Abstract
Th17 cells are implicated in CNS autoimmune diseases. We show that mice with targeted-deletion of STAT3 in CD4+ T-cells (CD4Stat3−/−) do not develop experimental autoimmune uveoretinitis (EAU) or experimental autoimmune encephalomyelitis (EAE). Defective Th17 differentiation noted in CD4Stat3−/− mice is compensated by exaggerated increases in Foxp3-, IL-10-, IL-4- and IFN-γ-expressing T-cells, suggesting critical roles of STAT3 in shaping Ag-specific CD4+ T-cell repertoire. In mice with EAU, a high percentage of IL-17-expressing-T-cells in their peripheral lymphoid organs also secretes IFN-γ while these double-expressors are absent in CD4Stat3−/− and WT mice without EAU, raising intriguing possibility that uveitis maybe mediated by Th17 and IL-17-expressing Th1 cells. Resistance of STAT3-deficient mice to EAU derives in part from an inability of uveitogenic Th17 and Th1 cells to enter eyes or brain of the CD4Stat3−/− mouse because of the reduction in the expression of activated α4/β1 integrins on CD4Stat3−/− T cells. Adoptive transfer of activated IRBP-specific uveitogenic T cells induced in CD4Stat3−/− mice a severe EAU characterized by development of retinal folds, infiltration of inflammatory cells into the retina and destruction of retinal architecture, underscoring our contention that the loss of STAT3 in CD4+ T cells results in an intrinsic developmental defect that renders CD4Stat3−/− resistant to CNS inflammatory diseases. STAT3 requirement for IL-17 production by Th17, generation of double positive T cells expressing IL-17 and IFN-γ and for T-cell trafficking into CNS tissues, suggests that STAT3 may be a therapeutic target for modulating uveitis, sceritis or multiple sclerosis.
Keywords: Th17, Th1, EAE, EAU, Stat3 knockout mice
Introduction
Uveitis is a group of sight-threatening intraocular inflammatory diseases that includes Behçet’s disease, birdshot retinochoroidopathy, Vogt–Koyanagi–Harada’s, sympathetic ophthalmia, ocular sarcoidosis (1). Studies of experimental autoimmune uveoretinitis (EAU), the model of uveitis (1) led to the conclusion that Th1 cells are the etiologic agent of uveitis because IFN-γ levels are elevated in the retina during uveitis and IL12p40 is required for EAU induction (2). However, subsequent studies revealed that IL-12 down-regulates EAU (3) and treatment of mice with EAU with anti-IFN-γ antibodies was found to exacerbate the disease (4), casting doubts on the role of this T cell subset as the etiologic agent of uveitis. Recent reports implicating Th17 cells in pathogenesis of human uveitis and scleritis has further complicated our understanding of the role of different T cell subsets in uveitis (5). Besides Th1 and Th17 cells, Th2 and Treg are also detected in the eye during uveitis (6, 7) and exact roles of these T cell subtypes in the immunopathogenic process are largely unknown. Thus in context of treating uveitis, it is important to note that presence of T cells in the retina compromises vision and preventing entry of all T cell types or limiting their expansion in the eye is of utmost importance (8).
Accordingly, identifying molecular pathways amendable to therapeutic targeting has attracted attention as a potential strategy for limiting expansion of uveitogenic T cells in the eye. Recent reports indicating requirement of STAT3 for commitment of naive T cells towards the Th17 developmental pathway (9, 10), suggest a potential involvement of STAT3 pathway in mediating CNS inflammatory diseases. In this study, we have generated mice with targeted deletion of STAT3 in the CD4+ T cell compartment (CD4Stat3−/−) and used them to examine whether STAT3 pathways are required for development of EAU, as well as, experimental autoimmune encephalomyelitis (EAE), another CNS disease that shares essential immunopathologic features as EAU (11). Here, we have shown that unlike the partial protection conferred by IL-17 blockage with IL-17 Abs (5), CD4stat3−/− mice are completely resistant to EAU or EAE and this dramatic outcome derives from combinatory mechanisms that include: IL-17 blockade; altered T cell homeostasis that favor expansion of anti-inflammatory responses; inhibition of T cell entry to CNS tissues. Resistance of STAT3 conditional knockout mice to development of EAE and EAU suggest that the STAT3 pathway is a potential therapeutic target for modulating these CNS inflammatory diseases.
Materials and Methods
Mice
Mice with conditional deletion of Stat3 in CD4+ T-cells (CD4Stat3−/−) were derived by breeding Stat3fl/fl with CD4-Cre mice (Taconic, Hudson, NY). Littermate Stat3fl/fl mice, in C57BL/6 background, were used as wild type (WT) controls. Animal care and experimentation conformed to NIH guidelines.
Induction of experimental autoimmune uveoretinitis (EAU) by active immunization
Mice were immunized with 150 μg interphotoreceptor retinoid-binding protein (IRBP) and 300μg of human IRBP peptide (1–20) in 0.2 ml emulsion 1:1 v/v with Complete Freund adjuvant (CFA) containing Mycobacterium tuberculosis strain H37RA (2.5 mg/ml). The mice also received Bordetella pertussis toxin (0.2 μg/mouse) concurrent with immunization and clinical disease was established by fundoscopy as described previously (12). Eyes for histological EAU evaluation were harvested 0, 7, 10, 14, and 21 days post-immunization, fixed in 10% buffered formalin, embedded in methacrylate, and stained with Hematoxylin and eosin (H&E).
