Abstract
Objective:
Evaluation of noninvasive stimulation modalities to augment cough and assist tracheostomy decannulation in high-level tetraplegia.
Study Design:
Single case study.
Methods:
A 65-year-old man with C4 ASIA C tetraplegia had delayed rehabilitation due to a tracheostomy and recurrent pneumonia primarily resulting from ineffective cough. Anterior surface electrical stimulation (SES) of the abdominal musculature was conducted to train an effective cough and enable decannulation. Training occurred daily for 4 weeks. The patient was tested 1 year later with posterolateral SES to determine the relative clinical effect of this delivery method.
Results:
At baseline, the addition of anterior SES increased maximal expiratory pressure (80%), maximal expiratory cough pressure (67%), and peak expiratory flow rate (11%). Three weeks after training began, the patient was decannulated following a program of SES and assisted and voluntary coughing. Upon testing 1 year later, SES with posterolaterally placed electrodes also produced an enhancement of voluntary cough attempts.
Conclusions:
Noninvasive SES can potentially assist decannulation of tracheostomies.
Keywords: Functional electrical stimulation, transcutaneous; Spinal cord injuries; Tetraplegia; Tracheostomy; Cough reflex; Pneumonia
INTRODUCTION
Individuals with spinal cord injury (SCI) are highly susceptible to respiratory compromise. During the acute phase after SCI, it is the leading cause of death for spinal patients at all levels, accounting for 28% of deaths in the first year (1). Clinicians augment respiratory hygiene using conventional chest physiotherapy, nebulizers and other pharmacotherapies, insufflation-exsufflation devices, noninvasive ventilation, tracheostomies, bronchoscopy, and stimulation techniques, including surface electrical stimulation (SES), implanted electrodes, and magnetic stimulation (2–6). Nevertheless, the incidence of respiratory illness after SCI remains high due to loss or impairment of voluntary control of muscles required for inhalation and forceful exhalation. Many of these patients are treated with tracheostomies to assist progression from intensive care to less acute settings. However, ongoing problems with secretion control can complicate the removal of these devices (7). This report outlines how SES training contributed to tracheostomy removal in a patient with high-level tetraplegia who was unable to be decannulated using conventional treatments.
CASE STUDY
A 65-year-old man with C4 ASIA C tetraplegia consented to participate in this study after 8 months of continuous postinjury care in an acute hospital spinal unit. The patient had no premorbid diagnosis of lung disease but had a 24 pack-year smoking history, although prior to admission he had not smoked for 15 years. The rehabilitation process was delayed due to recurrent respiratory infections stemming from an ineffective cough. This complication required frequent suctioning via a tracheostomy tube, which could only be provided in the acute setting. Adjuvant modalities unsuccessfully applied prior to the SES intervention included conventional chest physiotherapy techniques (implemented from admission and continued throughout this study), noninvasive ventilation performed with pressures of 12/6 cmH2O commenced 3 months post injury and ongoing through this SES clinical trial, a Passy–Muir valve trial 3 months prior to this study, and 2 documented uses of an insufflation-exsufflation device, one 3 months pretrial (1 week duration) and 1 during the SES trial (3 days duration).
METHODS
In both the ward and the laboratory, measurements were made during the maximal expiratory pressure (MEP) maneuver against a closed airway. Maximal expiratory cough pressure (MECP) was measured during a cough (open airway) through a mouthpiece. The SES was timed to occur at total lung capacity (TLC). Spirometry trials met American Thoracic Society requirements for reproducibility (8).
SES Training on the Ward
SES was applied through 2 pairs of surface electrodes (∼3 cm × 8 cm) placed anteriorly over the abdomen with the subject seated (Figure 1A). The stimuli were delivered via a commercially available stimulator (Multi-tems Pty Ltd, Sydney, Australia). Stimuli trains were delivered at 50 Hz (4 seconds on, 4 seconds off, 100 mA: maximum for this stimulator).
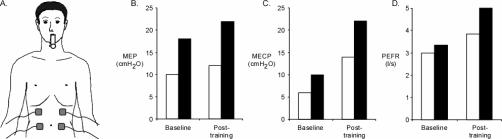
Figure 1. (A) Anterior electrode placement (ward testing); (B) Baseline and posttraining results for maximal expiratory pressure (MEP); (C) Maximal expiratory cough pressure (MECP); and (D) Peak expiratory flow rate (PEFR). Responses without stimulation (open bars) and with stimulation (filled bars) are shown.
