Abstract
Side effects from antiretroviral therapy (ART) for HIV disease can deter treatment, impact quality of life, and impede medication adherence. Individual differences in neuroticism may account for variations in the experience of side effects and perceptions of health status. Cross-sectional assessments were conducted with 258 HIV-infected participants with confirmed HIV infection and current ART regimen. Structural equation modeling (SEM) was used to evaluate a model of self-reported ART side effect frequency and severity and perceived health status, as related to symptoms of neuroticism. Symptoms of neuroticism were associated with greater reports of ART side effects and poorer perceived health but unrelated to reported CD4 count and viral load. A structural model was supported in which greater symptoms of neuroticism are linked to poorer perceived health through greater side effect frequency and severity. Individual differences in symptoms of neuroticism can explain variations in side effect reporting and consequential impairments in perceived health in the context of HIV treatment. Identification and intervention with individuals high in symptoms of neuroticism may be warranted to alleviate side effect-related concerns and maximize treatment benefit.
Keywords: Neuroticism, HIV, AIDS, Adherence, Compliance, Symptom Reporting, Structural Equation Modeling
While the life-extending benefits of antiretroviral therapies (ART) for HIV infection are well-documented, adverse side effects accompany drug benefit (Johnson & Gerber, 2000; Volberding, 2003). Adverse effects are predictable, undesirable, dose-related pharmacologic effects that occur within therapeutic dose ranges. The most common side effects from ART are gastro-intestinal problems such as diarrhea, nausea and vomiting, fat redistribution, and dermatological problems such as rashes. Additional “unseen” negative effects that become apparent over time include cardiac and liver problems, and increased triglyceride levels.
Research with patients undergoing treatments for a wide range of medical problems strongly links side effects to lower levels of quality of life (QOL) (Arana, 2000; Beisecker et al., 1997; Cameron, Leventhal, & Leventhal, 1993; Carruth & Boss, 1990; Johnson, Stallworth, & Neilands, 2003; Larsen & Gerlach, 1996; McElroy, Keck, & Friedman, 1995; Mohr et al., 1998; Ray-Chaudhuri, Abbott, & Millac, 1991; Richards & Martinson, 1987; Ritsner et al., 2002; Shapiro, Boggs, Rodrigue, & Urry, 1997; Turkkan, 1993). Side effects are often cited when evaluating the impact of ART on the HIV treatment arena (Bates, 1996; Johnson & Gerber, 2000; Volberding, 2003). In a nationally representative sample of 2267 HIV+ adults in the US, multiple symptoms (not separated by disease or treatment causes) were related to diminished QOL which was, in turn, significantly predictive of days on disability (Lorenz, Shapiro, Asch, Bozzette, & Hays, 2001). The impact of side effects on QOL is often cited as a primary deciding factor for when to start ART among HIV+ individuals with middle range CD4 counts (200-500) (Chene et al., 2002). Furthermore, side effects from ART are often linked to lower levels of medication adherence (Ammassari et al., 2001; Johnson et al., 2005) and the discontinuation of otherwise appropriate and effective therapy.
An area that has not been comprehensively explored in attempts to account for the experience of HIV-related side effects is the potential role of neuroticism in side effect reporting. In a seminal series of studies conducted in the late 1970s and early 1980s, Costa and McCrae theorized and tested whether a personality construct known as neuroticism was responsible for individual variations in somatic complaints, including reports of symptoms and side effects (Costa, Fleg, McCrae, & Lakatta, 1982; Costa & McCrae, 1987). Neuroticism, which has been defined as the “tendency to experience negative, distressing emotions and to possess associated behavioral and cognitive traits” (Costa & McCrae, 1987) (p.301), has been psychometrically validated (Costa & McCrae, 1992) and linked to symptom reporting and perceptions of health status. It is however, not consistently linked to long-term health status or mortality. For this reason, neuroticism appears to function according to what has been referred to as refer to as the “symptom perception hypothesis,” (Watson & Pennebaker, 1989) or hypochondriacal presentation (Costa & McCrae, 1985), which suggests that persons high in negative affectivity or neuroticism are more likely to attend to and complain about physical sensations. However, this does not necessarily reflect higher levels of objective symptomatology, deteriorated health, or increased likelihood of mortality from disease (Costa, 1987). There is evidence that neuroticism may influence nonspecific side effects in antidepressant treatment (Davis, Ralevski, Kennedy, & Neitzert, 1995) and other medical settings (Barsky, Saintfort, Rogers, & Borus, 2002), but there have been no published investigations of the role of neuroticism in the reporting of side effects from ART for HIV disease. While states of psychopathology such as depression and anxiety are frequently measured by clinicians, these states are transient and subject to more fluctuation than the trait of neuroticism (Costa & McCrae, 1988). By measuring symptoms of neuroticism, clinicians can better contextualize individual reports of side effects and symptoms.
The purpose of this study is to explore the associations among neuroticism, medication side effect reporting, and perceived health status among a sample of HIV-infected men and women taking ART. A structural equation model is tested in which greater symptoms of neuroticism are associated with greater reports of treatment side effects, which are in turn related to poorer appraisal of overall health. Specifically, it is hypothesized that higher symptoms of neuroticism will be associated with greater number and perceived severity of medication side effects. The choice of a mediation model was based on prior studies which found variables such as constructive thinking and health behaviors to mediate the relationship between neuroticism and subjective well-being and depression (Gallant & Connell, 2003; Harris & Lightsey, 2005). Consistent with prior findings, it is predicted that higher symptoms of neuroticism will be associated with lower ratings of perceived health status, but will be unrelated to objective measures of disease severity, as measured by CD4 count and HIV viral load. It is hypothesized that higher levels of neuroticism will be related to poorer perceived health status via perceived side effects. The secondary hypothesis was explored that neuroticism serves as a confounding variable that artificially inflates the association between subjective variables, as has been suggested in other contexts (Huebner, Nemeroff, & Davis, 2005).
