Abstract
Background/Objective:
Reports in the literature suggest that administration of intrathecal baclofen to control spasticity may have deleterious effects on erectile function in men with spinal cord injury (SCI). A prospective study was conducted to document any changes in perceived sexual function after implant of a baclofen pump.
Methods:
Seven adult men with SCI (ASIA A or B) who received intrathecal baclofen through an implantable pump for treatment of severe spasticity were followed for an average of 670 days (22.4 months) after implant. Perceived sexual function was assessed using the Brief Sexual Function Inventory. Severity of spasticity and overall health-related quality of life were also assessed.
Results:
Participants reported improvements in spasticity severity and overall health-related quality of life. Two of 7 participants reported some negative changes in perceived sexual function after baclofen pump implant, noted in the areas of reduced sex drive and problems with erections (frequency, rigidity, difficulty in achieving). However, most participants reported minimal effects on sexual function, and 2 participants reported marked improvement in perceived sexual function from pre- to post-implant. Analysis of changes in perceived sexual function over time suggest that problems may be associated with an increase in baclofen dose and may be reversible with a reduction in dose.
Conclusions:
Intrathecal baclofen may impact perceived sexual function particularly at higher doses. However, the effects seem to be reversible with withdrawal or reduction of baclofen administration.
Keywords: Spinal cord injuries, Baclofen, Spasticity, Sildenafil citrate, Sexual function, Erectile dysfunction
INTRODUCTION
Intrathecal administration of baclofen is widely recognized as an effective treatment for severe spasticity of spinal origin. Dramatic improvements in spasticity, the ability to independently complete activities of daily living, and bladder and urethral sphincter function have been noted (1–3). Adverse side effects have been minimal. Concerns have been raised, however, about the potential for impaired sexual function in men. Temporary loss of reflexogenic erection after a rapid increase in baclofen dose has been reported and presumed to result from inhibition of visceral afferent input to the lumbosacral spinal cord (2).
In a long-term follow-up study of the effects and side effects of intrathecal baclofen conducted by Meythaler et al (4), 2 of 7 participants reported a temporary decrease in penile erections. Denys et al (5) also reported changes in sexual function after initiation of intrathecal baclofen in a study of 9 men (5 with spinal cord injury [SCI]; 4 with multiple sclerosis [MS]). Results were mixed, with none of the participants reporting any change in libido or ability to achieve erections. However, 5 participants reported diminished strength or duration of erections, and 2 reported greater difficulty in reaching ejaculation. Denys et al (5) attribute the inhibitory effect of intrathecal baclofen on erection and ejaculation to its agonist effect on gamma-aminobutyric acid B (GABAB) receptors in the lumbosacral spinal cord. They also pointed out that the inhibitory effects of intrathecal baclofen seem to be reversible with withdrawal of medication.
The purpose of this study was to examine, prospectively and with a standardized measure of sexual function, the impact of intrathecal baclofen on perceived sexual functioning in men with severe spasticity of spinal origin.
METHODS
Participants
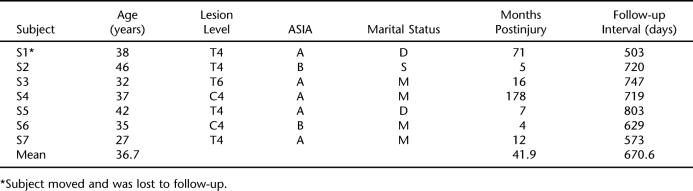
After approval of the study protocol by the hospital's institutional review board, 7 participants were recruited from among patients who had been referred for implant of a baclofen pump to control severe spasticity secondary to traumatic SCI. Inclusion criteria were (a) men 25 to 45 years of age; (b) SCI; (c) positive bulbocavernous reflex, indicating capacity for reflexogenic erections; and (d) reported history of sexual activity since injury. Table 1 presents the main characteristics of the study participants.
Table 1.