Cough training occurred daily for 20 to 30 minutes, for 4 weeks. Voluntary cough efforts were timed with the trains of SES. The patient inhaled to near TLC during the 4 seconds when no stimulation occurred. Just prior to stimulation, the patient closed the glottis and held it closed for 2 to 3 seconds into the stimulation period. About 1 second before stimulation ceased, the patient initiated a cough. The patient rested for 1 stimulation cycle and repeated the cough effort on the next cycle. Coordination of the voluntary cough with the SES was assisted by lights on the stimulator. To maximize inspiratory capacity, a thermoplastic abdominal plate and an abdominal binder were applied over the electrodes.
Lung function and cough-related measurements were performed both with and without SES, at baseline and after 4 weeks of cough training. A portable handheld spirometer (Microlab 3300, Micromedical Ltd, Kent, United Kingdom) measured peak expiratory flow rate (PEFR), forced expiratory volume in 1 second (FEV1), and functional vital capacity (FVC). A custom-designed manometer measured MECP (at the mouth) developed during an attempted cough and MEP during a maximal expiratory effort against a closed airway from TLC. The patient repeated each maneuver 3 times. A flanged mouthpiece was used to reduce the chance of air leaks around the lips for MEP and MECP measurements in the ward. Baseline measurements via the tracheostomy involved dressings as necessary to minimize air leaks. A tube mouthpiece placed between the lips was used for the other measurements.
Subsequent Measurements
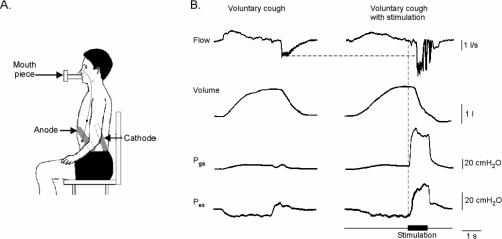
Approximately 1 year after hospital discharge, the patient returned to our respiratory physiology laboratory to participate in an experiment to test the effectiveness of SES in enhancing cough in SCI. In this study, electrodes were placed in the posterolateral position (Figure 2A). Trains of electrical stimuli (50 Hz, 1 second duration, 200 microseconds pulse width; 225 mA, DS7 Digitimer Co, Hertfordshire, United Kingdom) were delivered bilaterally and timed to coincide with a voluntary cough effort from TLC. The patient was seated and breathed on a mouthpiece connected to a pneumotachometer to measure expiratory flow and expired lung volume (Hans Rudolph, Kansas City, MO). Pressures developed during MEP maneuvers and coughs were measured using a gastroesophageal catheter (an intraesophageal tube mounted with pressure transducers located in the stomach and esophagus) to measure the abdominal (gastric) pressure (Pga) and thoracic (esophageal) pressure (Pes) (Gaeltec Ltd., Dunvegan, United Kingdom). All signals were stored on a computer (using Spike2 Cambridge Electronic Design, Cambridge, UK). Typical flow, volume, Pga, and Pes signals during a voluntary cough with and without posterolateral SES are shown in Figure 2B. A flanged mouthpiece was used to reduce the chance of air leaks around the lips. Additionally, a researcher manually positioned the mouthpiece to limit air leaks and maintained bilateral cheek pressure.
Figure 2. (A) Posterolateral electrode placement (laboratory testing); (B) Typical flow, volume, gastric pressure (Pga) and esophageal pressure (Pes) during a voluntary cough without (left panels) and with (right panels) posterolateral surface electrical stimulation (SES). Timing of stimulation is indicated by the thick horizontal bar.
RESULTS
Ward Assessment (Anterior SES)
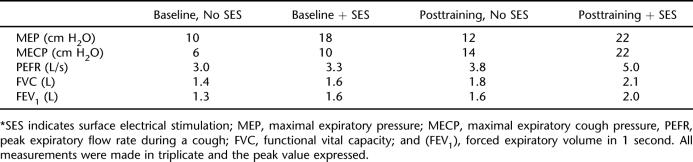
At baseline, the addition of SES produced increases in MEP (80%), MECP (67%), and PEFR (11%) (Table 1, Figure 1). There were also small increases in FVC and FEV1 with the addition of SES. After 4 weeks of training, testing with no SES showed a small increase in MEP, but MECP and PEFR had increased by 133% and 28%, respectively. Post-training, the addition of SES also produced increases in MEP (83%), MECP (57%), and PEFR (30%).
Table 1.
Baseline and Posttraining Measurements With and Without Anterior SES (Ward Testing)*
Clinically, after 2 weeks of training the patient did not require further suctioning but was able to manage his secretions with a combination of assisted coughs, SES, and voluntary coughing. After 3 weeks of training, the tracheostomy was removed, and the patient did not present with a chest infection in the remaining 11 months of his admission. Removal of the tracheostomy enabled the patient to commence rehabilitation. Respiratory SES assisted in secretion control where previous modalities were insufficient.