Induction of experimental autoimmune encephalomyelitis (EAE) by active immunization
EAE was induced by immunization with 200 μg myelin oligodendrocyte glycoprotein (MOG) peptide (35–55) (Sigma, ST Louis, MO) in CFA emulsion, containing 2.5 mg/ml of heat killed, pulverized Mycobacterium tuberculosis strain H37RA, by subcutaneous (s.c) injection into the tail base. The mice also received 200 ng Bordetella pertussis toxin concurrent with immunization. Spinal cord and brain were harvested 21 days post-immunization and stained with H & E as described above. Clinical signs of EAE were graded according to the following scale: 0, No clinical symptoms; 1, clumsiness, incontinence or atonic bladder, flaccid tail; 2, mild paraparesis (trouble initiating movement); 3, moderate paraparesis (hind limb weakness); 4, complete front and hind limb paralysis; 5, moribund state. CNS infiltrates were collected from the brain and spinal code at day-21 post-immunization and lymphocytes/mononulear cells were isolated by percoll gradient.
Induction of EAU in CD4Stat3−/− mice by adoptive transfer of WT uveitogenic lymphocytes
EAU was induced in WT C57BL/6 by immunization with IRBP in CFA and disease was confirmed by fundoscopy as described above. Donor mice were sacrificed and CD4+ T cells were isolated from drawing lymph nodes and spleen of mice with EAU and stimulated with IRBP (20ug/ml) and radiated APC from for 3 days. The IRBP-specific T cells were then transferred i.v. to naive CD4Stat3−/− recipient mice at 10 × 106 cells/mouse. Ten days after the T cell transfer, disease was assessed by fundoscopy and the CD4Stat3−/− recipient mice were then euthanized. Eyes were enucleated, fixed in 10% buffered formalin and sectioned for histopathological examination.
Isolation, propagation and characterization of naïve and activated CD4+ T-cells
CD4+ T-cells were purified from peripheral blood, thymus, spleens or lymph nodes and stimulated with 10X irradiated syngeneic splenocytes (as APC) in the presence of 20μg/ml MOG peptide or IRBP. Some cultures were activated in plate-bound anti-CD3 Ab (10 μg/ml) (BD BioScience) and anti-CD28 Ab (5 μg/ml) in complete medium. For propagation under Th1 condition, medium was supplemented with anti-IL-4 Ab (10 μg/ml) and IL-12 (10 ng/ml) (Pepro Tech Inc., Rocky Hill, NJ). For Th2 condition the medium contained IL-4 (10 ng/ml), anti-IFN-γ Ab (10 μg/ml) and anti-IL-12 Ab (10 μg/ml) while Th17 polarization medium contained TGF-β (10 ng/ml), IL-6 (10 ng/ml), anti-IFN-γ Ab (10 μg/ml) and anti-IL-4 Ab (10 μg/ml). Most cultures were stimulated for 4 days and some were subsequently expanded for 4 days in IL-2 before analysis. FACS analysis using anti-CD3, CD4, CD8, CD44, IFN-γ, IL-4, IL-10, IL-17, Foxp3, α4-integrin or β1-integrin mAbs and corresponding isotype control Abs (PharMingen) was performed on Becton Dickinson FACSCalibur (BD Biosciences, San Jose, CA) as previously described (5).
Proliferation, ELISA and intracellular cytokine staining assays
Draining lymph nodes were collected 14 days after immunization with IRBP. Cells were stimulated with PPD (5 μg/ml), PHA (10 μg/ml) or IRBP (0.1, 1.0 or 10 μg/ml) in presence or absence of exogenous cytokine. After 60 h, cultures were pulsed with 3H-thymidine (0.5 μCi/10 μl/well) for 12 additional hours. The presented data are mean CPM ± S.E. of responses of triplicate cultures. ELISA was performed using Pierce SearchLight technology (Pierce Woburn, MA). For intracellular cytokine detection, freshly isolated or cultured CD4+ T-cells were re-stimulated for 5 h with PMA (20ng/ml)/ionomycin (1μm) in the presence of Golgistop at the recommended concentrations (BD Pharmingen, San Diego, CA). The cells were surface stained with labeled Abs, fixed, permeabilized, and stained with the requisite Abs using the BD Biosciences Cytofix/Cytoperm kit according to the manufacturer’s instructions.
Quantitative and semi-quantitative RT-PCR analysis
All RNA samples were DNA free. Complementary DNA (cDNA) was generated as described previously (13); each gene-specific primer pair used for RT-PCR analysis spans at least an intron. qRT-PCR analysis was performed as previously described (14) using primers and probes from Applied Biosystems. The mRNA expression levels were normalized to the levels of ACTB (encoding β-actin) and GAPDH housekeeping genes.
