Abstract
Objectives:
Approximately one third to one half of the penis is embedded in the pelvis and can be felt through the scrotum and in the perineum. The main arteries and nerves enter the penis through this perineal part of the penis, which seems to represent a highly sensitive area. We investigated the hypothesis that percutaneous perineal stimulation evokes erection in patients with neurogenic erectile dysfunction.
Methods:
Percutaneous electrostimulation of the perineum (PESP) with synchronous intracorporeal pressure (ICP) recording was performed in 28 healthy volunteers (age 36.3 ± 7.4 y) and 18 patients (age 36.6 ± 6.8 y) with complete neurogenic erectile dysfunction (NED). Current was delivered in a sine wave summation fashion. Average maximal voltages and number of stimulations delivered per session were 15 to 18 volts and 15 to 25 stimulations, respectively.
Results:
PESP of healthy volunteers effected an ICP increase (P < 0.0001), which returned to the basal value upon stimulation cessation. The latent period recorded was 2.5 ± 0.2 seconds. Results were reproducible on repeated PESP in the same subject but with an increase of the latent period. Patients with NED recorded an ICP increase that was lower (P < 0.05) and a latent period that was longer (P < 0.0001) than those of healthy volunteers.
Conclusion:
PESP effected ICP increase in the healthy volunteers and patients with NED. The ICP was significantly higher and latent period shorter in the healthy volunteers than in the NED patients. PESP may be of value in the treatment of patients with NED, provided that further studies are performed to reproduce these results.
Keywords: Spinal cord injuries, Electrostimulation, Penis, Impotence, Perineum, Neurogenic erectile dysfunction
INTRODUCTION
The majority of the penile arteries, veins, and nerves enter or leave the penis through the perineum (1–4). The common penile artery, after piercing the urogenital diaphragm, gives rise to the cavernosal artery, which enters the corpus cavernosum at the hilum of the penis (3). The venous blood is drained from the penis by 3 systems: superficial, intermediate, and deep (1–3). The superficial system drains into the internal saphenous vein and the intermediate system into the periprostatic plexus. The deep drainage system is composed of the cavernosal and crural veins, which pass through the hilum of the penis and drain into the pudendal vein (5). The peripheral nerves that have a role in erectile function comprise the thoracolumbar sympathetic, lumbosacral parasympathetic, and lumbosacral somatic nerves (6–8). They innervate the penis through the perineum (6,7).
The penis consists of 2 parts: an external part that lies outside the pelvic cavity and an internal part inside the pelvis. Approximately one third to one half of the penis is internal (9,10). This portion of the penis can be felt through the scrotum and in the perineum; as mentioned above, the main arteries and nerves enter the penis through the perineal part of the penis, and the veins leave the penis through it. For these reasons, the perineum behind the scrotum supposedly represents a highly sensitive area. We hypothesized that percutaneous stimulation of the perineum would evoke erection in patients with neurogenic erectile dysfunction (NED). This hypothesis was investigated in the current study.
MATERIALS AND METHODS
Study Participants
Twenty-eight healthy male volunteers (mean [± SD] age 36.3 ± 7.4 y, range 28–46 y) were enrolled in the study; all were married. The study also included 18 married patients with complete NED due to multiple sclerosis (MS). Mean age was 36.6 ± 6.8 years (range 29–42 y). Duration of erectile dysfunction (ED) was 9 to 16 years (mean 11.6 ± 2.2 y). The study participants signed informed consents to enter the study after they had been informed about both its purpose and its protocol.
Laboratory work including urinalysis, blood count, and liver and kidney function tests as well as electrocardiography showed normal findings. The Cairo University Faculty of Medicine Review Board and Ethics Committee approved the study.
Methods
Percutaneous electrostimulation of the perineum (PESP) was performed, and penile erection was evaluated by recording the intracorporeal pressure. PESP was performed by means of the same probe used in rectal electroejaculation (11). With the study participant lying in the lithotomy position and the scrotum elevated and strapped to the abdomen, the penile bulb in the perineum was palpated. The probe was applied to the perineum in the area between the anal orifice and the bulb of corpus spongiosum. The probe was connected to an electrical stimulator, and current was delivered in a sine wave summation fashion. Average maximal voltages and number of stimulations delivered per session were 15 to 18 volts and 15 to 25 stimulations, respectively. Stimulation above this level was uncomfortable in the healthy volunteers and the patients with MS. The stimulation period varied from 15 to 20 minutes each time (mean 16.4 ± 1.2 min). The intracavernosal pressure (ICP) was measured by means of a 27-gauge butterfly needle that was inserted into one corpus cavernosus (CC). The needle was connected to a strain gauge pressure transducer (Statham 230 B, Oxnard, CA). The measurements were started 20 minutes after needle insertion into the CC to ensure that the CC had adapted to the inserted needle.