Method
Study Respondents
A total of 258 HIV-positive individuals were screened for recruitment into the Balance Project, a clinical trial of a coping intervention for HIV+ men and women on antiretroviral therapy. Brochures, posters, and project descriptions, as well as direct contact with staff in clinical and social service agencies were used to recruit respondents. In addition, advertisements were placed in newspapers serving HIV-positive and gay/bisexual populations, and potential respondents learning of the study by word of mouth were eligible to be screened. Interested persons who provided verbal consent were briefly screened by project personnel to determine their self-reported HIV status as well as basic treatment, demographic and contact information, and then, if they wished to participate, scheduled for an in-person interview.
Respondents were required to be at least 18 years of age, to provide written informed consent and documentation of their HIV infection and medication regimen, to be free of severe neuropsychological impairment or psychosis, and not be currently involved in another behavioral intervention study related to HIV. They were required to be taking antiretroviral medications for at least the past 30 days as documented by prescription bottles or official list of pharmacologic regimen provided by healthcare provider or pharmacy. Participants must have answered affirmatively when asked whether they had ever experienced side effects from their HIV treatment regimen. Screening for impairment and psychosis was conducted informally by trained staff with oversight by senior staff and clinical personnel. If, during the course of obtaining informed consent or during the conduct of the interview, a participant’s behavior or responses suggested difficulty understanding questions, the interviewer was instructed to consult with senior staff. In those cases in which sufficient concern of impairment was evidenced, the interview data were not used in analysis. Note, this was an informal screening rather than a diagnostic assessment of pathology or impairment. A total of 8 cases were excluded based on this procedure.
Overview of assessment procedures
All procedures and forms were reviewed and approved by the Institutional Review Boards (IRB) at the University of California, San Francisco. Assessment interviews were conducted in private settings in research offices. Written informed consent to participate in the study was obtained from each respondent prior to the administration of the baseline interview. The interview was then conducted over a period of 45 minutes to one hour.
Procedures involved a combination of Audio Computer Assisted Self-Interviewing (ACASI) and Computer Assisted Personal Interviewing (CAPI) using Questionnaire Development System (QDS), Nova Research Company. ACASI allows the respondent to listen to an item via headphones while reading the item on a computer monitor. The respondent then enters his or her response directly into the computer. This approach has been proposed as an effective method of decreasing social desirability and thereby enhancing veracity of self-report of sensitive behaviors and attitudes (Gribble, Miller, Rogers, & Turner, 1999; Turner et al., 1998). With CAPI, an interviewer reads items from a computer and the respondent verbally gives responses that the interviewer enters directly into the computer. All respondents responded to survey items in the same modality (CAPI vs ACASI) and measures described below indicate which form of interview administration was used. CAPI and ACASI procedures have been widely used in HIV-related research (Bangsberg, Bronstone, & Hofmann, 2002; Johnson, Catz et al., 2003; Lightfoot et al., 2005; Weinhardt et al., 2004)
Respondents were compensated US$25 for completing the interview and those needing child care were also eligible to receive US$10 to defray child care costs.
Interview training and quality assurance
Interviewers were trained with the use of a detailed assessment manual, practice with the computer programs, and review and certification of audio-recorded mock interviews based on standardized criteria. All interviews were audio-recorded and labeled with the respondent’s study identification number, date of the interview, and the interviewer’s identification number. A sample of recordings was reviewed for protocol adherence and feedback was provided to all interviewers on a regular basis.
Measures
Demographic and background data included items such as respondent age, race/ethnicity, gender, sexual orientation, relationship status, educational level, and employment status. Self-reported recent CD4 count (a laboratory measure of immune system deterioration) and HIV viral load (detectable vs. nondetectable) were also assessed, which have been found reliably linked to chart reviews of these laboratory assays in other contexts (Cunningham, Rana, Shapiro, & Hays, 1997; Kalichman, Rompa, & Cage, 2000). (Combination of CAPI and ACASI).
Neuroticism was assessed using the NEO Five Factor Inventory (Avia et al., 1995; Costa & McCrae, 1992; McCrae & Costa, 1991). The measure gives summary scores for each of the “Big Five” personality factors: Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness and has sound psychometric properties (e.g., internal consistency from .74 to .89). For the Neuroticism scale, all three component subscales of anxiety (sample item: “I am not a worrier,” reverse scored), depression (sample item: “Too often, when things go wrong, I get discouraged and feel like giving up”), and self-reproach, (sample item: “Sometimes I feel completely worthless”) were used. The NEO-FFI demonstrates high correlations with the larger NEO-PI parent inventory and both measures have evidence of convergent validity with other measures of neuroticism (Avia et al., 1995). (ACASI).
