp (*; p = 0.012).
A two-way repeated measures ANOVA did not reveal any effect of conditioning on the latency of C-TS responses between Aδ-C and C-only in Experiment 1.
Effect of C on Aδ cerebral responses
We observed a significant attenuating effect of C-CS on Aδ-TS (Figure 4, Figure 5 and Table 4). Table 4 gives the statistical summary of the two-way ANOVA analyses, showing a significant main effect of both C37 and C50 conditioning across sites (p = 0.003) and the greater effect of C50 compared to C37 conditioning (C37 vs C50; p = 0.019) on peak evoked potential amplitude; the main effects are confirmed for the integrated amplitude (not shown; intensity, p = 0.016; across sites, p = 0.006). As expected, the TS response to the 2pp stimulus exceeded that to the pp stimulus, but this did not apparently affect conditioning. There is no interaction of site with stimulus intensity. A separate analysis (2 way ANOVA without repeated measures) shows that both the C37 and C50 conditioning stimuli (CS) are effective at both intensities of test stimulus intensity (pp, 2pp) when compared with the unconditioned Aδ-mediated responses of experiment 1 (p < 0.0001; only pp intensity used for Inter- and Contra- test stimuli (TS) in experiment 2).
Fig. 4.

Waveforms of C-Aδ experiment in subject 6. Same general format as Figure 1 except that the C conditioning stimulus is shown in each record and was applied at 37°C (upper records) and at 50°C (lower record) in each condition. In addition, the intensity of the Aδ test stimulus was applied at both 2x pin prick (2pp) and pin prick (pp) intensities during intrasegmental (Intra) conditioning but only at pp intensities during Inter- and Con- condtioning. The peak and integrated amplitudes of the Aδ-P2 response was smaller during C conditioning at 50°C than during C conditioning at 37°C in all conditions.
Fig. 5.
Graphical summary of the analysis of the effect of C conditioning on the mean (± s.e.m.) Aδ-mediated cortical response amplitude (Aδ -P2; see Table 4). The Aδ-mediated response is more attenuated by the C50 than by the C37 conditioning stimulus (left graph; **; p = 0.003) but there is no difference across sites. The C50 stimulus intensity is more effective than the C37 stimulus (right graph; *; p = 0.02) but there is no difference across test stimulus intensities (pp, 2pp). A separate analysis (ANOVA without repeated measures) shows that the C37 and C50 conditioning stimuli (CS) are effective at both intensities of test stimulus intensity (pp, 2pp) when compared with the unconditioned Aδ-mediated responses of experiment 1 (p < 0.0001; only pp intensity used for Inter- and Conrta- test stimuli (TS) in experiment 2).
TABLE 4.
Statistical summary of C-Aδ experiment
| Effect on P2 peak amplitude of Aδ | |||
|---|---|---|---|
| dF | F | p | |
| Main effects - TS site | |||
| CS Intensity (C37 vs. C50) | 1,4 | 38.9 | 0.003 |
| Site (intra, inter, contra) | 2,8 | 1.0 | 0.39 |
| Site (intra, inter, contra)2 | 1.2, 4.7 | 1.0 | 0.39 |
| Interactions | |||
| CS Intensity (C37 vs. C50) × Site | 2,8 | 0.3 | 0.65 |
| CS Intensity (C37 vs. C50) × Site2 | 1.1, 4.6 | 0.3 | 0.65 |
| Main effects - TS and CS intensity | |||
| CS Intensity (C37 vs. C50) | 1,4 | 14.5 | 0.019 |
| TS Intensity (2pp, pp) | 1,4 | 14.4 | 0.019 |
| Conditioning (Aδ only vs. C37- Aδ)1 | 1.24 | 16.7 | <0.0001 |
| Interactions | |||
| CS intensity × TS intensity | 1,4 | 0.1 | 0.79 |
| Conditioning (Aδ only vs. C37- Aδ) × TS intensity | 1.24 | 0.0 | 0.93 |
Not repeated measures because of different subjects in experiment 1 & experiment 2
Greenhouse-Geisser corrected
dF - degrees of freedom, F - F statistic, t - t statistic, p - p-value (bold if < 0.05)
Figure 5 is a graphical presentation of these results.