Laboratory Assessment: 1 Year After Discharge (Posterolateral SES)
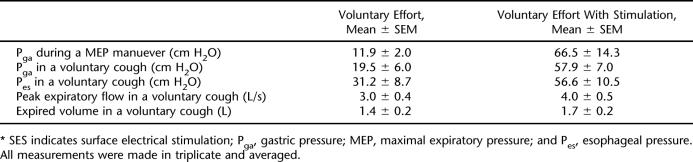
The combined voluntary MEP maneuver and train of stimuli, compared to a MEP maneuver with no stimulation, resulted in a more than fivefold increase in Pga. During a cough, the SES produced a 33% increase in peak expiratory flow, a 21% increase in expired volume, a threefold increase in Pga and a twofold increase in Pes (Figure 2, Table 2). The patient developed Pga pressures of 10 cmH2O passively during inspiration (to TLC), but during voluntary cough, did not develop further Pga, suggesting that he had no preserved ability to voluntarily activate his abdominal muscles.
Table 2.
Measurements 1 Year After Discharge With and Without Posterolateral SES (Laboratory Testing)
DISCUSSION
In this case study, the acute hospital stay was prolonged due to the inability of conventional respiratory techniques to address the problem of an ineffective cough. A program of SES almost doubled the MEP during the acute management phase. This could be a result of a training effect after 4 weeks of inpatient training, as has been noted by Lin et al after magnetic stimulation (9). Measurement differences resulting from intercurrent decannulation could potentially also have contributed, however. Subsequent modifications to the technique of stimulation led to a more than 5 times increase (of Pga) in MEP. This may reflect the posterolateral positioning of electrodes (10) and an increased stimulus intensity. These later laboratory-based results were unaffected by training effects or intercurrent decannulation.
Although flanged mouthpieces have been related to measurement error in this population group (11), the authors minimized this error in the laboratory-based results by using a researcher to position the mouthpiece to limit air leaks and maintain bilateral cheek pressure. The laboratory-based results demonstrate that the Pga and Pes values (Table 2), both of which would have been unaffected by mouthpiece type, also improved.
Several studies describe the effective use of FES to assist with enhancing cough in tetraplegic patients (3,12–15), with limited success in producing high enough expiratory pressures for effective cough. Other studies have used magnetic stimulation over the T9-T10 spinous process to activate the spinal nerve roots (T8-T12) that supply abdominal muscles (9,16–21). Despite the relative success of the magnetic stimulation in activating a large portion of abdominal muscles, the stimulators are large, expensive, and not appropriate for application in any subsequent community maintenance therapy. Additional improvements in airway pressure and flow rate have been described by DiMarco et al (22), although this involves an invasive procedure, using surgically implanted electrodes positioned on the thecal sac in the midline epidural space at the T9, T11, and L1 levels.
We have recently shown that posterolateral SES is 2 to 3 times more efficient at producing abdominal muscle contraction and abdominal pressure than stimulation over the anterior wall of the abdomen and was equally as good as magnetic stimulation over the T9-T10 spinous process (10).
Problems specific to SES include the potential to cause local skin burns or irritation, electrode sensitivity, autonomic dysreflexia, and problems with use over healing wounds (23). It is contraindicated in patients with cardiac pacemakers and has precautions for use with metallic implants (23) and unstable spinal columns. Setup requirements include adequate training of patient and staff for electrode placement and coordination of glottis closure prior to stimulation, testing to ensure that the patient can tolerate the stimulation and does not suffer thermal injury or autonomic dysreflexia, and increasing awareness that bowel and bladder programs may be affected (3). It is important to recognize that this patient was cognitively capable, motivated, and able to tolerate lower (ward-based) levels of stimulation prior to progressing to higher-intensity studies using posterolateral electrode placement.
CONCLUSION
Surface functional electrical stimulation has potential application as an adjuvant respiratory technique in acute SCI to assist respiratory management such as tracheostomy decannulation through its ability to augment cough and thus improve respiratory hygiene. The effectiveness is improved by the use of posterolateral SES techniques.
Acknowledgments
We are grateful to John Lawrence and Lyndall Katte for assistance with the ward-based measurements.