Western blot analyses
Preparation of whole cell lysates was as described (12). Blots were probed with rabbit polyclonal STAT3 or β-actin specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Pre-immune serum was used in parallel as controls and signals were detected with HRP conjugated-secondary F(ab′)2 antibodies (Ab) (Zymed Laboratories, San Francisco, CA) using the ECL system (Amersham, Arlington Heights, IL).
Statistical analysis
All experiments were performed at least twice and were highly reproducible. Figures show data from representative experiments or from combined experiments as indicated. The Student’s t test was performed on the data.
Results
Mice with targeted deletion of STAT3 in CD4 compartment do not develop EAU
Th17 cells (Th17) are implicated in pathogenic mechanisms of scleritis and uveitis (5) and recent reports have suggested that STAT3 is required for development of naive T cells to the Th17 phenotype (9, 10). To investigate potential involvement of STAT3 pathway in mediating CNS inflammatory diseases, we generated mice with targeted deletion of Stat3 in their CD4+ T cells. Western blot analysis of purified CD4+ T cells confirms absence of Stat3 expression in CD4Stat3−/− T cells (Fig. 1A). The CD4Stat3−/− mice harbor fewer CD4+ T cells with slightly elevated numbers of CD8+ T cells in peripheral lymphoid tissues (data not shown). In line with other Stat3 conditional mice generated using CD4-Cre mice (9), CD4Stat3−/− appeared healthy and normal. EAU was induced in wild type (WT) or CD4Stat3−/− by immunization with IRBP in CFA and consistent with previous studies, all WT mice developed severe EAU characterized by targeted destruction of photoreceptor cells (Fig. 1B) and very high clinical EAU scores (Fig. 1C). In contrast, CD4Stat3−/− mice do not develop EAU; in three separate experiments involving more than 24 mice, none of the mice developed EAU (Fig. 1B, 1C). WT and CD4Stat3−/− T cells respond to PPD, PHA or IRBP in a dose dependent manner (Fig. 1D), suggesting that resistance of CD4Stat3−/− to EAU cannot be solely attributed to inability to respond to the autoantigen. It is of note that the higher proliferative response of WT cells is observed only in which antigen-experienced T cells are stimulated with IRBP but not if naïve T cells from these mouse strains are stimulated with anti-CD3 and anti-CD28 antibodies (data not shown). This suggests that the higher proliferative responses of the WT cells is not due to an intrinsic developmental defect that renders the CD4 T cells less responsive to antigen (see ahead). Similar results were obtained in delayed type hypersensitivity assays (Fig. 1E), underscoring the fact that CD4Stat3−/− T cells recognize and respond to IRBP but do not develop EAU.
Figure 1.
STAT3-deficient mice do not develop EAU. (A) Western blot analysis confirming the deletion of STAT3 in CD4Stat3−/− mice. (B) Six-week old mouse was immunized with IRBP in CFA and 14 days later eyes were enucleated. Five microns thick sections were cut through the retina and stained with H&E (100x). The retinas are from 2 different CD4STAT3−/− (bottom panel) and 2 WT (top panel) mice. (C) Two groups of 16 WT and CD4Stat3−/− mice were immunized with IRBP in CFA and EAU clinical scores were established 14 days after immunization by fundoscopy. (D) Lymph node cells of IRBP-immunized mice were stimulated with either PPD, PHA or IRBP for 3 days and proliferative responses of the cells was assessed. Results are presented in CPM units and indicate mean values of triplicate cultures. (E) Delayed type hypersensitivity (DTH) responses were assessed following injection of IRBP to ear lobes; DTH units are indicated as mean ear-thickness (μM). Data shown are 8 mice per group and representative of at least two experiments. Error bars are SD.