To test whether PESP had induced its effect through excitation of the nerves in the perineum, lidocaine block of these nerves was performed. Five milliliters of 2% lidocaine were subcutaneously injected into the perineum, behind the penile bulb. The latter was palpated and the lidocaine was injected 1.5 to 2 cm posterior to it and in front of the anal orifice. PESP was performed 30 minutes after lidocaine injection and 3 hours later when the anesthetic effect had waned, and the ICP was measured after each PESP. On the second day, the test was repeated using normal saline instead of lidocaine. To ensure reproducibility of the results, the aforementioned readings were repeated at least twice in each individual study participant and the mean value was calculated. Statistical analysis was performed using the paired Student's t test, and values were given as the mean ± standard deviation. Significance was ascribed to P < 0.05.
RESULTS
No adverse side effects were encountered in the healthy volunteers or the patients with MS during or after performing the tests.
Healthy Volunteers
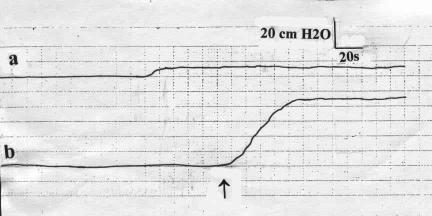
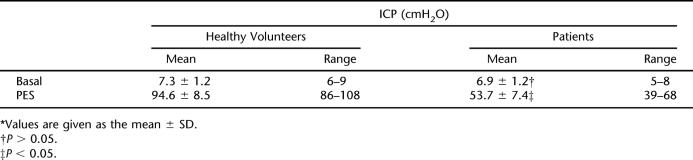
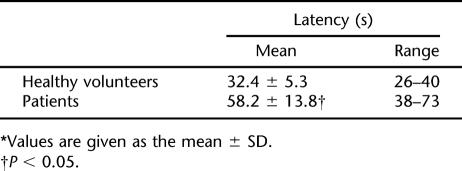
The ICP measured prior to PESP (basal) recorded a mean of 7.3 ± 1.2 cmH2O (Figure 1, Table 1). PESP using the aforementioned parameters effected an increase of the ICP to a mean of 94.6 ± 8.5 cmH2O (P < 0.0001, Figure 1, Table 1). The ICP started to increase 2 to 3 seconds (2.6 ± 0.2 s) after the start of PESP. This period represented the latent period. The ICP reached its maximum after 26 to 40 seconds (mean 32.4 ± 5.3 s). The elevated ICP was maintained as long as PESP was continuous. After cessation of PESP, the ICP remained elevated for a mean period of 38.2 ± 6.8 seconds (range 22–46 s), followed by return to the basal values. On repetition of the PESP, the latent period, which is the time from the start of PESP to the start of ICP elevation, was progressively prolonged while the pressure elevation was the same. With perineal electrostimulation 1 minute after return of the ICP to the basal values, the ICP elevation occurred after 70 to 110 seconds (mean 96.4 ± 11.3). This latent period increased to 3 to 6 minutes (mean 4.6 ± 1.1 min) after the sixth to eighth repetitions of the PESP. From the eighth repetition onward, the ICP did not respond to PESP. These results were reproducible with no significant difference (P > 0.05) when the tests were repeated in an individual subject.
Figure 1. The intracavernosal pressure of a healthy volunteer measured (a) at rest, and (b) on percutaneous perineal electrostimulation. ↑ = electrostimulation.
Table 1.
Intracavernosal Pressure (ICP) Measurements of Healthy Volunteers and Neurogenic Erectile Dysfunction Patients: Basal and During Percutaneous Electrostimulation (PES)*
Patients With NED
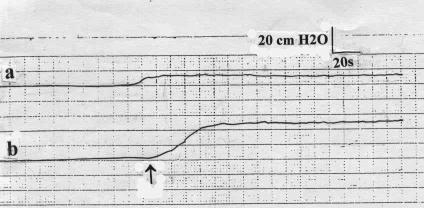
The basal ICP did not show a significant difference from that of the healthy volunteers (Table 1). Upon PESP, the ICP recorded a mean of 53.7 ± 7.4 cmH2O, which was significantly lower than that of the healthy volunteers (P < 0.05, Table 1, Figure 2). The latent period recorded a mean of 14.6 ± 2.2 seconds (range 12–18 s), which was significantly longer (P < 0.0001) than that of the healthy volunteers. The maximal pressure elevation occurred 38 to 66 seconds (mean 53.6 ± 8.2 s) after the start of PESP. The ICP returned to the basal value 12 to 26 seconds (mean 18.2 ± 4.8 s) after PESP cessation. PESP 1 minute after return of the ICP to the basal value effected an ICP increase similar to the aforementioned value after a mean period of 56 to 93 seconds (mean 78.2 ± 10.8 s). Repetition of the PESP did not produce ICP elevation. When in the individual study participant the aforementioned tests were repeated, the results were reproducible with no significant difference (P > 0.05).