Main Characteristics of Study Participants
Data Collection
Rather than focus solely on sexual function, which may have overly sensitized participants to concerns in this area, the study was presented as an investigation of the effectiveness of intrathecal baclofen in treating spasticity and common side effects associated with its use. Measures of perceived sexual function were imbedded in a patient questionnaire that also addressed severity of spasticity and overall health-related quality of life. The questionnaire was compiled from existing standardized measures. Respondents were instructed to respond to all questions based on problems, symptoms, and experiences during the previous month.
Perception of spasticity severity was assessed using items from the performance scales developed by Schwartz et al (6) as a self-report measure of impairment and disability resulting from MS. The spasticity items included a rating of severity on a 6-point numeric scale, ranging from 0 = normal (“I have noticed no problems with spasticity.”) to 5 = total spasticity disability (“Every day spasticity problems prevent me from doing my daily activities.”). A second item asked the respondent to assign a general utility rating (from 0 = worst health to 100 = perfect health) of their spasticity's impact on health and well being.
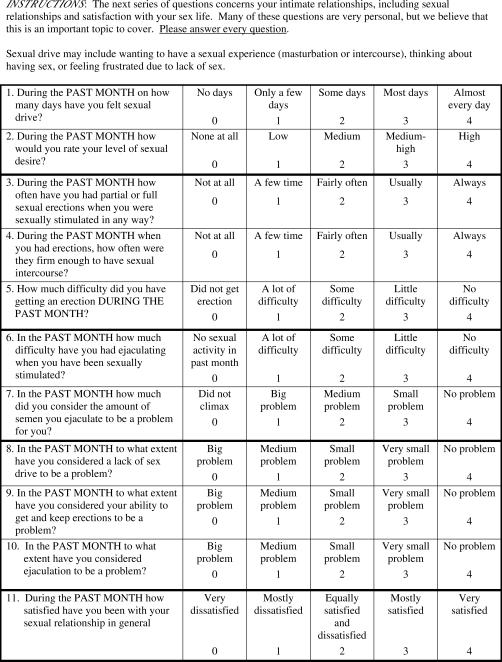
Perceived sexual functioning was assessed using the Brief Sexual Function Inventory (BSFI) developed by O'Leary et al (7) for use in urology research and practice. The BSFI is presented in Figure 1 and consists of 11 items covering 5 aspects of sexual functioning: sexual drive (2 items), erection (3 items), ejaculation (2 items), perception of problems with sexual function in each of these areas (3 items), and an overall satisfaction rating (1 item). Each item is scored on a 5-point rating scale with individualized anchor point descriptors.
Figure 1. The BSFI adapted from O'Leary et al (7).
Health-related quality of life was assessed using version 2.0 of the SF-36 Health Survey developed by Ware and Sherbourne (8). The 36-item survey yields an 8-scale profile of functional health and well-being scores, as well as psychometrically based physical and mental health summary measures and a preference-based health use index.
Procedures
Questionnaires were either given to participants to complete during an outpatient clinic visit or mailed to respondents to complete and return to the study investigators. A research nurse was available to assist with completing the questionnaire, either in person in the clinic or by telephone. To serve as a baseline measure, participants were asked to complete the questionnaire at least once before the date on which they were admitted for implant of a baclofen pump. Participants were asked to complete the questionnaire more than once, on a monthly basis, if more than 1 month elapsed from the time of study enrollment to pump implant. After pump implant, participants were asked to complete the questionnaire approximately monthly for 3 months and then approximately every 4 months for up to 2 years after implant.
Implantation of the baclofen pump followed standard clinical protocol and consisted of the following procedures. The patient received a trial administration of intrathecal baclofen, consisting of a 50-, 75-, or 100-μg bolus injection. Adverse and therapeutic effects were monitored during this trial administration. Patients who showed a favorable (and no adverse) effect underwent surgical implantation of a programmable infusion pump in the subcutaneous space of the upper abdominal wall. A catheter was tunneled through subcutaneous tissue to the L3–L4 vertebral level. The catheter was inserted into the subarachnoid space with the tip positioned at the T12–L1 vertebral level. The pump was filled with a 500-μg/mL solution of baclofen, and a daily dose was initiated approximately 1.5 times the lowest effective bolus dose used in the trial administration. As needed, dose was adjusted over time (but no greater than once per 24 hours) to maintain therapeutic effectiveness. Participants in this study received daily doses ranging from 100 to 990 μg/d.