Resistance of CD4Stat3−/− to EAU correlates with a paucity of Th17 and increase in Foxp3+ T cells
During EAU in mice, both Th1 and Th17 cells are expanded and recruited into the retina and their levels vary during the course of the disease (12). In WT mice, initial clinical signs of EAU is observed 7–12 days after immunization with full-blown clinical disease characterized by substantial increase in proinflammatory cytokines occurring by post-immunization days-12 to 14 (Fig. 1B, 1C) (12). As Th17 cells have recently been implicated in uveitis, we examined whether the resistance of CD4Stat3−/− to development of EAU, derives from defects in generation of Th17 cells. Freshly isolated cells from the lymph node and spleen of IRBP-immunized WT or CD4Stat3−/− mice on day 0, 10 and 21 post-immunization were therefore analyzed (without stimulation) to determine the relative abundance of Th17 and Th1 cells. In line with published report (5), onset of EAU pathology is temporally correlated with increase of Th17 and Th1 in these peripheral lymphoid tissues (Fig. 2A). However, there are notable differences between WT and CD4Stat3−/− in their T cell population dynamics elicited by immunization with IRBP. Compared to WT, numbers of Th17 in spleen and lymph nodes of IRBP-immunized CD4Stat3−/− is markedly reduced and remained low at all time points analyzed. Unlike the Th17 population, we observe progressive increase in the percentage of IFN-γ-expressing CD4Stat3−/− T cells (Fig. 2A), suggesting that Th1 cells do not cause EAU. We also analyzed PBMC (Fig. 2B) and spleen/lymph node CD4+ T cells (Fig. 2C) from both mouse strains and across the board, we detected substantially lower percentage of Th17 cells in these tissues of the CD4Stat3−/− mouse compared to the WT strain. It is also remarkable that pathology in the WT is associated with significant increase in the numbers of cells expressing both IFN-γ and IL-17 (referred to here as double positive or DP cells). Interestingly, expansion of the purified lymph node cells by IL-2 preferentially increased the percentage of the double positive cells (Fig. 2D) and consistent with previous reports (5), the cells that are predominantly expanded by IL-2 are memory cells (Fig. 2E). It is also of note that a majority of the CD4Stat3−/− cells exhibit an activated T cell phenotype, suggesting that the defect in Th17 developmental pathway does not interfere with capacity of the CD4Stat3−/− T cells to respond to activation signals. In contrast to the increase in IL-17-expressing and DP cells in WT lymph node and spleen, the percentage of Foxp3-expressing cells are increased in CD4Stat3−/− compared to WT cells (Fig. 2F). Lymph node and spleen cells isolated from mice 7 or 14 days post-immunization were stimulated with IRBP and analysis of cytokine secretion by ELISA reveals marked increase in IL-17, TNF-α, IL-23 and IL-1α in the WT but not the CD4Stat3−/− cells (Fig. 2G).
Figure 2.
Naïve CD4Stat3−/− T cells do not efficiently differentiate into Th17 cells. (A) Freshly isolated cells from lymph nodes and spleen of IRBP-immunized WT or CD4Stat3−/− mice on day 0, 10 or 21 post-immunization were analyzed by intracellular cytokine staining assay to determine relative abundance of IL-17- and IFN-γ-expressing CD4+ T cells. PBMC (B) or spleen/lymph node CD4+ T cells (C–F) isolated from mice at day-14 post-immunization were analyzed to determine percentage of cells expressing IL-17 and IFN-γ (B, C, D); CD44 and IL-17 (E) or Foxp3 or IL-17 (F). Freshly isolated cells were analyzed without prior stimulation with IRBP (A, B, C, E, F) or after stimulation for 4 days with IRBP and expansion in IL-2 for 3 days (D). (G) Lymph node and spleen cells isolated from mice 7 or 14 days post-immunization were stimulated with IRBP for 3 days and cytokine secretion was quantified by ELISA.
Expression of Th1, Th2 and Th17 signature cytokines is dysregulated in absence of STAT3
In vivo intracellular cytokine-staining assays of T cell populations of the PBMC, lymph nodes and spleen elicited by immunization of mice with the IRBP autoantigen reveal a marked diminution of IL-17-expressing T cells in CD4Stat3−/− mice (Fig. 2). To further characterize the function of STAT3, purified naive CD4+ T cells isolated from lymph nodes and spleen were stimulated with anti-CD3 and anti-CD28 Abs under Th1, Th2 or Th17 polarization conditions and RNA from the cells was analyzed by RT-PCR and patterns of cytokine expression of cells from the two mouse strains was determined by intracellular cytokine-staining assays and ELISA. Substantial reduction in IL-23R and RORγt expression (Fig. 2A), further underscores the Th17-developmental defects in CD4Stat3−/− mice (9, 10). Similar to results of analysis of freshly isolated lymph node and spleen cells (Fig. 2F), Foxp3-expressing CD4+ T cells are markedly elevated in the CD4Stat3−/− mouse (Fig. 3B). Consistent with in vivo results obtained in freshly isolated tissues of mice immunized with IRBP, the percentage of IL-17-expressing CD4+ T cells is also much higher in in vitro stimulated T cells of the WT compared to CD4Stat3−/− mouse (Fig. 3C). Interestingly, we observed an important distinction between T cells that are specific to the IRBP autoantigen and those that were non-specifically stimulated with anti-CD3 and anti-CD28 antibodies. In more than 3 independent studies, we consistently observe significant numbers of DP cells in cultures that were stimulated with IRBP while all cultures stimulated by anti-CD3 and anti-CD28 antibodies contain relatively low numbers of the DP cells. Interestingly, similar increase in DP cells has also been observed in EAE (15, 16). Surprisingly, intracellular cytokine analysis of the purified CD4+ T cells reveal tremendous increase in IL-4- and IL-10-expressing cells in CD4Stat3−/− lymph nodes and spleen (Fig. 3D). Stimulation of the cells under Th1, Th2 or Th17 polarizing condition further confirm that the secretion of IL-4 is markedly increased in CD4Stat3−/− (Fig. 3E), suggesting that deletion of STAT3 in CD4+ cells may induce a compensatory increase in Th1, Th2 and Treg subsets.
Figure 3.