ART Side Effects
Based on the AIDS Clinical Trials Group symptom checklist (Justice et al., 2001), a physical complaint checklist was developed, in which participants indicated whether they have experienced each of a list of 25 symptoms in the past 30 days and how much the problem bothered them (0 = not present; 1= present but does not bother me; ranging to 4 = present and bothers me terribly). For items endorsed on the symptom checklist, participants were asked whether they believe each was caused by HIV medications, HIV, or something unrelated (respondents were allowed to choose as many causes as appropriate). Other studies using variations this measure have reported meaningful relationships with QOL, mood, and medication adherence have been observed (Johnson et al., 2005; Johnson & Folkman, 2004; Johnson, Stallworth et al., 2003). For the current study, count of problems attributed to ART and average bother for those symptoms were used as indicators of presence and severity of ART side effects. (CAPI).
Perceived Health Status was assessed using the general health (“In general, would you say your health is: excellent, very good, good, fair, or poor?”) and health transition (“Compared to one year ago, how would you rate your health in general now?”) items of the SF-36 from the Medical Outcomes Study (Ware, Gandek, & Group, 1994; Ware, 1999). The measure has been widely used and well-validated in a variety of health contexts and populations (Keller, Majkut, Kosinski, & Ware, 1999; McHorney, Ware, Lu, & Sherbourne, 1994; McHorney, Ware, & Raczek, 1993; Ware & Gandek, 1998). (CAPI).
Data Analysis
Initial analyses consisted of one-way frequency tables for categorical variables and standard measures of central tendency (e.g., mean, median) and variability for continuous scale scores. Pearson product-moment correlations were computed to assess bivariate associations between HIV clinical marker variables (e.g., CD4 T-cell counts; detectable viral load) and neuroticism scale scores. A primary goal of this study was to evaluate the associations of neuroticism with ART side effects and perceived health. Moreover, an aim was to evaluate whether neuroticism had a direct association with perceived health or whether experiences of ART side effects would mediate the relationship between neuroticism and perceived health via an indirect route wherein neuroticism impacted ART side effects which in turn exerted influence on perceived health. Given the presence of multiple measures of neuroticism, ART side effects, and perceived health, and the interest in assessing the indirect effect of neuroticism on perceived health mediated by experiences of ART side effects, a structural equation modeling (SEM) approach was used to investigate the relationships among these variables. Unlike standard regression approaches, which assume that observed explanatory and mediating variables are measured without error, SEMs that feature multiple measures of important latent constructs (e.g., neuroticism, perceived health) yield regression coefficients among latent variables that are cleansed of measurement error, resulting in more accurate regression coefficients and more powerful tests of those coefficients. Unlike most regression methods, SEMs allow exact testing of the model’s fit to the data and provide various descriptive model fit measures that enable the researcher to assess the goodness of model fit to the sample data (Bollen, 1989).
SEMs also allow straightforward evaluation of mediation hypotheses in which an initial variable impacts a second variable indirectly by way of a third variable. This last variable is labeled a mediating variable if a previously significant relationship between the first and second variables is reduced or eliminated once the indirect influence of the first variable on the second variable by way of the third variable is considered (Baron & Kenny, 1986). SEMs allow researchers to test whether an intervening variable between two others mediates their association. While it is possible to use standard regression methods to assess mediation among observed variables (Preacher & Hayes, 2004), conceptualizing a mediating variable as a latent variable removes bias in the analysis that arises from lack of perfect reliability of observed mediating variables (Hoyle & Kenny, 1999). Bias in the mediating variable can weaken the statistical power of indirect effect tests, causing investigators to miss important research findings. Moreover, an attractive feature of the SEM approach for assessing statistical mediation is that all direct and indirect effects may be tested simultaneously with the same sample in the presence of all other hypothesized effects.
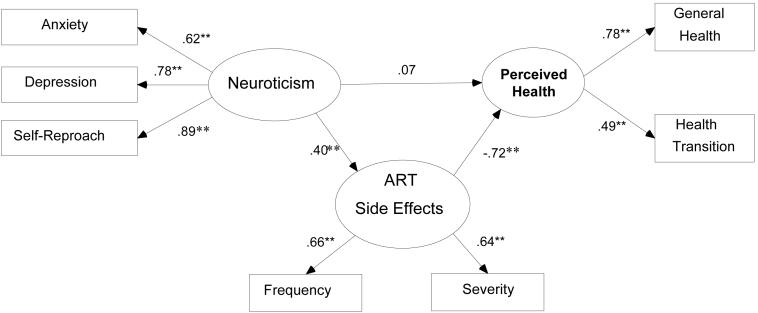
Figure 1 depicts the proposed structural equation model. Rectangles refer to observed scales of measurement whereas ovals refer to unobserved (or latent) variables. A neuroticism latent variable is measured by anxiety, depression, and self-reproach scale scores. Similarly, an ART side effects latent variable is measured by observed indices of frequency and severity of side effects. Finally, a perceived health latent variable is measured by general health and health transition scales. Single-headed arrows denote expected directional relations among variables. For instance, neuroticism impacts both perceived health and ART side effects. The figure depicts a direct relationship between neuroticism and perceived health (a direct effect); the diagram also includes an implied indirect relationship between neuroticism and perceived health by way of the potential mediating ART side effects latent variable. The resulting indirect effect is the product of the direct effects of neuroticism on ART side effects and ART side effects on perceived health.
Structural Model of Neuroticism, ART Side Effects, and Perceived Health.