RESULTS
Average pre- and post-implant questionnaire responses were calculated for each participant. Differences in pre- and post-implant responses were analyzed using the Wilcoxon signed-rank test for paired scores.
Participants reported marked improvement in their ratings of severity of spasticity from pre- to post-implant. On the 6-point spasticity severity scale, ratings improved from a baseline average of 3.14 ± 1.46(SD; range = 1.0–4.0) to a post-implant average of 1.63 ± 0.83 (range = 0.6–2.5). Statistically reliable differences (P = 0.018) were noted in average utility ratings, from a baseline average of 37.14 ± 24.30 (range = 10–70) to an average of 71.0 ± 15.42 (range = 42–89) post-implant.
As a group, participants reported improved health status scores on all but 1 scale (Physical Function) of the SF 36 Health Survey version 2.0. This might be expected given the nature of their disability. The most notable improvements were observed with the Social Functioning and Mental Health scales. Individually, all but 1 participant (S7) reported general improvement on the SF 36 Health Survey.
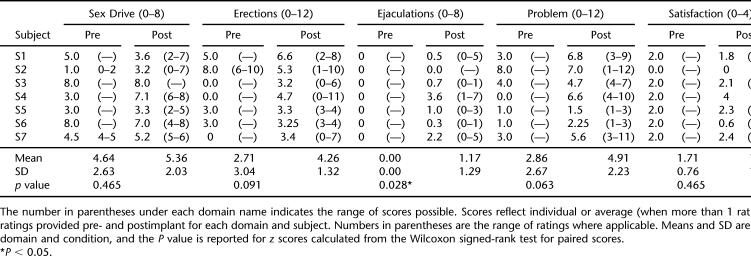
Participants also indicated generally unchanged or improved ratings of perceived sexual functioning after implant. Table 2 presents average scores and ranges, pre- and post-implant, for each subject on each of the 5 domains of the BSFI. The right column presents the total change in pre- to post-implant scores summed across all domains. Two subjects (S1 and S6) noted decreased sex drive (BFSI items 1 and 2) after baclofen pump implant, whereas 2 others (S2 and S4) indicated an increase in sex drive after implant. Subject 2 also reported increased difficulty with erections (items 3–5). All other participants reported no change or a modest improvement on the 3 items related to erections.
Table 2.
Domain Scores of the BSFI
All participants reported either no change or a slight improvement on items 6 and 7 of the BFSI related to ejaculation. As noted in Table 2, this was the only domain for which a statistically significant (P < 0.05) difference was noted between pre- and post-implant ratings. However, the domain score for all participants was “0” pre-implant, suggesting no sexual activity or climax in the previous month. Moreover, the improvements noted do not seem to be functionally meaningful, moving at best from “no sexual activity/climax” to “some difficulty/medium problem.”
With the exception of subject 2, all subjects reported a modest to quite dramatic (S4) improvement in their perception of problems with sexual function. On individual items (8–10 of the BFSI), subject 4 reported the greatest improvement in lack of sex drive and ejaculation as problems. Finally, participants reported essentially no change in their satisfaction with sexual relationships from before to after pump implant.