CD4 T-helper cell lineage-specific pattern of cytokine expression is dysregulated in CD4Stat3−/− mice. Purified naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 Abs under Th17 polarizing condition. (A) RNA isolated from the cells was subjected to RT-PCR analysis using primers specific to RORγt or IL-23 receptor (IL-23R). Relative abundance of the activated CD4+ T cells expressing Foxp3 (B), IL-17 and IFN-γ (C) or IL-4 and IL-10 (D) was quantified by intracellular cytokine staining assay. (E) ELISA analysis of cytokine secretion by naïve CD4 cells cultured under Th1, Th2 or Th17 polarization condition. Data shown are representative of at least three experiments.
CD4Stat3−/− mice do not develop experimental autoimmune encephalomyelitis (EAE)
Essential immunopathogenic features of EAU are very similar to those observed in EAE and in both diseases, Th17 and Th1 are implicated (17). We show here that targeted deletion of Stat3 in CD4+ T cells also confers protection from developing EAE (Fig. 4). Representative tissue sections of spinal cord from mice immunized with MOG reveal presence of inflammatory cell infiltrates in the white matter and perivascular lesions of WT (Fig. 4A–4C) but not CD4Stat3−/− mice (Fig. 4D–4F); in two separate experiments, sixteen WT mice developed classic symptoms of EAE ranging from hind limb weakness, ascending paralysis, to complete front and hind limb paralysis. In contrast, all sixteen CD4Stat3−/− mice remain normal after 1 month of observation (Fig. 4G) with no sign of CNS inflammation. Similar to EAU, development of EAE correlates with increase in IL-17-expressing T cells while the MOG-specific immune response of the CD4Stat3−/− mouse strain is dominated by IFN-γ-expressing T cells (Fig. 4H). These results further underscore the role of STAT3 pathways in shaping the T cell repertoire during CNS inflammatory diseases and suggest that whereas activation of STAT3 in vivo is required for generation of Th17 lineage, it limits expansion of Th1 and possibly other CD4+ subsets.
Figure 4.
Mice with targeted deletion of STAT3 in CD4+ T cells do not develop EAE. (A–F) Histological analysis of sections cut through the spinal cord of mice immunization with MOG. H&E-stained sections reveal inflammatory cell infiltrates in perivascular lesions of WT (AC) but not the CD4Stat3−/− strain (D–F). (G) Clinical scores of WT and CD4Stat3−/− mice immunized with MOG were graded as outlined in Methods section. (H) CD4+ T cells of day-14 immunized mouse lymph nodes were stimulated for 4 days with MOG and percentage of IL-17- and IFN-γexpressing T cells was assessed by intracellular cytokine staining assay. Data is representative of at least two experiments of 8 mice per group.
Resistance of CD4Stat3−/− to EAU derives from inability of uveitogenic T cells to enter the retina
In addition to its roles in skewing differentiation of naive T-cells towards the Th17 developmental pathway, STAT3 pathways also regulate expression of adhesion molecules and may therefore have critical roles in regulating trafficking of uveitogenic or encephalitogenic T-cells into the CNS during EAU or EAE. As indicated on Figure 4B and 4C, there is significant infiltration of the spinal cord of WT mice with EAE but not in CD4Stat3−/− mice immunized with MOG (Fig. 4E, 4F). Analysis of the eyes of both mouse strains 21 days post-immunization reveal the presence of Th17 and Th1 cells in retina of WT mice. In contrast, T cells are not detectable in the retina of CD4Stat3−/− mice immunized with IRBP. We further show that inability to recruit uveitogenic Th1 and Th17 cells into the eyes of CD4Stat3−/− mice following immunization with IRBP correlates with substantial decrease in retinal expression of genes coding for VLA-4 or α4 integrin (CD49d) and β1 integrin (CD29) (Fig. 5B); proteins that promote trans-endothelial migration of leukocytes into the CNS and entry of activated lymphocytes into the retina (18, 19). To further confirm this observation at the protein level, freshly isolated PBMC or spleen cells at days, 0, 7 or 14 post-immunization with IRBP were immediately analyzed for the expression of activated α4 integrin (CD49d) and β1 integrin (CD29). In PBMC (Fig. 5C), as well as, spleen cells (Fig. 5D) there is substantial elevation of percentage of cells co-expressing α4 and β1 integrin in WT compared to reduced levels in CD4Stat3−/− mice. These results suggest that decreased levels of activated integrins resulting from STAT3 deficiency may inhibit trans-endothelial migration of leukocytes and recruitment of T cells into the retina of CD4Stat3−/− mice. However, because expression of these integrins is not completely extinguished, the inhibition of these integrins may not be solely responsible for inability of CD4Stat3−/− T cells to enter the eye.
Figure 5.

Resistance to EAU derives from inability of uveitogenic T cells to enter the retina. (A) Detection of IL-17- and IFN-γ-expressing CD4+ T cells in eyes of WT mice with EAU but not in CD4Stat3−/− mouse eye enucleated 21 days after immunization with IRBP. Freshly isolated cells from lymph nodes/spleen (B, D) or PBMC (C) of IRBP-immunized WT or CD4Stat3−/− mice on day 0, 7 or 14 post-immunization and were analyzed by real-time RT-PCR for expression of α4 integrin mRNA (B) or cell surface Flow cytometry (C, D) for detection of T cells expressing α4 and β1 integrin.