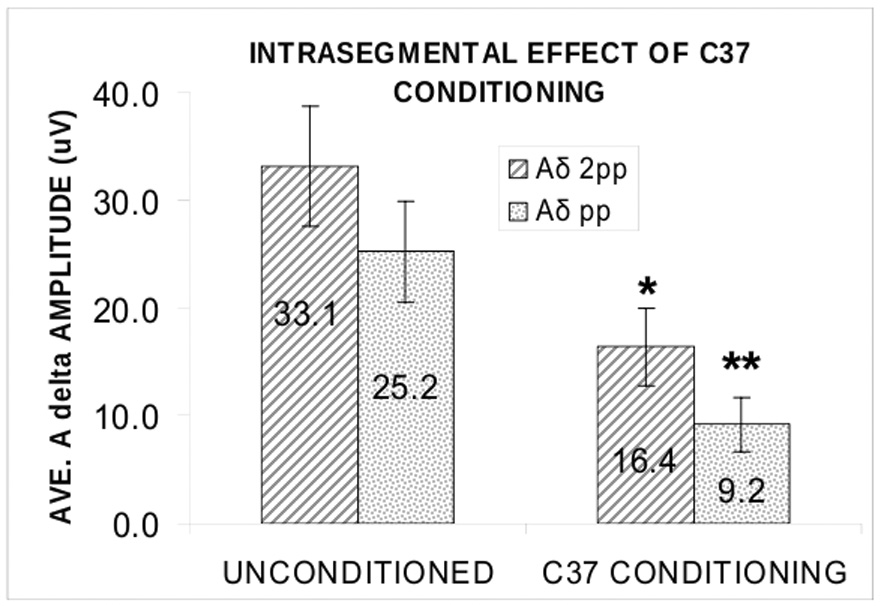
Because we used only pp stimulus intensity for the TS during Inter and Contra conditioning in this experiment, we wished to test independently the effect of C37 Intra conditioning when both pp and 2pp TS were used. We used a 2-way ANOVA to compare the group means of the amplitudes of the unconditioned intrasegmental Aδ responses in experiment 1 with those of the conditioned intrasegmental Aδ responses only during intrasegmental conditioning in experiment 2 when both pp and 2pp TS intensities were used. The results (Fig. 6) show a significant main effect of C37 conditioning (F1 = 15.9; p = 0.001) on the peak amplitudes of the TS Aδ responses to both pp and 2pp stimulus intensities, no interaction of conditioning with the effect of TS on evoked potential amplitude (F1 = 0.093; p = 0.764), and the expected main effect of stimulus intensity on TS amplitude (F1 = 4.9; p = 0.039). Inspection of the data suggests a trend for a weaker conditioning effect on the stronger TS (Fig.6).
Fig. 6.
Suppressive effect of intrasegmental C conditioning (37°C stimulation) on Aδ cerebral responses to test stimulation (TS) at pp and 2pp intensities. Average (± s.e.m.) unconditioned peak amplitude of Aδ evoked potential (left pair of bars) compared with average conditioned Aδ evoked potential amplitude (right pair of bars). Hatched bars from 6 participants in experiment 1 (Aδ-C); stippled bars from 5 participants in experiment 2 (C-Aδ). The 37°C stimulus attenuated the Aδ responses to both 2pp (*, p < 0.025) and pp (**, p < 0.008) stimulation and conditioning did not alter the effect of TS stimulus intensity on evoked potential amplitude (p = 0.764; 2-way ANOVA).
Normalized values of the peak and integrated amplitude were < 1 in all conditions and all subjects except 1 (Fig. 7). The ANOVA applied to these values showed that the peak amplitudes of Aδ-P2 of C50-Aδ are significantly smaller than those of C37- Aδ in all conditions (F4,28 = 5.50; p = 0.002). There is no effect of test stimulus intensity or of stimulus site, Intra pp being no more effective than Inter or Contra pp.
Fig. 7.

Suppressive effect of C50 vs C37 conditioning stimulation on normalized Aδ-mediated cerebral responses to stimulation across each of 4 conditions for each individual. Group mean (± s.e.m.) shown as solid line. Format is the same as for Figure 3. Normalized values of peak amplitude were < 1 in all conditions for all subjects but one (F4,28 = 5.5; p = 0.002). There is no effect of stimulus site or of test stimulus intensity.