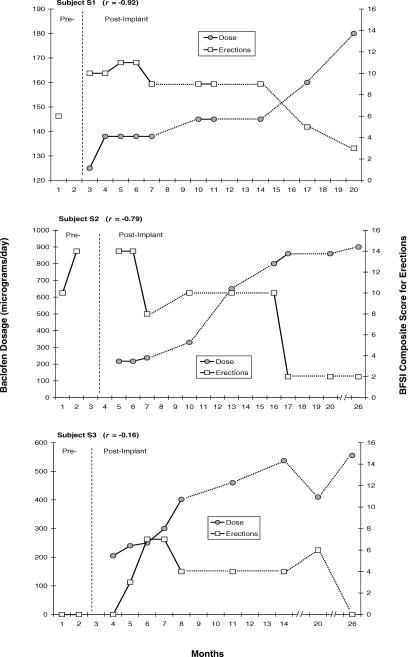
Changes over time were observed in subjects' ratings of perceived sexual function, particularly related to erections. For 3 subjects (S1, S2, and S3), these changes seemed to be related to changes in the dose of intrathecal baclofen. Figure 2 presents, for each of these subjects, the changes noted in baclofen dose and their composite scores on all items related to erections (3, 4, 5, and 9) on the BFSI. As baclofen dose was increased to maintain clinical effectiveness in managing spasticity, BFSI scores related to erections decreased quite dramatically. Fairly robust correlations were noted (ranging from −0.16 to −0.92) between baclofen dose and perceived problems with erections reported by these subjects.
Figure 2. Changes over time in dose of intrathecal baclofen (dark circles) and composite scores on the BSFI items (shaded squares) related to erectile function (items 3, 4, 5, and 9) for subjects S1, S2, and S3, respectively. For these 3 subjects, there seems to be a dose-related effect of baclofen on perceived erectile functioning. Break lines in x-axis indicate noncontinuous months.
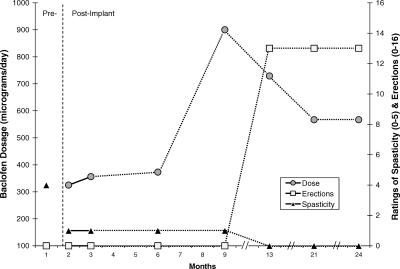
An apparent dose-response relationship was also observed for subject 4, who reported the most dramatic changes in ratings of perceived sexual functioning from pre- to post-implant, across all 5 domains of the BSFI. Figure 3 presents changes over time in baclofen dose, BSFI composite scores for items related to erections, and ratings of spasticity severity (on a 0–5 scale). Ratings of spasticity severity improved from a pre-implant baseline severity of 4 (severe disability) to a score of 1 (minimal disability) with administration of baclofen doses ranging from 325 to 373 μg/d. Baclofen dose was increased to 900 μg/d and then titrated back to 567 μg/d over a 12-month period. Further improvement in ratings of spasticity severity (to 0 or “normal”) was reported after the increase in baclofen dose. Moreover, a dramatic improvement in ratings of sexual function was reported subsequent to the increase and later tapering in baclofen dose.
Figure 3. Changes over time in dose of intrathecal baclofen (dark circles), composite scores on the BSFI items related to erectile function (shaded squares), and ratings of spasticity severity (black triangles) for subject S4. Ratings of perceived erectile functioning improved only after a marked increase and then decrease in dose of intrathecal baclofen. Break line on x-axis indicates noncontinuous months.
Finally, in examining relationships between dependent variables with respect to pre- and post-implant changes, we noted modest correlations between change scores on the BSFI and SF 36 (r = 0.60) and between the BSFI and spasticity use ratings (r = 0.58). A statistically significant relationship was noted between change scores on spasticity utility ratings and the SF 36 pre- and post-implant (r = 0.92, P < 0.01).
DISCUSSION
Reports in the literature of adverse effects of intrathecal baclofen on sexual functioning are corroborated to some degree by our findings. Three of 7 participants reported negative changes from pre- to post-implant on at least 1 dimension of sexual function. In general, however, the extent to which sexual function was impaired by intrathecal baclofen seems to be minimal. All 7 participants reported improvement in perceived sexual function in 1 or more areas. Only 1 participant (S2) noted a net negative change over all 5 domains.