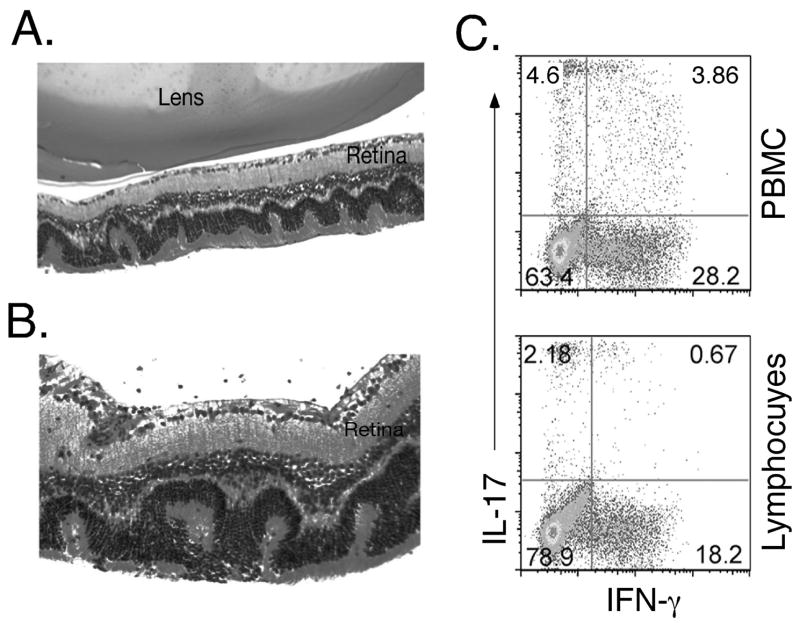
Adoptive transfer of IRBP-specific WT uveitogenic T cells induces EAU in CD4Stat3−/− mice
We performed adoptive transfer experiments to further confirm that the resistance of CD4Stat3−/− mice to EAU is a direct consequence of absence of STAT3 expression by CD4+ T cells. Activated IRBP-specific CD4+ T cells from WT mice with EAU were adoptively transferred into CD4Stat3−/− mice. The mice exhibited signs of EAU 12 days after adoptive transfer of the WT cells, as determined by fundoscopy and histology (Figures 6A and 6B). Similar to immunized WT mice, EAU in CD4Stat3−/− mice is characterized by development of retinal folds, disruption of the retinal architecture, and infiltration of inflammatory cells into the retina. In addition, disease development in the CD4Stat3−/− mice correlates with detection of substantial percent of donor Th17 cells and substantial percentage of PBMC are DP T cells expressing IL-17 and IFN-γ (Fig. 6C). It is also of note that we also adoptively transferred Stat3 deficient immune cells to WT mice but these mice did not develop EAU (data not shown), further underscoring the involvement of Stat3 pathways in development of EAU.
Figure 6.
Adoptive transfer of IRBP-specific WT uveitogenic T cells induces EAU in CD4Stat3−/− mice. (A, B) Histological section through the eye of CD4Stat3−/− mouse that received by i.v injection, IRBP-activated CD4+ cells isolated from WT mice with EAU. (C) Analysis of freshly isolated PBMC and lymph node CD4+ T cells of recipient CD4Stat3−/− mouse for IL-17- or IFN-γ expressing T cells by the intracellular cytokine-staining assay.
Discussion
In this study, we have provided direct experimental evidence that expression of STAT3 in CD4+ T cells is essential for the development of two CNS inflammatory diseases, EAU and EAE. In contrast to mice with conditional deletion of STAT3 in their T cell CD4 compartment (CD4Stat3−/−) that are completely resistant to either EAU or EAE (Fig. 1 and 4), WT (Fig. 1B) and CD4Stat3−/−mice injected with activated IRBP-specific uveitogenic T cells from WT mice with EAU (Fig.6), developed EAU characterized by massive infiltration of inflammatory cells into the retina and targeted destruction of photoreceptor cells. WT mice also developed severe EAE characterized by massive infiltration of inflammatory cells into the spinal cord and developed classic symptoms of EAE ranging from hind limb weakness, ascending paralysis, to complete front and hind limb paralysis. In contrast to the WT mouse strain where onset of CNS pathology is temporally correlated with increase of Th17 in peripheral lymphoid tissues, numbers of Th17 in spleen and lymph nodes of IRBP- or MOG-immunized CD4Stat3−/− mice is markedly reduced due to inability of naïve Stat3-deficient T cells to differentiate into Th17 phenotype. Of particular note is our finding that the level of cells expressing IFN-γ is markedly elevated in the CD4Stat3−/− compared to WT mice, suggesting that paucity of Th17 cells is compensated for by substantial increases in Th1 cells (Fig. 2D, 2E) and that etiology of EAU or EAE cannot be attributed to Th1 cells alone. Consistent with the role of TNF-α and IL-1α production by Th17 cells in EAU pathology (5), we observe significant secretion of TNF-α IL-17 and IL-1α by WT lymph node T cells of mice with EAU but not T cells of CD4Stat3−/− mice immunized with IRBP. The inherent defect in Th17 differentiation pathway of CD4Stat3−/− T cells does not appear to derive from failure to respond to T cell activation signals, as CD4Stat3−/− T cells exhibit a highly activated T cell phenotype compared to WT cells (Fig. 2E) and respond to the autoantigen (Fig. 1D and 1E). Interestingly, antigen-experienced WT T cells exhibit much higher in vitro proliferative responses to IRBP (Fig. 1D) while naïve WT and CD4Stat3−/− T cells are similar in their proliferative responses when stimulated with anti-CD3 and anti-CD28 antibodies (data not shown). Thus, the higher proliferative responses of the WT cells to IRBP (Fig. 1D and 1E) may derive, in part, from the fact that IRBP-specific immune responses in WT mice is dominated by Th17, Th1 and DP (IL-17 and IFN-γ-expressing) T cells, while responses of CD4stat3−/− T cells is characterized by absence of Th17 and DP cells and increase in cells expressing IL-10 and Foxp3.