REFERENCES
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Slack RS, Shucart W. Respiratory dysfunction associated with traumatic injury to the central nervous system. Clin Chest Med. 1994;15:739–749. [PubMed] [Google Scholar]
- Jaeger RJ, Turba RM, Yarkony GM, Roth EJ. Cough in spinal cord injured patients: comparison of three methods to produce cough. Arch Phys Med Rehabil. 1993;74:1358–1361. doi: 10.1016/0003-9993(93)90093-p. [DOI] [PubMed] [Google Scholar]
- Slonimski M, Aguilera EJ. Atelectasis and mucus plugging in spinal cord injury: case report and therapeutic approaches. J Spinal Cord Med. 2001;24:284–288. doi: 10.1080/10790268.2001.11753586. [DOI] [PubMed] [Google Scholar]
- Viroslav J, Rosenblatt R, Tomazevic SM. Respiratory management, survival, and quality of life for high-level traumatic tetraplegics. Respir Care Clin N Am. 1996;2:313–322. [PubMed] [Google Scholar]
- Winck JC, Gonçalves MR, Lourenço C, Viana P, Almeida J, Bach JR. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest. 2004;126:774–780. doi: 10.1378/chest.126.3.774. [DOI] [PubMed] [Google Scholar]
- Kent C. Tracheostomy decannulation. Respir Care. 2005;50:538–541. [PubMed] [Google Scholar]
- American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Lin VW, Hsiao IN, Zhu E, Perkash I. Functional magnetic stimulation for conditioning of expiratory muscles in patients with spinal cord injury. Arch Phys Med Rehabil. 2001;82:162–166. doi: 10.1053/apmr.2001.18230. [DOI] [PubMed] [Google Scholar]
- Lim J, Gorman RB, Saboisky JP, Gandevia SC, Butler JE. Optimal electrode placement for non-invasive electrical stimulation of human abdominal muscles. J Appl Physiol. 2007;102:1612–1617. doi: 10.1152/japplphysiol.00865.2006. [DOI] [PubMed] [Google Scholar]
- Tully K, Koke K, Garshick E, Lieberman S, Tun C, Brown R. Maximal expiratory pressures in spinal cord injury using two mouthpieces. Chest. 1997;112:113–116. doi: 10.1378/chest.112.1.113. [DOI] [PubMed] [Google Scholar]
- Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest. 1993;103:166–169. doi: 10.1378/chest.103.1.166. [DOI] [PubMed] [Google Scholar]
- Lin KH, Lai YL, Wu HD, Wang TQ, Wang YH. Effects of an abdominal binder and electrical stimulation on cough in patients with spinal cord injury. J Formos Med Assoc. 1998;97:292–295. [PubMed] [Google Scholar]
- Stanic U, Kandare F, Jaeger R, Sorli J. Functional electrical stimulation of abdominal muscles to augment tidal volume in spinal cord injury. IEEE Trans Rehabil Eng. 2000;8:30–34. doi: 10.1109/86.830946. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Tromans AM, Harris KR, Swain ID. Electrical stimulation of abdominal muscles for control of blood pressure and augmentation of cough in a C3/4 level tetraplegic. Spinal Cord. 2002;40:34–36. doi: 10.1038/sj.sc.3101250. [DOI] [PubMed] [Google Scholar]
- Kyroussis D, Polkey MI, Mills GH, Hughes PD, Moxham J, Green M. Simulation of cough in man by magnetic stimulation of the thoracic nerve roots. Am J Respir Crit Care Med. 1997;156:1696–1699. doi: 10.1164/ajrccm.156.5.9702008. [DOI] [PubMed] [Google Scholar]
- Lin VW, Hsieh C, Hsiao IN, Canfield J. Functional magnetic stimulation of expiratory muscles: a noninvasive and new method for restoring cough. J Appl Physiol. 1998;84:1144–1150. doi: 10.1152/jappl.1998.84.4.1144. [DOI] [PubMed] [Google Scholar]
- Lin VW, Singh H, Chitkara RK, Perkash I. Functional magnetic stimulation for restoring cough in patients with tetraplegia. Arch Phys Med Rehabil. 1998;79:517–522. doi: 10.1016/s0003-9993(98)90065-x. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Luo Y, Guleria R, Hamnegard CH, Green M, Moxham J. Functional magnetic stimulation of the abdominal muscles in humans. Am J Respir Crit Care Med. 1999;160:513–522. doi: 10.1164/ajrccm.160.2.9808067. [DOI] [PubMed] [Google Scholar]
- Singh H, Magruder M, Bushnik T, Lin VW. Expiratory muscle activation by functional magnetic stimulation of thoracic and lumbar spinal nerves. Crit Care Med. 1999;27:2201–2205. doi: 10.1097/00003246-199910000-00022. [DOI] [PubMed] [Google Scholar]
- Estenne M, Pinet C, De Troyer A. Abdominal muscle strength in patients with tetraplegia. Am J Respir Crit Care Med. 2000;161:707–712. doi: 10.1164/ajrccm.161.3.9906020. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce an effective cough in spinal cord injured patients. Am J Respir Crit Care Med. 2006;173:1386–1389. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman PH. An update on functional electrical stimulation after spinal cord injury. Neurorehabil Neural Repair. 2000;14:251–263. doi: 10.1177/154596830001400402. [DOI] [PubMed] [Google Scholar]