Notes: N = 246. ** = p < .01
The hypothesized structural model was fit using Mplus version 3.13 (Muthén & Muthén, 2004) using the weighted least-squares estimator with mean and variance adjustment (WLSMV) to accommodate the non-normal ordered categorical SF-36 general health and health transitions outcome measures (Flora & Curran, 2004; Muthen & Curran, 1997). Global fit of the model to the data was assessed via the robust chi-square test of exact model fit. Because robust chi-square tests do not fully correct for data non-normality, the following approximate fit indices are also reported: Bentler’s comparative fit index (CFI: (Bentler & Bonnett, 1980); the root mean square error of approximation (RMSEA: (Browne & Cudek, 1993), and the weighted root mean square residual (WRMR: (Yu, 2002). To attain adequate fit a model’s CFI should meet or exceed .90 (Vandenberg & Lance, 2000 ), RMSEA should be .06 or lower (Hoyle & Kenny, 1999; Hu & Bentler, 1999), and WRMR should be 1.00 or lower (Yu, 2002). For each parameter estimate the unstandardized regression coefficient (B), the bootstrap-based bias-corrected 95% confidence interval of B based on 5920 bootstrap samples (Hox, 2002), and the standardized regression coefficient (Beta) (MacKinnon, Lockwood, & Williams, 2004; Shrout & Bolger, 2002) are reported.
Results
Demographic characteristics of the sample are described in Table 1. In general, the sample was well educated (>89% with a high school degree or higher), white (52%), male (87%) and self-identified as homosexual (69%). Of note, only 30% of the sample was employed. Table 2 provides summary measures of central tendency for the continuous measures that appear in the structural model, depicted in Figure 1. The median most recent CD4 count was 419 and the range of time since CD4 count testing is .39 to 12.5 months, with a median of 2 months since most recent assay. In addition to the primary explanatory latent variable of interest, neuroticism, the following covariates were included in the model: biological gender (male = 0; female = 1), age at study entry, ethnicity, CD4 T-cell count, detectable viral load (undetectable = 0; detectable = 1), maximum number of ART medications used, and maximum length of time a participant used ART medications. Due to small numbers of participants who were not Black or White, these participants were grouped in an Other ethnicity category in the structural model to ensure stable coefficients for the ethnicity parameter estimates.
Table 1.
Demographic characteristics of study sample
| Variable | Level | N (%) |
|---|---|---|
| Education | <High School | 26 (10.1) |
| HS Graduate | 63 (24.4) | |
| AA Degree | 91 (35.3) | |
| College Degree | 56 (21.7) | |
| Master’s Degree | 18 (7.0) | |
| PhD/MD/JD | 4 (2) | |
| Employed | Yes | 78 (30.2) |
| No | 180 (69.8) | |
| Gender | Male | 225 (87.2) |
| Female | 33 (12.8) | |
| Sexual Orientation | Heterosexual | 49 (19) |
| Homosexual | 179 (69.4) | |
| Bisexual | 25 (9.7) | |
| Unsure | 2 (.8) | |
| Other | 3 (1.2) | |
| SF-36 General Health | Poor | 12 (4.7) |
| Fair | 67 (26) | |
| Good | 97 (37.6) | |
| Very Good | 67 (26) | |
| Excellent | 15 (5.8) | |
| SF-36 Health Transition | Much worse | 5 (1.9) |
| Somewhat worse | 43 (16.7) | |
| About the same | 92 (35.7) | |
| Somewhat better | 52 (20.2) | |
| Much better | 66 (25.6) | |
| Race/Ethnicity | Native American | 5 (1.9) |
| Asian | 9 (3.5) | |
| Pacific Islander | 3 (1.2) | |
| Black | 68 (26.4) | |
| White | 134 (52) | |
| Multiracial | 21 (8.1) | |
| Other | 3 (1.2) | |
| Hispanic/Latino | 15 (5.8) | |
| Maximum number ART meds | 0 | 3 (1.2) |
| 1 | 1 (0.4) | |
| 2 | 55 (21.3) | |
| 3 | 123 (47.7) | |
| 4 | 64 (24.8) | |
| 5 | 12 (4.7) | |
| Detectable HIV Viral Load | Yes | 71 (28.4) |
| No | 179 (71.6) |
Notes: N = 258. Eight participants did not report HIV viral load.
Table 2.
Means, Medians, and Standard Deviations of Continuous Variables
| Variable | N | Mean (SD) | Median |
|---|---|---|---|
| Age (years) | 258 | 46.33 (7.77) | 45.50 |
| CD4 T-Cell Count | 257 | 440.64 (253.46) | 419.00 |
| Neuroticism: Anxiety | 258 | 10.02 (2.72) | 10.00 |
| Neuroticism: Depression | 258 | 9.86 (2.81) | 10.00 |
| Neuroticism: Self-Reproach | 258 | 14.27 (4.48) | 14.00 |
| Count of ART Symptoms | 258 | 5.77 (4.62) | 5.00 |
| Mean bother of ART symptoms | 258 | 2.34 (0.84) | 2.50 |
| Maximum ART Tx Length (years) | 255 | 4.03 (3.37) | 2.99 |
Notes: One participant did not report CD4; three participants did not report ART treatment length.
Bivariate correlations between the measures of neuroticism and CD4 and HIV viral load were used to determine whether neuroticism was correlated with reported clinical markers of HIV infection. The correlations between the measures of neuroticism and CD4 were not significant (bivariate correlations less than |.08|), and the neuroticism scores among those reporting detectable viral loads were not significantly different than those with undetectable viral loads. Taken collectively, these results suggest no association between neuroticism indicators and HIV infection measures.