Stimulated CD4Stat3−/− T cells secrete exaggerated levels of IFN-γ and IL-4 (Fig. 3C, 3D, 3E), suggesting that whereas STAT3 positively regulates IL-17 expression, it negatively regulates cytokines that antagonize the development and functions of the Th17 subset. Similarly, IL-10- and Foxp3-expressing cells are higher in CD4Stat3−/− compared to WT mice (Fig. 3B, 3D), suggesting that compensatory increase in Tr1 and inducible Treg cells may represent another mechanism that restrains development of pathogenic CNS autoimmune diseases in CD4Stat3−/− mice. Decrease in regulatory T cell subsets and marked increase in Th17 signature cytokines during EAU in WT mice (Fig. 2G) may provides a mechanistic link between activation of STAT3 pathways and development of pathogenic CNS autoimmune diseases. It is however paradoxical that STAT3 has recently been shown to induce rapid expression of anti-inflammatory genes (20, 21) and is also required for differentiation and expansion of Th17 cells that mediate a number of human inflammatory diseases (5). The role of STAT3 pathways of CD4+ T cells in mediating CNS inflammatory diseases appear to extend beyond promoting Th17 developmental pathway as STAT3 pathways also regulate expression of adhesion molecules that regulate trafficking of uveitogenic or encephalitogenic T cells into the CNS during EAU and EAE. Although we detect Th17 and Th1 cells in WT eyes, they are not detectable in retina of CD4Stat3−/− mice immunized with IRBP (Fig. 5A). We show that inability to recruit uveitogenic T cells into CD4Stat3−/− retina following immunization with IRBP correlates with substantial decrease in the number cells expressing activated of α4/β1-integrin (Fig. 5B-4D). On the other hand, enhanced expression and expression of these adhesion molecules correlates with entry of activated lymphocytes into the retina and brain (Fig. 5A) (22, 23).
With regards to uveitis and other ocular inflammatory diseases, there is compelling evidence for involvement of both Th1 and Th17 cell types. Th17 are most abundant in retina at early stages of the disease, increase during active uveitis/scleritis and decrease following treatment (5). On the other hand, greatest numbers of Th1 in the retina coincides with recovery phase of EAU, and together these observations have led to the suggestion that Th1 may confer protection while Th17 may promulgate EAU pathology (5). It is however premature to surmise that Th1 cells are not etiologic agents of uveitis since adoptive transfer of an IRBP-specific T cell line, depleted of IL-17-expressing T cells, induces EAU (24). Moreover, IL-17−/− mice are partially protected from EAU (R. Caspi; personal communication) and only partial remediation of EAU is achieved by neutralizing IL-17 with anti-IL17 Abs (5). These and other studies underscore involvement of other etiologic mechanisms and continue to fuel debate over the exact role of Th1 and Th17 subtypes in uveitis. In fact, in a very recent report published while our manuscript was in revision, STAT3 signaling was found to be required in the development of Th17-dependent autoimmunity and consistent with data noted in this study, ablation of STAT3 signaling in CD4 cells increased Th1responses, suggesting that STAT3 signaling may skew Th responses away from the Th1 pathway and toward the Th17 pathway (25). In our study, analysis of IRBP-specific T cells from lymph node of mice with EAU further reveal presence of substantial numbers of T cells co-expressing IL-17 and IFN-γ (Fig. 2D and 6C) and recent reports have identified these double-expressors as Th1-like cells (26). Considering that evidence linking Th17 subset to etiology of CNS inflammatory diseases are largely based on correlations between IL-17 expression in CNS target tissue and initiation of CNS disease, our results raise the intriguing possibility that uveitis may indeed be mediated not only by Th17 but also by DP cells expressing IFN-γ and IL-17. Similar analysis of IRBP-immunized CD4Stat3−/− mice reveal a virtual absence of the DP cells (Fig. 2D) and establishes for the first time that STAT3 may be required for their generation. As many inflammatory cytokines activate STAT3 pathways in lymphocytes, we cannot rule out the possibility that generation of DP cells expressing both IL-17 and IFN-γ does occur in situ at target sites of inflammation and may therefore contribute to the disease process.