A two-way repeated measures ANOVA did not reveal any effect of conditioning on the latency of Aδ-TS responses between C50-Aδ and C37-Aδ in Experiment 2.
Sensation Rating (SR) score
In both experiments, subjects experienced a pin prick sensation with Aδ stimulation and a heat sensation with C stimulation. On average, none of the stimuli was rated as painful. The mean SR of the CS was 3.1 +/− 0.55 sd for the Aδ fiber stimulus and 2.9 +/− 0.61 sd for the C fiber stimulus. In experiment 1, there was a trend for the C-mediated response to be perceived as more intense during intrasegmental Aδ conditioning (post hoc t test, t6 = −3.2; Bonferroni corrected p = 0.06). In experiment 2, C-mediated conditioning had no significant effect on the rating of the Aδ test stimulus at either location or intensity. The Aδ test stimulus was, however, rated as stronger at 2pp than at pp stimulus intensities (F1,6 = 9.8; p = 0.02).
Discussion
Our data provide neurophysiological evidence for a mutually suppressive interaction between stimuli that preferentially excite Aδ and C afferents in humans. The neurophysiological characteristics of the cortical responses to these stimuli were nearly identical to those reported by Inui et al (Inui et al., 2002) and by Granovsky et al (Granovsky et al., 2005) for Aδ and C fiber mediated evoked potentials. The cortical response to preferential Aδ or C fiber stimulation was suppressed whenever either cortical response preceded the other. The Aδ and C suppressive conditioning occurred when applied at all conditioning sites and, for Aδ conditioning, intra-segmental interaction was differentially effective as shown in the examination of the normalized responses. The suppressive effect also varied in magnitude with the intensity of a conditioning stimulus in both Aδ-C and C-Aδ experiments. In the C- Aδ experiment, we showed that the 50°C, compared with the 37°C stimulus, was differentially effective in attenuating the Aδ-mediated response. We also showed, by a group comparison of the unconditioned and conditioned Aδ responses, that the 37°C conditioning stimulus attenuated the Aδ response.
We could not detect any significant difference of SR score and peak latency in either experiment. The lack of perceived intensity difference may be because the brevity of each stimulus did not provide the temporal summation necessary for accurate intensity estimation (Torebjork et al., 1984; Magerl et al., 1999); in addition, the short intervals between the CS and TS may have impaired the ability of our subjects to discriminate clearly between them and to evaluate intensity differences easily. Finally, the evoked potentials we recorded at Cz are probably generated within or near the anterior cingulate gyrus (Lenz et al., 1998) and their amplitudes may not be specifically and exclusively related to perceived stimulus intensity.
Selectivity of the stimulation
It is never possible to be certain that cutaneous stimuli completely exclude one or another fiber type. Tactile stimuli, for example may activate both C and Aβ fibers (Vallbo et al., 1999). However, our methods provided a strongly predominant, if not absolutely exclusive, stimulation of one particular fiber type. We used an intradermal electrode method that evokes a cerebral potential and waveform that is morphologically similar to the well-known LEP and that is mediated by Aδ fibers (Inui et al., 2002). We found no electrophysiological evidence, using high gain recordings, for the activation of Aβ; fibers, and our subjects clearly perceived the pin prick sensation that is strongly associated with the Aδ-mediated LEP. For the C fiber stimulation, we used a method that has been shown to be C fiber-mediated by conduction velocity measurements and that evokes only the sensation of warmth without a tactile or pin prick component when applied to glabrous skin (Granovsky et al., 2005). The shorter latency of this response, compared to C fiber-mediated potentials evoked by infrared laser stimulation (Tran et al., 2001; Opsommer et al., 2001), is probably due to several factors, including a longer stimulus duration (approximately 10 to 300 times longer) and a much larger stimulation area (30 to 3000 times greater). We could not detect electrophysiological evidence for the activation of Aδ fibers with this stimulus. Indeed, when this contact heat stimulus is applied to hairy skin at temperatures sufficient to produce a slightly painful pin prick sensation, only waveforms consistent with Aδ fiber stimulation appear. Therefore, for C fiber activation, we intentionally used painless warm stimuli at temperatures well below those necessary to stimulate the heat nociceptors innervated by Aδ fibers (Treede et al., 1995).