Of the 258 participants who completed the screener, 246 provided complete data on all measures included in the structural equation model. Though the chi-square test of absolute fit was significant (χ2 (25) = 43.67, p = .01), the approximate fit indices indicated very good model fit to the data (CFI = .90, RMSEA = .06; WRMR = .88). Unstandardized direct effects and 95% confidence intervals for each explanatory variable on each outcome variable are shown in Table 3. The corresponding standardized direct effects are displayed on the diagram of the structural model, shown in Figure 1. The indirect effects and 95% confidence intervals of neuroticism and each of the covariates listed above on perceived health, mediated by ART Side Effects were also computed. Neuroticism mediated by ART Side Effects was negatively associated with perceived health (B = -0.14; 95% CI = -0.26, -0.06; Beta = -.29).
Table 3.
Unstandardized regression weights (B), 95% confidence intervals (CI), and from structural model
| Outcome | Explanatory | B | CI |
|---|---|---|---|
| Anxiety | Neuroticism | 1.00 | (1.00, 1.00) |
| Depression | Neuroticism | 1.30 | (1.04, 1.72)** |
| Self-Reproach | Neuroticism | 2.35 | (1.72, 3.55)** |
| Sum of ART Symptoms | ART Side Effects | 1.00 | (1.00, 1.00) |
| Mean bother of ART Symptoms | ART Side Effects | 0.18 | (0.12, 0.26)** |
| SF-36 General Health | Perceived Health | 1.00 | (1.00, 1.00) |
| SF-36 Health Transitions | Perceived Health | 0.62 | (0.14, 1.08)** |
| ART Side Effects | Gender | -0.84 | (-2.75, 0.89) |
| ART Side Effects | Age | 0.05 | (-0.01, 0.11) |
| ART Side Effects | Black Race | -1.00 | (-2.40, 0.29) |
| ART Side Effects | Other Race | 0.33 | (-0.84, 1.48) |
| ART Side Effects | CD4 | 0.00 | (-0.01, 0.01) |
| ART Side Effects | Detectable VL | 0.93 | (-0.08, 2.05) |
| ART Side Effects | Number ART Used | 0.35 | (-0.22, 0.95) |
| ART Side Effects | ART Tx Length | -0.14 | (-0.30, 0.01) |
| ART Side Effects | Neuroticism | 0.73 | (0.38, 1.21)** |
| Perceived Health | Gender | -0.25 | (-0.71, 0.27) |
| Perceived Health | Age | 0.01 | (-0.01, 0.03) |
| Perceived Health | Black Race | -0.01 | (-0.46, 0.35) |
| Perceived Health | Other Race | 0.28 | (-0.07, 0.62) |
| Perceived Health | CD4 | 0.00 | (0.00, 0.01) |
| Perceived Health | Detectable VL | -0.31 | (-0.62, -0.01)* |
| Perceived Health | Number ART Used | 0.03 | (-0.15, 0.22) |
| Perceived Health | ART Tx Length | -0.01 | (-0.06, 0.03) |
| Perceived Health | Neuroticism | 0.04 | (-0.08, 0.15) |
| Perceived Health | ART Side Effects | -0.20 | (-0.30, -0.12)** |
Notes: N = 246 respondents who provided complete data on all measures. Outcome refers to dependent variables in the structural model; Explanatory refers to explanatory or independent variables in the structural model. Black Race and Other Race coefficients compare Black and other non-White ethnic groups to the reference group of White participants. 95% confidence intervals are bias-corrected and originate from 5920 bootstrap samples.
95% confidence interval does not include zero, signifying statistical significance at p < .05.
99% confidence interval does not include zero, signifying statistical significance at p < .01.
Discussion
Overall, findings provide support for the symptom perception hypothesis of neuroticism in a new context: side effects from antiretroviral therapy for HIV infection. Results indicate that those individuals who report greater symptoms of neuroticism also report greater frequency and severity of side effects from their medications. Further, higher levels of neuroticism are associated with poorer perceived health indirectly through side effect perceptions. However, reported laboratory measures of disease progression do not suggest that their level of disease is more advanced than those with lower symptoms of neuroticism.
The findings are consistent with previous research on neuroticism and symptom reporting. However, the present work extends this body of knowledge. With the exception of work in coronary artery disease (Barefoot, Beckham, Peterson, Haney, & Williams, 1992; Costa, 1987) and depression (Davis et al., 1995), many of the previous studies linking symptoms of neuroticism to symptom reports were in the context of healthy populations and often assessed medically unexplained symptoms. The present study is rare in its focus on a sample with a confirmed medical condition and with an emphasis on detecting perceptions of adverse effects from pharmacologic treatment. The findings suggest that symptoms of neuroticism may have a central role in the inefficient utilization of health care that is characteristic of “worried well” and the placebo and nocebo phenomena (Barsky et al., 2002; Hahn, 1997; Kennedy, 1961).
Findings indicate that individuals higher in symptoms of neuroticism are more likely to report impaired health status, despite an absence of objective support for poorer health. This finding has implications for both health-related research and the provision of health care for people living with HIV and other conditions. It may be that self-reported health status and quality of life are influenced by factors such as neuroticism in a way that bias clinical and research findings. While this supposition has been made previously by others (Costa, 1987; Costa & McCrae, 1985; Watson & Pennebaker, 1989), to the knowledge of the authors, this is the first study supporting the notion in the context of HIV disease, an increasing and costly area of medical care. In research contexts, neuroticism should be taken into account statistically and/or methodologically when evaluating patients’ reports of side effects and perceived health. Clinical implications of findings include direction to help identify in advance patients who are more likely to perceive distressing side effects from treatment. The construct of neuroticism suggests that high symptoms of neuroticism are associated with a higher likelihood of experiencing negative affect, which may result in symptoms of depression of anxiety (Clarke, 2004). While symptoms of anxiety and depression fluctuate, neuroticism is more stable. Therefore, assessing level of neuroticism may allow the detection of future problems with side effect reporting and the increased likelihood of future elevations of symptoms of anxiety and depression. Subsequent interventions may involve proactively addressing patient expectations about side effects and using multi-disciplinary informational and supportive approaches to help patients understand and manage adverse effects from treatment.