One advantage of this study design was the ability to examine changes over time in ratings of sexual function. As a result, study findings shed some light on possible dose effects of baclofen on sexual function that have been suggested previously by Denys et al (5). Dose-effect relationships were noted for 3 participants (Figure 3), and for a fourth participant (Figure 3), changes in ratings of perceived sexual function occurred only after the dose of baclofen was increased (perhaps beyond a threshold level at which problems were perceived) and then decreased. This finding lends support to previous reports that the effects of baclofen on sexual function seem to be transitory and reversible with withdrawal or reduction in dose.
It should be noted as a limitation of this study that we did not control for the use of medications for treatment of erectile dysfunction and, in fact, 3 subjects (S3, S4, and S5) were prescribed Viagra (sildenafil citrate, Pfizer, Inc. USA), at some point during their participation in the study. Subject 5 was prescribed sildenafil citrate throughout the entire study, both pre- and post-implant. Subjects 3 and 4 received prescriptions for sildenafil citrate on only 1 occasion each. Subject 3 was given sildenafil citrate in month 21 (Figure 2), soon after beginning an upward titration of baclofen to maintain spasticity management. Subject 4 received sildenafil citrate in month 15 (Figure 3), 2 months after reporting a dramatic increase in perceived sexual function. We were unable to verify why these subjects requested and received prescriptions for sildenafil citrate at these points in time, but it does suggest that any adverse effects of intrathecal baclofen on sexual function may be ameliorated with intervention.
A second limitation of this study was use of the BSFI as a measure of perceived sexual function. Because most men with SCI experience ejaculatory dysfunction, the BSFI has limitations as a measure of change in ejaculatory function with this patient population. It would also have been useful to document qualitative (eg, orgasmic sensation) and quantitative (eg, erective rigidity) aspects of erectile function as they were affected by the use of baclofen.
CONCLUSION
Despite the limitations noted, this study supports previous research in this area and may have practical implications for practitioners. In a post-study questionnaire, all 7 participants indicated that improvements in spasticity management clearly outweighed any problems noted in memory, cognition, or sexual function. Thus, this study further corroborates findings of Denys et al (5), in that none of the participants requested treatment interruption. Patients who are considering intrathecal baclofen for treatment of spasticity should be informed that problems with sexual function may occur, particularly at higher doses. However, patients should also be counseled that these problems are reversible with a reduction in baclofen dose and may also be ameliorated with medications for treatment of erectile dysfunction.
Acknowledgments
The authors thank Kathy Mattox, RN, and Dianne McPherson for assistance with data collection.
REFERENCES
- Penn R, Savoy S, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. New Engl J Med. 1989;320:1517–1521. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- Steers W, Meythaler J, Haworth C, Herrell D, Park T. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;92:1849–1855. doi: 10.1016/s0022-5347(17)37048-9. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Mane M, Thiebaut J-B, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996;77:35–39. doi: 10.1016/s0003-9993(96)90217-8. [DOI] [PubMed] [Google Scholar]
- Meythaler J, Steers W, Tuel S, Cross L, Haworth C. Continuous intrathecal baclofen in spinal cord spasticity. Am J Phys Med Rehabil. 1992;71:321–327. doi: 10.1097/00002060-199212000-00003. [DOI] [PubMed] [Google Scholar]
- Denys P, Mane M, Azouvi P, Chartier-Kastler E, Thiebaut J-B, Bussel B. Side effects of chronic intrathecal baclofen on erection and ejaculation in patients with spinal cord lesions. Arch Phys Med Rehabil. 1998;79:494–496. doi: 10.1016/s0003-9993(98)90061-2. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999;52:63–70. doi: 10.1212/wnl.52.1.63. [DOI] [PubMed] [Google Scholar]
- O'Leary M, Fowler F, Lenderking W, et al. A brief male sexual function inventory for urology. Urology. 1995;46:697–706. doi: 10.1016/S0090-4295(99)80304-5. [DOI] [PubMed] [Google Scholar]
- Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]