Consideration of attentional factors
The effects of attention and expectation are difficult to eliminate completely in these experiments. However, in the Aδ-C experiments, the attenuation effect of CS on TS, although evoked from all conditioning sites, was significantly greater during intrasegmental Aδ-C conditioning; this result would not be expected if attentional or habituation factors were responsible for our results. Stimulus expectation might contribute to the reduced amplitude of Aδ mediated laser-evoked potentials (LEP) as shown in the conditioning-test paradigm of Truini and colleagues where the paired laser stimuli were delivered repetitively at fixed intervals (Truini et al., 2004). However, these investigators did not measure expectation and did not require the subjects to attend to and rate each stimulus. In our experiment, subjects were asked to rate each stimulus, an attentional factor that should have increased, or at least attenuated the decrease of, the LEP (for review, see (Lorenz and Garcia-Larrea, 2003)). This conjecture is supported by the results of Mouraux and colleagues (Mouraux et al., 2004) who required stimulus ratings of each of two consecutive laser stimuli with randomly varying stimulus onset and interstimulus intervals and found that the late-LEP response to the second stimulus was not altered. It is unlikely also that the response attenuation is due primarily to a shift of attention toward the CS and from the TS. First, in the Aδ-C experiment, intradermatomal conditioning uniquely enhanced the attenuation effect. If attention had played a role here, the attenuation effect should have been at least as strong during contralateral conditioning because the subjects’ attention would have been directed toward the contralateral side (Legrain et al., 2002; Lorenz and Garcia-Larrea, 2003). Secondly, Ward and colleagues (Ward et al., 1996) have presented evidence that visual attentional shifts to a second stimulus do not occur earlier than 500 ms after the first stimulus. In our study, the signals of the CS were designed to reach the cortex no earlier than 500 ms before that of the TS. Assuming that somatosensory attentional shifts occur on average no earlier than those in the visual system, the weight of the evidence presented here favors the interpretation that the attenuation effect found in the present study is not due to expectation effects or to shifts of attention from the TS to the CS but rather results from the physiological interaction of signals mediated by Aδ and C fibers. Although we did not find a differential effect of intra-segmental conditioning in the C-Aδ experiments, the 500 ms interstimulus interval was the same as in the Aδ-C experiment and thus favors a physiological interaction mechanism rather than purely attentional or related cognitive effects.
Intra- and extrasegmental components of the interaction
Our neurophysiological data suggests an intrasegmental component for the Aδ-C interaction, which is consistent with a possible spinal site of interaction. However, this somatotopic effect could also be mediated through somatotopically organized ascending or descending processes at all supraspinal levels. In addition, the extrasegmental and contralateral effects of conditioning in both the Aδ-C and C-Aδ experiments suggest that the interaction must also be mediated through a widely distributed, non-somatotopic mechanism. Overall, the interpretation favors an interaction mediated through both somatotopic and nonsomatotopic mechanisms that receive convergent input from Aδ and C fibers.
The interaction of Aδ and C signals is intensity dependent
The suppressive interaction varies in magnitude with the intensity of a conditioning stimulus in both Aδ-C (pp vs. 2pp) and C-Aδ (37°C vs. 50°C) experiments. The Aδ-C interaction may explain why, in previous experiments, painful laser stimulation could evoke only the late-LEP Aδ response although Aδ and C fibers were probably simultaneously activated. To elicit an ultralate-LEP or C response, many methods of selective activation of C fiber have been proposed (for review see (Plaghki and Mouraux, 2003)). All of these methods are designed to avoid concomitant activation of Aδ fibers. Although waveforms consistent with an ultralate (C-mediated) LEP have been recorded in the immediate wake of a late (Aδ-mediated) LEP, (Magerl et al., 1999) a fiber-specific origin of the suspected ultralate response could not be established; nor could an incomplete suppressive effect of the preceding Aδ response be ruled out. Indeed, Towell and colleagues (Towell et al., 1996) noticed that the ultra-late LEPs could be recorded most frequently when the stimulus intensity was low and the late LEPs less well identified. These observations support our finding that the mutually suppressive interaction depends on the magnitude of the conditioning stimulus.