Neuroticism has been proposed as a confounder of the relationships among subjective factors (Huebner et al., 2005) in which neuroticism is viewed as an ancillary background characteristic that influences the perceptions of stress and subsequent subjective outcomes with little regard to the logical sequential order in which these constructs emerge. By contrast, the proposed model posits that emergence of neuroticism precedes substantial side effect perceptions and that those perceptions in turn negatively impact perceptions of health. Of particular interest is the absence of a significant direct linkage between neuroticism and quality of life: the absence of a direct relationship between neuroticism and perceived health suggests that in at least some circumstances, multiple regression models that do not take into account the indirect impact of neuroticism on outcome variables mediated by key intermediary variables such as side effect reports may not adequately and accurately capture the complex impact of neuroticism on key psychological outcomes such as perceived health.
Although not explored in this study, adverse effect complaints from ART are associated with nonadherence, which is a persistent problem in the treatment of HIV disease (Ammassari et al., 2001; Fogarty et al., 2002; Johnson et al., 2005). To achieve maximal clinical benefit from ART, adherence levels as high as 95% are necessary (Paterson et al., 2000). To the degree that perceived side effects impede adherence, the role of neuroticism may have important implications. For example, patients who are high in neuroticism may be candidates for intervention so that their perceptions of treatment side effects do not jeopardize treatment benefit via nonadherence or early discontinuation of therapy. Consequential increases in treatment costs may result from unnecessarily discontinuing or changing regimens or prescribing additional drugs to treat the side effects.
There are limitations of note in the current study, namely, reliance on self-reported data, cross-sectional design, use of a convenience sample, and the selection of measures. To minimize the biases inherent in self-reported data, measures with validation support in the literature were used, including the reliance on patient-reported CD4 count and viral load (Cunningham et al., 1997; Kalichman et al., 2000). The use of cross-sectional data with a non-probability sample limits the degree to which causal inferences and generalizations can be made from these findings. Specifically, the sample includes substantial representation of people living with HIV who are unemployed, homosexual-identified, and white. These demographic breakdowns are consistent with other published data with HIV-infected samples, (Johnson, Catz et al., 2003; Johnson, Elliott, Neilands, Morin, & Chesney, 2006) but result in limited generalizability of study findings. Follow up studies are needed with other populations, such as employed persons, women, and persons of color to evaluate the findings in these groups.
In the present study with its moderate sample size, it was not possible to rule out competing models that suggest alternate causal sequences. Future research efforts will engage in selective, empirically-driven investigations of the differential impacts of ethnicity, age, and gender on the relationships between neuroticism and clinical outcomes of interest such as adherence to medications. Future work will also explore causal relationships in prospective, experimental research designs. Finally, because the present investigation was a screening for enrollment in a clinical trial, the measures included factors relevant to determining trial eligibility. For that reason, comprehensive appraisals of medication adherence and objective health status were not collected. Likewise, although other covariates such as length of time since HIV diagnosis, time on current treatment regimen, and estimates of the difficulty and complexity of each medication regimen may be of interest, they are not available with this dataset.
Acknowledgments
This research was funded by R01MH068208 from the National Institutes of Health. The authors would like to thank the men and women who participated in the research and the interviewers and recruiters who worked on the project.
References
- Ammassari A, Murri R, Pezzotti P, Trotta M, Ravisio L, DeLonis P. Self reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- Arana GW. An overview of side effects caused by typical antipsychotics. Journal of Clinical Psychiatry. 2000;61:5–11. [PubMed] [Google Scholar]
- Avia MD, Sanz J, Sanchezbernardos ML, Martinezarias MR, Silva F, Grana JL. The 5-Factor Model .2. Relations of the Neo-Pi With Other Personality Variables. Personality and Individual Differences. 1995;19:81–97. [Google Scholar]
- Bangsberg DR, Bronstone A, Hofmann R. A computer-based assessment detects regimen misunderstandings and nonadherence for patients on HIV antiretroviral therapy. AIDS Care. 2002;14:3–15. doi: 10.1080/09540120220097892. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Beckham JC, Peterson BL, Haney TL, Williams RB., Jr. Measures of neuroticism and disease status in coronary angiography patients. J Consult Clin Psychol. 1992;60:127–132. doi: 10.1037//0022-006x.60.1.127. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. Jama. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- Bates CM. HIV medicine: drug side effects and interactions. Postgraduate Medical Journal. 1996;72:30–36. doi: 10.1136/pgmj.72.843.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisecker AE, Cook MR, Ashworth J, Hayes J, Brecheisen M, Helmig L, et al. Side effects of adjuvant chemotherapy: Perceptions of node-negative breast cancer patients. Psycho-Oncology. 1997;6:85–93. doi: 10.1002/(SICI)1099-1611(199706)6:2<85::AID-PON247>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonnett DG. Significance Tests and Goodness of Fit in the Analysis of Covariance Structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York, NY, USA: 1989. [Google Scholar]