Figure 2. The intracavernosal pressure of a patient with neurogenic erectile dysfunction (a) at rest and (b) on percutaneous perineal electrostimulation. ↑ = electrostimulation.
Results of Lidocaine Perineal Injection
PESP 30 minutes after perineal anesthetization did not produce significant changes (P > 0.05) in the ICP. Repetition of the test 3 hours after anesthetization, when the anesthetic effect had waned, produced an ICP response similar to that before anesthetization (P > 0.05). When the test was repeated using normal saline instead of lidocaine, the results were similar to those before saline administration.
DISCUSSION
There are various methods of inducing erection in addition to the normal erection induced by sexual stimulation. Yohimbine and alpha 2-adrenoreceptor antagonists have a weak effect (12,13). Intracavernous injection and penile implants are helpful to many patients (14,15). However, phosphodiesterase type 5 inhibitors have replaced most of the aforementioned modalities of treatment of ED (16).
Erection and ejaculation could be induced by means of electrovibration of the glans penis or rectal probe electrostimulation (17). While various sensory stimuli may produce erection and ejaculation in a healthy man, glans penis or rectal probe electrostimulation may result in peripherally mediated erection and ejaculation (18).
The penile arteries and nerves enter the penis through the posterior part of the corporal tissue in the perineum (10). Afferent sensory stimuli are initially relayed from the perineum by way of the pudendal nerve to the cerebral cortex (11). The efferent neural fibers travel down the anterolateral column of the spinal cord to terminate in the lower thoracic and upper lumbar cord. Therefore, stimulation of the perineum would supposedly stimulate the penile innervation and may enhance the penile vascularity. The classical method of penile electrostimulation is through the glans penis or anal route (18). The electrostimulator is applied to the glans penis or introduced into the anal canal through the anal orifice. It acts by stimulating not only the postganglionic, short adrenergic fibers to the male reproductive tract, but also the pudendal nerve fibers. It may also enhance the penile vascularity through the arteries entering the penis through the perineum. Thus when the stimulator is applied to the perineum, it apparently stimulates the nerves and arteries in this area.
In the current study, PESP produced significant increases of the ICP in both the healthy volunteers and the NED patients, with more increase in the former than the latter. The erection in the healthy volunteers was maintained as long as PESP was continuous and remained for approximately 40 seconds after cessation of PESP. These results were reproducible in the patients with NED, but with lower values compared to the healthy volunteers. Lidocaine block of these nerves inhibited the electrostimulation-induced erection, indicating that PESP had induced its effect through excitation of the nerves in the perineum. It remains to be determined whether perineal electrostimulation can be used clinically to induce erection in patients with ED.
The treatment of NED is challenging. In our laboratory, phosphodiesterase type 5 inhibitors effect erection in approximately 40% of these patients, using the 5 different phosphodiesterase inhibitors at their maximal tolerated dosages. The remaining patients who do not respond to phosphodiesterase type 5 inhibitors may use intracavernous injections or embedded penile implants (14,15). The latter methods are invasive and have complications. NED patients do not respond in a high percentage of instances to the vacuum tumescence enhancement system. PESP appears to represent a simple and noninvasive method for producing erection in patients with NED and complete lesions, such as subjects with complete spinal cord injury in whom the other methods fail. However, the rectal probe electrostimulator is not practical for use in the bedroom. A system needs to be developed that could be miniaturized and applied to the body or implanted.
CONCLUSION
In conclusion, PESP effected ICP increase in the healthy volunteers and patients with NED. The ICP was significantly higher and latent period shorter in the healthy volunteers than in the ED patients. After failure of other modalities, PESP is suggested for possible use for the treatment of patients with NED, provided that further studies reproduce the current results.
Table 2.
The Latency Period of Healthy Volunteers and Patients With Neurogenic Erectile Dysfunction*
Acknowledgments
Margot Yehia assisted in preparing the manuscript.