Although the stimuli we used were not painful, our results are consistent with psychophysical evidence that innocuous warm stimuli can attenuate heat pain sensation (Casey et al., 1993) and that electrocutaneous stimulation of Aδ and C fibers can strongly attenuate perceived heat and mechanical pain (Nilsson and Schouenborg, 1999; Nilsson et al., 2003). However, as in the Aδ-C experiment, the magnitude of the C-Aδ suppressive interaction depends on the intensity of the CS. Thus, the 50°C stimulus was more effective than the 37°C stimulus in suppressing the Aδ-TS response. Taken together, the results show that the conditioned cerebral responses to the preferential stimulation of either Aδ or C fibers depend on the relative input activity of the Aδ and C signals.
Mechanisms mediating the suppressive interaction
Based on a time-frequency analysis of electroencephalographic epochs, Mouraux et al (Mouraux et al., 2003) hypothesized that the brain processes first engaged by Aδ fibers could not be reactivated by a subsequent C fiber volley. However, as noted above, Mouraux and colleagues also found that the second of two Aδ LEP responses was not altered at interstimulus intervals ranging between 280 and 2100 ms (Mouraux et al., 2004). If, according to the latter results, similar inputs do not interact within the time interval we tested, then the interaction we observed between two distinctly different fiber types must be mediated through a neuronal mechanism that receives converging input from Aδ and C fibers and differentiates between them to suppress responses evoked from a different, delayed afferent source. We did not, however, examine the effect of predominant C fiber conditioning on the cortical response to the same C fiber stimulus. It is therefore possible that C fiber conditioning produces a more generalized attenuating effect on the amplitude of cortical somatosensory responses1. In addition, we did not investigate the effect of interactions with tactile stimuli, so our experiments do not provide information about mechanisms related to interactions with Aβ-mediated responses.
Physiological and clinical significance
Our results demonstrate an early inhibitory interaction between the two fiber types that provide the major inputs to spinothalamic pathways mediating pain and temperature sensations1. Although these brief stimuli were not painful, their suppressive interactions may nonetheless be relevant for pain-related sensations and behaviors because heat pain requires the neural substrates for painless heat and cold (Defrin et al., 2002) and the just-supraliminal stimulation of heat nociceptors evokes painless warmth (Green and Cruz, 1998). In this broader context, the suppressive cortical interaction between Aδ and C fiber pathways may reflect a) the well-established suppressive interaction of noxious stimuli (Bouhassira et al., 1993;Reinert et al., 2000) and b) the activation of contrasting and perhaps mutually exclusive behaviors: an Aδ-mediated channel for rapid escape from threat and a slow C-mediated channel that promotes avoidance and the protection of injured tissue.
Acknowledgments
This research was supported by NIAMS AR46045 and the Dept. of Veteran’s Affairs. (KLC); The Research Council of Norway (DM); John J. Bonica (IASP) ,IBRO, and INS Fellowship (NIH/WHO) F05 NS 048581-01 (TDT). The authors have no actual or potential conflicts of interest related to any aspect of this report.