- Browne MW, Cudek R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Sage Publications; Newbury Park, CA: 1993. [Google Scholar]
- Cameron L, Leventhal EA, Leventhal H. Symptom representations and affect as determinants of care seeking in a community-dwelling, adult sample population. Health Psychology. 1993;12:171–179. doi: 10.1037//0278-6133.12.3.171. [DOI] [PubMed] [Google Scholar]
- Carruth AK, Boss BJ. More than they bargained for: Adverse drug effects. Journal of Gerontological Nursing. 1990;16:27–31. doi: 10.3928/0098-9134-19900701-07. [DOI] [PubMed] [Google Scholar]
- Chene G, May M, Costagliola D, Monforte A. d. A., Junghans C, Wolf F. d., et al. ART Cohort Collaboration Prognosis of HIV-1 infected drug naïve patients starting potent antiretroviral therapy; Paper presented at the XIV International AIDS Conference; Barcelona. 2002. [Google Scholar]
- Clarke D. Neuroticism: Moderator or mediator in the relation between locus of control and depression? Personality and Individual Differences. 2004;37:245–258. [Google Scholar]
- Costa PT, Fleg JL, McCrae RR, Lakatta EG. Neuroticism, coronary artery disease, and chest pain complaints: Cross-sectional and longitudinal studies. Experimental Aging Research. 1982;8:37–44. [Google Scholar]
- Costa PT., Jr. Influence of the normal personality dimension of neuroticism on chest pain symptoms and coronary artery disease. Am J Cardiol. 1987;60:20J–26J. doi: 10.1016/0002-9149(87)90679-5. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. The NEO Personality Inventory-R: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Costa PT, Jr., McCrae RR. Hypochondriasis, neuroticism, and aging. When are somatic complaints unfounded? Am Psychol. 1985;40:19–28. doi: 10.1037//0003-066x.40.1.19. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? Journal of Personality. Special Issue: Personality and physical health. 1987;55:299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Personality in adulthood: A six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. Journal of Personality & Social Psychology. 1988;54:853–863. doi: 10.1037//0022-3514.54.5.853. [DOI] [PubMed] [Google Scholar]
- Cunningham WE, Rana HM, Shapiro MF, Hays RD. Reliability and validity of self-report CD4 counts-in persons hospitalized with HIV disease. J Clin Epidemiol. 1997;50:829–835. doi: 10.1016/s0895-4356(97)00061-9. [DOI] [PubMed] [Google Scholar]
- Davis C, Ralevski E, Kennedy SH, Neitzert C. The role of personality factors in the reporting of side effect complaints to moclobemide and placebo: A study of healthy male and female volunteers. Journal of Clinical Psychopharmacology. 1995:15. doi: 10.1097/00004714-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Flora DB, Curran PJ. An Empirical Evaluation of Alternative Methods of Estimation for Confirmatory Factor Analysis With Ordinal Data. Psychological Methods. 2004;9:466–491. doi: 10.1037/1082-989X.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports [Review] Patient Education & Counseling. 2002;46:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Gallant MP, Connell CM. Neuroticism and depressive symptoms among spouse caregivers: Do health behaviors mediate this relationship? Psychology and Aging. 2003;18:587–592. doi: 10.1037/0882-7974.18.3.587. [DOI] [PubMed] [Google Scholar]
- Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: Methodological issues. Journal of Sex Research. 1999;36:16–24. doi: 10.1080/00224499909551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26:607–611. doi: 10.1006/pmed.1996.0124. [DOI] [PubMed] [Google Scholar]
- Harris PR, Lightsey OR., Jr. Constructive Thinking as a Mediator of the Relationship Between Extraversion, Neuroticism and Subjective Well-Being. European Journal of Personality. 2005;19:409–426. [Google Scholar]
- Hox JJ. Multilevel Analysis: Techniques and Applications. Lawrence Erlbaum and Associates; Mahwah, New Jersey: 2002. [Google Scholar]
- Hoyle RH, Kenny DA. Sample Size, Reliability, and Tests of Statistical Mediation. In: Hoyle RH, editor. Statistical Strategies for Small Sample Research. Sage Publications; Thousand Oaks, California: 1999. [Google Scholar]
- Hu L.-t., Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6 [Google Scholar]
- Huebner DM, Nemeroff CJ, Davis MC. Do hostility and neuroticism confound associations between perceived discrimination and depressive symptoms? Journal of Social & Clinical Psychology. 2005;24:723–740. [Google Scholar]
- Johnson MO, Catz SL, Remien RH, Rotheram-Borus MJ, Morin SF, Charlebois ED, et al. Theory guided, empirically supported avenues for intervention on HIV medication nonadherence: Findings from the Healthy Living Project. AIDS Patient Care STDS. 2003;17:645–656. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Charlebois E, Morin SF, Catz SL, Goldstein RB, Remien RH, et al. Perceived Adverse Effects of Antiretroviral Therapy. Journal of Pain & Symptom Management. 2005;29:193–205. doi: 10.1016/j.jpainsymman.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Elliott TR, Neilands TB, Morin SF, Chesney MA. A Social Problem Solving Model of Adherence to HIV Medications. Health Psychology. 2006;25:355–363. doi: 10.1037/0278-6133.25.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MO, Folkman S. Side effect and disease related symptom representations among HIV+ adults on antiretroviral therapy. Psychology, Health & Medicine. 2004;9:139–148. [Google Scholar]