Identifying pathways that mediate pathogenic autoimmune diseases of the CNS is potentially applicable to other organ-specific autoimmune diseases and of practical importance in development of organ-specific anti-inflammatory therapy. In context of ocular inflammatory diseases, it should be emphasized that all major T cell subsets (Th1, Th2, Th17, Tr1 and Treg) enter the eye during uveitis. Regardless of whether these cells confer protection or induce uveitis, prolonged presence of any T cell type in the eye compromises vision by interfering with light refraction and is therefore undesirable. Thus, the therapeutic goal is to inhibit or limit expansion of T cells that enter the eye during ocular inflammation; knowing the T cell subset that initiates the disease process is irrelevant in context of restoring normal vision. Here, we have shown that in contrast to the partial protection conferred by IL-17 blockage with IL-17 Abs, CD4stat3−/− mice are completely resistant to EAU or EAE. This dramatic outcome derives from combinatory mechanisms that include: IL-17 blockade; altered T cell homeostasis that favor expansion of anti-inflammatory responses; inhibition of T cell entry to CNS tissues. Although recent studies suggest that cytokines such as IL-21 that promote Th17 development may be effective targets for modulating CNS disease (15, 16), we believe that STAT3 is a more attractive target for therapeutic modulation of uveitis and possibly other CNS inflammatory diseases; its deletion impacts not only Th17 but also IL-17-expressing Th1 cells and prevents access of T cells into immunologically privileged tissues of the CNS.
Acknowledgments
Authors thank P. Silver (NEI, NIH) for assistance with the EAU studies; D. Levy (New York University) for providing the Stat3fl/fl mice; Igal Gery (NEI, NIH) for assistance with the EAU studies and reading the manuscript.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Nussenblatt RB. Proctor Lecture. Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest Ophthalmol Vis Sci. 1991;32:3131–3141. [PubMed] [Google Scholar]
- 2.Tarrant TK, Silver PB, Chan CC, Wiggert B, Caspi RR. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. J Immunol. 1998;161:122–127. [PubMed] [Google Scholar]
- 3.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–230. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspi RR, Silver PB, Chan CC, Sun B, Agarwal RK, Wells J, Oddo S, Fujino Y, Najafian F, Wilder RL. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol. 1996;157:2668–2675. [PubMed] [Google Scholar]
- 5.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 6.Dullforce PA, Seitz GW, Garman KL, Michael JA, Crespo SM, Fleischman RJ, Planck SR, Parker DC, Rosenbaum JT. Antigen-specific accumulation of naive, memory and effector CD4 T cells during anterior uveitis monitored by intravital microscopy. Cellular immunology. 2006;239:49–60. doi: 10.1016/j.cellimm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 8.Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273–289. doi: 10.1080/08830180212067. [DOI] [PubMed] [Google Scholar]
- 9.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 10.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 11.Calder VL, Lightman SL. Experimental autoimmune uveoretinitis (EAU) versus experimental allergic encephalomyelitis (EAE): a comparison of T cell-mediated mechanisms. Clinical and experimental immunology. 1992;89:165–169. doi: 10.1111/j.1365-2249.1992.tb06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–127. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 14.Yu CR, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, Gery I, Egwuagu CE. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173:737–746. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 15.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. 2007 doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. 2007 doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke MA, Segal BM. Th17 and Th1 responses directed against the immunizing epitope, as opposed to secondary epitopes, dominate the autoimmune repertoire during relapses of experimental autoimmune encephalomyelitis. J Neurosci Res. 2007;85:1685–1693. doi: 10.1002/jnr.21291. [DOI] [PubMed] [Google Scholar]
- 18.Martin AP, de Moraes LV, Tadokoro CE, Commodaro AG, Urrets-Zavalia E, Rabinovich GA, Urrets-Zavalia J, Rizzo LV, Serra HM. Administration of a peptide inhibitor of alpha4-integrin inhibits the development of experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2005;46:2056–2063. doi: 10.1167/iovs.04-0418. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. off. Immunology. 1995;86:408–415. [PMC free article] [PubMed] [Google Scholar]
- 20.El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, Smith AM, Rutschman R, Kaushal D, Shen Y, Suda T, Donnelly RP, Myers MG, Jr, Alexander W, Vignali DA, Watowich SS, Ernst M, Hilton DJ, Murray PJ. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880–7888. doi: 10.4049/jimmunol.177.11.7880. [DOI] [PubMed] [Google Scholar]
- 21.Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans. 2006;34:1028–1031. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. 2003;142:47–57. doi: 10.1016/s0165-5728(03)00258-3. [DOI] [PubMed] [Google Scholar]
- 23.Wooten DK, Xie X, Bartos D, Busche RA, Longmore GD, Watowich SS. Cytokine signaling through Stat3 activates integrins, promotes adhesion, and induces growth arrest in the myeloid cell line 32D. The Journal of biological chemistry. 2000;275:26566–26575. doi: 10.1074/jbc.M003495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan CC, Caspi RR. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156:1654–1660. [PubMed] [Google Scholar]
- 25.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]