Abbreviations
- ANOVA
analysis of variance
- CHEPS
contact heat evoked potential stimulator
- Con
contralateral
- CS
conditioning stimulus
- EP
evoked potential
- Inter
intersegmental
- Intra
intrasegmental
- LEP
laser evoked potential
- N2
second negative wave
- P2
second positive wave
- pp
pin prick intensity
- 2pp
twice pin prick intensity
- SD
standard deviation
- SE
standard error of the mean
- SR
sensory rating
- TS
testing stimulus
Footnotes
While this report was under review, Truini and colleagues published a report showing that C fiber conditioning attenuates the amplitude of both Aδ and C-mediated responses evoked by spatially adjacent laser stimuli. Their results and ours are consistent with a suppressive interaction that may in part reflect a relative refractoriness of cortical mechanisms mediating orienting responses. Our study additionally demonstrates extrasegmentally and, specifically for Aδ conditioning only, intrasegmentally mediated components of this interaction (Truini et al., 2007).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akyuz G, Guven Z, Ozaras N, Kayhan O, Akyuz G, Guven Z, Ozaras N, Kayhan O. The effect of conventional transcutaneous electrical nerve stimulation on somatosensory evoked potentials. EEG clin Neurophysiol. 1995;35:371–376. [PubMed] [Google Scholar]
- Apkarian AV, Stea RA, Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: A touch gate. Somatosens Mot Res. 1994;11:259–267. doi: 10.3109/08990229409051393. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Stea RA, Manglos SH, Szeverenyi NM, King RB, Thomas FD. Persistent pain inhibits contralateral somatosensory cortical activity in humans. Neurosci Lett. 1992;140:141–147. doi: 10.1016/0304-3940(92)90088-o. [DOI] [PubMed] [Google Scholar]
- Beitel RE, Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey's face. J Neurophysiol. 1976;39:1160–1175. doi: 10.1152/jn.1976.39.6.1160. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Le Bars D, Bolgert F, Laplane D, Willer J-C. Diffuse noxious inhibitory controls in humans: A neurophysiological investigation of a patient with a form of Brown-Sequard syndrome. Ann.Neurol. 1993;34:536–543. doi: 10.1002/ana.410340406. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Human cerebral potentials evoked by CO2 laser stimuli causing pain. Exp Brain Res. 1987a;67:153–162. doi: 10.1007/BF00269463. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Pain related cerebral potentials: late and ultralate components. Int J Neurosci. 1987b;33:15–23. doi: 10.3109/00207458708985926. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibers responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL, Zumberg M, Heslep H, Morrow TJ. Afferent modulation of noxious and innocuous heat sensation in the human hand. Somatosens Motor Res. 1993;10:327–337. doi: 10.3109/08990229309028841. [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A. The substantia gelatinosa of the spinal cord. Brain Res. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Chung JM, Fang ZR, Hori Y, Lee KH, Willis WD. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain. 1984a;19:259–275. doi: 10.1016/0304-3959(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Chung JM, Kenshalo DR, Jr, Gerhart KD, Willis WD. Excitation of primate spinothalamic neurons by cutaneous C-fiber volleys. J Neurophysiol. 1979;42:1354–1369. doi: 10.1152/jn.1979.42.5.1354. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain. 1984b;19:277–293. doi: 10.1016/0304-3959(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Cruz F, Lima D, Coimbra A. Several morphological types of terminal arborizations of primary afferents in laminae I–II of the rat spinal cord as shown after HRP labeling and Golgi impregnation. J Comp Neurol. 1987;261:221–236. doi: 10.1002/cne.902610205. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- Falinower S, Willer JC, Junien JL, Le Bars D. A C-fiber reflex modulated by heterotopic noxious somatic stimuli in the rat. J Neurophysiol. 1994;72:194–213. doi: 10.1152/jn.1994.72.1.194. [DOI] [PubMed] [Google Scholar]