- Johnson MO, Stallworth T, Neilands TB. The drugs or the disease? Causal attributions of symptoms held by HIV positive adults on HAART. AIDS and Behavior. 2003;7:109–117. doi: 10.1023/a:1023938023005. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Gerber JG. Advances in HIV/AIDS therapy. Advances in Internal Medicine. 2000;45:1–40. [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54:S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Rompa D, Cage M. Reliability and validity of self-reported CD4 lymphocyte count and viral load test results in people living with HIV/AIDS. International Journal of STD & AIDS. 2000;11:579–585. doi: 10.1258/0956462001916551. [DOI] [PubMed] [Google Scholar]
- Keller SD, Majkut TC, Kosinski M, Ware JE., Jr. Monitoring health outcomes among patients with arthritis using the SF-36 Health Survey: overview. Medical Care. 1999;37:MS1–9. doi: 10.1097/00005650-199905001-00001. [DOI] [PubMed] [Google Scholar]
- Kennedy WP. The nocebo reaction. Med Exp Int J Exp Med. 1961;95:203–205. [PubMed] [Google Scholar]
- Larsen EB, Gerlach J. Subjective experience of treatment, side-effects, mental state and quality of life in chronic schizophrenia out-patients treated with depot neuroleptics. Acta Psychiatrica Scandinavica. 1996;93:381–388. doi: 10.1111/j.1600-0447.1996.tb10664.x. [DOI] [PubMed] [Google Scholar]
- Lightfoot M, Rogers T, Goldstein R, Rotheram-Borus MJ, May S, Kirshenbaum S, et al. Predictors of substance use frequency and reductions in seriousness of use among persons living with HIV. Drug Alcohol Depend. 2005;77:129–138. doi: 10.1016/j.drugalcdep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Lorenz KA, Shapiro MF, Asch SM, Bozzette SA, Hays RD. Associations of symptoms and health-related quality of life: findings from a national study of persons with HIV infection. Ann Intern Med. 2001;134:854–860. doi: 10.7326/0003-4819-134-9_part_2-200105011-00009. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. The NEO Personality Inventory: Using the Five-Factor Model in counseling. Journal of Counseling & Development. 1991;69:367–372. [Google Scholar]
- McElroy SL, Keck PE, Friedman LM. Minimizing and managing antidepressant side effects. Journal of Clinical Psychiatry. 1995;56:49–55. [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Likosky W, Boudewyn AC, Marietta P, Dwyer P, Van der Wende J, et al. Side effect profile and adherence to in the treatment of multiple sclerosis with interferon beta-1a. Multiple Sclerosis. 1998;4:487–489. doi: 10.1177/135245859800400605. [DOI] [PubMed] [Google Scholar]
- Muthen BO, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2:371–402. [Google Scholar]
- Muthén LK, Muthén B. Mplus User’s Guide. Muthen and Muthen, Inc.; Los Angeles, CA: 2004. [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Ray-Chaudhuri K, Abbott RJ, Millac PA. Subcutaneous apomorphine for parkinsonian patients with psychiatric side effects on oral treatment. Journal of Neurology, Neurosurgery & Psychiatry. 1991;54:372–373. doi: 10.1136/jnnp.54.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards ME, Martinson IM. Patients’ perceptions of the impact of chemotherapy side effects and their methods of coping with this impact. Loss, Grief & Care. 1987;1:53–70. [Google Scholar]
- Ritsner M, Ponizovsky A, Endicott J, Nechamkin Y, Rauchverger B, Silver H, et al. The impact of side-effects of antipsychotic agents on life satisfaction of schizophrenia patients: A naturalistic study. European Neuropsychopharmacology. 2002;12:31–38. doi: 10.1016/s0924-977x(01)00128-6. [DOI] [PubMed] [Google Scholar]
- Shapiro DE, Boggs SR, Rodrigue JR, Urry HL. Stage II breast cancer: Differences between four coping patterns in side effects during adjuvant chemotherapy. Journal of Psychosomatic Research. 1997;43 doi: 10.1016/s0022-3999(97)80001-3. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in Experimental and Nonexperimental Studies: New Procedures and Recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Turkkan JS. Antihypertensive pharmacotherapy side effects: Behavioral measurement and quality-of-life issues in clinical trials. Experimental & Clinical Psychopharmacology. 1993;1 [Google Scholar]
- Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science. 1998;280:867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Lance CE. A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organizational Research Methods. 2000;3:4–69. [Google Scholar]
- Volberding PA. HIV therapy in 2003: consensus and controversy. Aids. 2003;17(Suppl 1):S4–11. doi: 10.1097/00002030-200304001-00002. [DOI] [PubMed] [Google Scholar]
- Ware JE, Gandek B, Group IP. The SF-36 Health Survey: Development and use in mental health research and the IQOLA Project. International Journal of Mental Health. 1994;23:49–73. [Google Scholar]
- Ware JE., Jr. SF-36 Health Survey. In: Mark E, Maruish E, editors. The use of psychological testing for treatment planning and outcomes assessment. 2nd ed. Mahwah, NJ, US: 1999. pp. 1227–1246. [Google Scholar]
- Ware JE, Jr., Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. Journal of Clinical Epidemiology. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Kelly JA, Brondino MJ, Rotheram-Borus MJ, Kirshenbaum SB, Chesney MA, et al. HIV Transmission Risk Behavior Among Men and Women Living With HIV in Four U.S. Cities. Journal of AIDS and Human Retrovirology. 2004;36:1057–1066. doi: 10.1097/00126334-200408150-00009. [DOI] [PubMed] [Google Scholar]
- Yu CY. Evaluating cutoff criteria of model fit indices for latent variable models with binary and continuous outcomes. University of California; Los Angeles: 2002. [Google Scholar]