REFERENCES
- Paick J, Lue T. Anatomy of the penis. In: Hashmat A, Das S, editors. The Penis. Philadelphia, Pa: Lea & Febiger; 1993. p. 12. [Google Scholar]
- Goldstein AMB, Padma-Natham H. The microarchitecture of the intracavernosal smooth muscle and the cavernosal fibrous skeleton. J Urol. 1990;144:1144–1146. doi: 10.1016/s0022-5347(17)39677-5. [DOI] [PubMed] [Google Scholar]
- Breza J, Aboseif SR, Orvis BR, et al. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437–443. doi: 10.1016/s0022-5347(17)40789-0. [DOI] [PubMed] [Google Scholar]
- Wagner HFH, Andresen R, Knispel HH, et al. Evaluation of penile arteries with colored duplex sonography: prevalence and possible therapeutic implications of connections between dorsal and cavernous arteries in impotent men. J Urol. 1995;153:1469–1471. doi: 10.1016/s0022-5347(01)67435-4. [DOI] [PubMed] [Google Scholar]
- Krane RJ, Goldstein I, Saenz de Tejada I. Impotence. N Engl J Med. 1989;321:1648–1659. doi: 10.1056/NEJM198912143212406. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Steers W. Neuroanatomy and neurophysiology of penile erection. In: Tanagho EA, Lue TF, McClure D, editors. Contemporary Management of Impotence and Infertility. Baltimore, Md: Williams & Wilkins; 1988. pp. 3–27. [Google Scholar]
- Steers WD. Neural control of penile erection. Semin Urol. 1990;8:866–879. [PubMed] [Google Scholar]
- Lue TF, Takamura T, Schmidt RA, et al. Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol. 1984;131:273–280. doi: 10.1016/s0022-5347(17)50344-4. [DOI] [PubMed] [Google Scholar]
- Basu SC. Management of erectile dysfunction. In: Basu SC, editor. Male Reproductive Dysfunction. 1st ed. New Delhi, India: Jaypee Brothers; 2005. pp. 61–96. [Google Scholar]
- Skandalakis JE, Colborn GL, Weidman TA, et al. Perineum. Skandalakis' Surgical Anatomy. In: Skandalakis JE, Colborn GL, Weidman TA, et al., editors. The Embryologic and Anatomic Basis of Modern Surgery. 1st ed. Athens, Greece: Paschalidis Medical Publications; 2004. pp. 1618–1640. [Google Scholar]
- Shaban SF, Lipshultz LI. Electroejaculation. In: Rajfer J, editor. Common Problems in Infertility and Impotence. Chicago, IL: Year Book Medical Publishers, Inc; 1990. pp. 126–131. [Google Scholar]
- Morales A, Condra M, Owen JA, et al. Is yohimbine effective in the treatment of organic impotence? Results of a controlled trial. J Urol. 1987;137:1168–1172. doi: 10.1016/s0022-5347(17)44436-3. [DOI] [PubMed] [Google Scholar]
- Smith ER, Lee RL, Schnur SL, et al. Alpha 2-adrenoreceptor antagonists and male sexual behaviour: erectile and ejaculatory reflexes. Physiol Behav. 1987;41:15–19. doi: 10.1016/0031-9384(87)90124-7. [DOI] [PubMed] [Google Scholar]
- Juenemann KP, Lue TF, Fournier GR, Jr, et al. Hemodynamics of papaverine and phentolamine-induced penile erection. J Urol. 1986;136:158–161. doi: 10.1016/s0022-5347(17)44763-x. [DOI] [PubMed] [Google Scholar]
- Montague DK. Penile prostheses. In: Montague DK, editor. Disorders of Male Sexual Function. Chicago, IL: Year Book Medical Publishers; 1988. pp. 179–196. [Google Scholar]
- Lowy M, Collins S, Bloch M, et al. Quality of erection questionnaire correlates: change in erection quality with erectile function, hardness, and psychosocial measures in men treated with sildenafil for erectile dysfunction. J Sex Med. 2007;4:83–92. doi: 10.1111/j.1743-6109.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Bennett CI, Seager SW, McGuire EJ. Electroejaculation for recovery of semen after retroperitoneal lymph node dissection. Case report. J Urol. 1987;137:513–515. doi: 10.1016/s0022-5347(17)44094-8. [DOI] [PubMed] [Google Scholar]
- Shaban SF, Lipshultz LI. Clinical electroejaculation. Med Instrum. 1988;22:77–81. [PubMed] [Google Scholar]