- Gobel S, Falls WM, Humphrey E. Morphology and synaptic connections of ultrafine primary axons in lamina I of the spinal dorsal horn: candidates for the terminal axonal arbors of primary neurons with unmyelinated (C) axons. J Neurosci. 1981;1:1163–1179. doi: 10.1523/JNEUROSCI.01-10-01163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovsky Y, Matre D, Sokolik A, Lorenz J, Casey KL. Thermoreceptive innervation of human glabrous and hairy skin: a contact heat evoked potential analysis. Pain. 2005;115:238–247. doi: 10.1016/j.pain.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Green BG, Cruz A. "Warmth-insensitive fields": evidence of sparse and irregular innervation of human skin by the warmth sense. Somatosens Mot Res. 1998;15:269–275. doi: 10.1080/08990229870682. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R. After-effect of transcutaneous electrical nerve stimulation (TENS) on pain-related evoked potentials and magnetic fields in normal subjects. Clin Neurophysiol. 2000;111:717–724. doi: 10.1016/s1388-2457(99)00299-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Asai T, Murase K. Robust Changes of Afferent-Induced Excitation in the Rat Spinal Dorsal Horn After Conditioning High-Frequency Stimulation. J Neurophysiol. 2000;83:2412–2420. doi: 10.1152/jn.2000.83.4.2412. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Handwerker HO, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of A[delta] fibers by intra-epidermal needle electrode in humans. Pain. 2002;96:247–252. doi: 10.1016/S0304-3959(01)00453-5. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Shibasaki H. Mechanisms of pain relief by vibration and movement. J Neurol Neurosurg Psychiatry. 1992;55:282–286. doi: 10.1136/jnnp.55.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99:21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979a;186:117. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979b;186:133. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Honda T, Shimada Y, Ozaki N, Shiraishi Y, Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Liu XG, Morton CR, Azkue JJ, Zimmermann M, Sandkuhler J. Long-term depression of C-fibre-evoked spinal field potentials by stimulation of primary afferent A delta-fibres in the adult rat. Eur J Neurosci. 1998;10:3069–3075. doi: 10.1046/j.1460-9568.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Garcia-Larrea L. Modulation attentionnelle et cognitive des reponses evoquees par laser. Neurophysiol Clin. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Magerl W, Ali Z, Ellrich J, Meyer RA, Treede RD. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain. 1999;82:127–137. doi: 10.1016/S0304-3959(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Marchand S, Bushnell MC, Duncan GH. Modulation of heat pain perception by high frequency transcutaneous electrical nerve stimulation (TENS) Clin Jour Pain. 1991;7:122–129. doi: 10.1097/00002508-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Giesler GJ, Besson JM. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977;27:15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A delta and C-fibre afferent volleys. Clin Neurophysiol. 2003;114:710–722. doi: 10.1016/s1388-2457(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Refractoriness cannot explain why C-fiber laser-evoked brain potentials are recorded only if concomitant A[delta]-fiber activation is avoided. Pain. 2004;112:16–26. doi: 10.1016/j.pain.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Nahra H, Plaghki L. Modulation of perception and neurophysiological correlates of brief CO2 laser stimuli in humans using concurrent large fiber stimulation. Somatosens Mot Res. 2003;20:139–147. doi: 10.1080/0899022031000105172. [DOI] [PubMed] [Google Scholar]
- Nilsson HJ, Psouni E, Schouenborg J. Long term depression of human nociceptive skin senses induced by thin fibre stimulation. Eur J Pain. 2003;7:225–233. doi: 10.1016/S1090-3801(02)00120-9. [DOI] [PubMed] [Google Scholar]
- Nilsson HJ, Schouenborg J. Differential inhibitory effect on human nociceptive skin senses induced by local stimulation of thin cutaneous fibers. Pain. 1999;80:103–112. doi: 10.1016/s0304-3959(98)00205-x. [DOI] [PubMed] [Google Scholar]
- Opsommer E, Weiss T, Plaghki L, Miltner WH. Dipole analysis of ultralate (C-fibres) evoked potentials after laser stimulation of tiny cutaneous surface areas in humans. Neurosci Lett. 2001;298:41–44. doi: 10.1016/s0304-3940(00)01718-3. [DOI] [PubMed] [Google Scholar]
- Perl ER. Pain and nociception. In: Darian-Smith I, editor. Handbook of Physiology. Section 1: The Nervous System. Bethesda: American Physiological Society; 1984. pp. 915–975. [Google Scholar]
- Plaghki L, Bragard D, Le Bars D, Willer J-C, Godfraind JM. Facilitation of a Nociceptive Flexion Reflex in Man by Nonnoxious Radiant Heat Produced by a Laser. J Neurophysiol. 1998;79:2557–2567. doi: 10.1152/jn.1998.79.5.2557. [DOI] [PubMed] [Google Scholar]
- Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Neurophysiol Clin. 2003;33:269–277. doi: 10.1016/j.neucli.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Reinert A, Treede RD, Bromm B. The pain inhibiting pain effect: an electrophysiological study in humans. Brain Res. 2000;862:103–110. doi: 10.1016/s0006-8993(00)02077-1. [DOI] [PubMed] [Google Scholar]
- Rethelyi M, Light AR, Perl ER. Synaptic ultrastructure of functionally and morphologically characterized neurons of the superficial spinal dorsal horn of cat. J Neurosci. 1989;9:1846–1863. doi: 10.1523/JNEUROSCI.09-06-01846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J, Chen JG, Cheng G, Randic M. Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997;17:6483–6491. doi: 10.1523/JNEUROSCI.17-16-06483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Weidner C, Handwerker HO, Torebjork HE. Innervation Territories of Mechano-Insensitive C Nociceptors in Human Skin. J Neurophysiol. 2002;88:1859–1866. doi: 10.1152/jn.2002.88.4.1859. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Synaptic mediation from cutaneous mechanical nociceptors. J Neurophysiol. 1994;72:612–621. doi: 10.1152/jn.1994.72.2.612. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yoshimura M, Baba H, Shimoji K, Higashi H. Role of A δ afferent fibers in modulation of primary afferent input to the adult rat spinal cord. Brain Res. 1995;691:92–98. doi: 10.1016/0006-8993(95)00619-2. [DOI] [PubMed] [Google Scholar]
- Svensson P, Hashikawa CH, Casey KL. Site- and modality-specific modulation of experimental muscle pain in humans. Brain Res. 1999;851:32–38. doi: 10.1016/s0006-8993(99)02073-9. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, LaMotte RH, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol. 1984;51:325–339. doi: 10.1152/jn.1984.51.2.325. [DOI] [PubMed] [Google Scholar]
- Towell AD, Purves AM, Boyd SG. CO2 laser activation of nociceptive and non-nociceptive thermal afferents from hairy and glabrous skin. Pain. 1996;66:79–86. doi: 10.1016/0304-3959(96)03016-3. [DOI] [PubMed] [Google Scholar]
- Tran TD, Hoshiyama M, Inui K, Kakigi R. Electrical-induced pain diminishes somatosensory evoked magnetic cortical fields. Clin Neurophysiol. 2003;114:1704–1714. doi: 10.1016/s1388-2457(03)00151-2. [DOI] [PubMed] [Google Scholar]
- Tran TD, Lam K, Hoshiyama M, Kakigi R. A new method for measuring the conduction velocities of Abeta-, Adelta- and C-fibers following electric and CO(2) laser stimulation in humans. Neurosci Lett. 2001;301:187–190. doi: 10.1016/s0304-3940(01)01639-1. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Galeotti F, Cruccu G, Garcia-Larrea L. Inhibition of cortical responses to A[delta] inputs by a preceding C-related response: Testing the "first come, first served" hypothesis of cortical laser evoked potentials. Pain. 2007;131:341–347. doi: 10.1016/j.pain.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Truini A, Rossi P, Galeotti F, Romaniello A, Virtuoso M, Lena C, Leandri M, Cruccu G. Excitability of the Ad nociceptive pathways as assessed by the recovery cycle of laser evoked potentials in humans. Exp Brain Res. 2004;155:120–123. doi: 10.1007/s00221-003-1785-x. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Li Q-J, Matsui A, Matsui Y. Inhibition of nociceptive responses of wide-dynamic-range neurons by peripheral nerve stimulation. Brain Res Bull. 1990;25:387–392. doi: 10.1016/0361-9230(90)90224-n. [DOI] [PubMed] [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated Afferents Constitute a Second System Coding Tactile Stimuli of the Human Hairy Skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Ward R, Duncan J, Shapiro K. The Slow Time-Course of Visual Attention. Cog Psychol. 1996;30:79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Svensson P, Arendt-Nielsen L, Watanabe I, Svensson P, Arendt-Nielsen L. Influence of segmental and extra-segmental conditioning, stimuli on cortical potentials evoked by painful electrical stimulation. Somatosens Motor Res. 1999;16:243–250. doi: 10.1080/08990229970492. [DOI] [PubMed] [Google Scholar]
- Willis WD, Trevino DL, Coulter JD, Maunz RA. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974;37:358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]
- Willis WDJ. Sensory Mechanisms of the Spinal Cord. vol. 1 & 2. New York: Academic/Plenum; 2004. pp. 1–962. [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Zoppi M, Voegelin MR, Signorini M, Zamponi A. Pain threshold changes by skin vibratory stimulation in healthy subjects. Acta Physiol Scand. 1991;143:439–443. doi: 10.1111/j.1748-1716.1991.tb09256.x. [DOI] [PubMed] [Google Scholar]


