Abstract
Objective
To examine the brain’s sensitivity to monetary rewards of different magnitudes in cocaine abusers and to study its association with motivation and self-control.
Method
Sixteen cocaine abusers and 13 matched healthy comparison subjects performed a forced-choice task under three monetary value conditions while brain activation was measured with functional magnetic resonance imaging. Objective measures of state motivation were assessed by reaction time and accuracy, and subjective measures were assessed by self-reports of task engagement. Measures of trait motivation and self-control were assessed with the Multidimensional Personality Questionnaire.
Results
The cocaine abusers demonstrated an overall reduced regional brain responsivity to differences between the monetary value conditions. Also, in comparison subjects but not in cocaine abusers reward-induced improvements in performance were associated with self-reports of task engagement, and money-induced activations in the lateral prefrontal cortex were associated with activations in the orbitofrontal cortex. For cocaine subjects, prefrontal cortex sensitivity to money was instead associated with motivation and self-control.
Conclusions
These findings suggest that in cocaine addiction (1) activation of the corticolimbic reward circuit to gradations of money is altered; (2) lack of a correlation between objective and subjective measures of state motivation may be indicative of disrupted perception of motivational drive, which could contribute to impairments in self-control; and (3) the lateral prefrontal cortex modulates trait motivation and deficits in self-control, and a possible underlying mechanism may encompass a breakdown in prefrontal-orbitofrontal cortical communication.
Introduction
Drug addiction is characterized by Impaired Response Inhibition and Salience Attribution (I-RISA) where the motivation to procure drugs overpowers the drive to attain most other non drug-related goals (1). In clinical practice, a similar notion that motivated, goal-directed, behavior is limited to drug-related rewards has been integrated into the core diagnostic definition of substance dependence (DSM-IV), prompting the use of Motivational Interviewing as a brief therapeutic intervention (2). However, although cocaine addicted subjects show lower corticolimbic activations when viewing non drug as compared to drug rewards (an erotic video versus a cocaine video, (3)), there is still a paucity of work on the underlying neurobiological markers of motivation and response to reward in human drug addiction.
The brain reward circuit has classically included the mesocorticolimbic dopaminergic network spanning the prefrontal cortex (PFC), amygdala, and mesencephalon but also the thalamus and cerebellum, which are associated with the processing of salient stimuli (4). Within the PFC, the orbitofrontal cortex (OFC) and anterior cingulate cortex mediate the sustained activation of goal-directed (including drug seeking) behavior (5). The lateral PFC has been implicated in the cognitive aspects of reward expectancy, possibly integrating cognitive and motivational operations (6) by attending to internally generated emotional (7) and crucial feedback (8) information.
In the current study comparing drug addicted individuals to non addicted subjects, our goals were to: (1) quantify the neural responsivity to a non drug reward (money); (2) examine intercorrelations between measures of state motivation; and (3) examine associations between the PFC and trait motivation and self-control. We hypothesized that in cocaine abusers (1) neural sensitivity to different levels of money would be reduced; (2) a disrupted perception of internally generated motivational drives will be indicated by a discrepancy between self-reported motivation and actual task performance; and (3) decreased PFC sensitivity to reward would be related to impaired perception of inner motivation and decreased self-control.
Methods
Subjects
Subjects were 16 cocaine abusers and 13 comparison subjects matched on gender, race, English as first language, handedness, education, socio-economic status, general intellectual functioning, and self-reported depression. Significant group differences were observed in age and cigarette smoking (Table 1s). Initial screening by phone and subsequent on site evaluation by a neurologist and a clinical psychologist ensured that the cocaine abusers were not using marijuana, barbiturates, amphetamines, or opiates (this was ensured by pre scan urine tests in all subjects), that they were free of illnesses that required hospitalization or regular monitoring, and that they had a DSM-IV diagnosis for Substance Dependence or Abuse (see Table 1s for drug use histories). Subjects were fully informed of the nature of the research and provided written consent for their involvement in this study in accordance with the local Institutional Review Board.
Table 1.
SPM results of the monetary reward effect (45¢ or 1¢ > 0¢) in all subjects (A: comparison subjects, N=13, and cocaine abusers, N=16) and in direct group comparisons (B: comparison subjects > cocaine abusers). Statistical thresholds were p<0.005 uncorrected for A and p<0.05 uncorrected for B (third order analyses and a priori hypothesis), minimum cluster size was 5 contiguous voxels (135 mm3). Side (hemisphere) and Brodmann’s areas (BA) are listed in the left column; Talairach coordinates, size (number of voxels), T values, and p values (cluster level corrected) are also provided. The average signal in regions in boldface is used for the ROI analyses in Figures 2–3.
| (A) Voxel-by-voxel: All subjects | (B) Voxel-by-voxel: Comparison subjects > Cocaine subjects | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | x, y, z | Size1 | T | p | x, y, z | Size1 | T | p |
| PREFRONTAL CORTICAL | ||||||||
|
1. Left Orbitofrontal Cortex, insula, amygdala
BA 47, 13 |
1. −39,12, −15 | 114 | 4.7 | .000 | −36,18, −9 | 579 | 3.1 | 0.000 |
| 2. −36,6, −3 | 3.9 | |||||||
| 3. −27, −3, −15 | 2.6° | |||||||
| 2. Right Orbitofrontal Cortex, insula
BA 11, 47, 13, 45, 44 |
4. 39,33, −12 | 10 | 4.0 | .002* | 30,24,6 | 554 | 3.6 | 0.000 |
| 5. 39,12, −12 | 2.8° | 48,12,18 | 3.2 | |||||
| 6. 42,39,0 | 2.7° | 57,18,6 | 3.1 | |||||
| 7. 42,21, −12 | 2.5° | 33,18,12 | 3.2 | |||||
| 42,12,3 | 3.2 | |||||||
|
3. Lateral Prefrontal Cortex
BA 10, 9 |
8. −36,48,18 | 106 | 4.8 | .005 | −27,39,24 | 579 | 3.2 | 0.000 |
| 9. −18,51,33 | 3.3 | |||||||
| 10. 30,45,27 | 2.8° | |||||||
|
4. Anterior Cingulate Cortex, rostral dorsolateral PFC
BA 9, 32, 10 |
11. 3,45,21 | 8 | 3.9 | .003* | ||||
| 12. 3,54,36 | 2.9 | |||||||
| 13. 6,57,33 | 3.0 | |||||||
| 14. −6,51,12 | 3.5 | |||||||
| 15. 0,48,18 | 3.1 | |||||||
| Other: Right Precentral Gyrus
BA 6, 9 |
48,3,36 | 554 | 4.1 | 0.000 | ||||
| 42, −6,57 | 3.2 | |||||||
|
| ||||||||
| SUBCORTICAL | ||||||||
|
| ||||||||
| 5. Right Mesencephalon | 16. 3, −18, −9 | 196 | 4.5 | .000 | ||||
| 17. 6, −21, −18 | 3.2 | |||||||
| 6. Left Mesencephalon | 18. −12, −18, −3 | 196 | 3.8 | .000 | ||||
| 19. −15, −12, −6 | 3.3 | |||||||
| 7. Thalamus | 20. −6, −9,6 | 196 | 4.4 | .000 | −6, −6,12 | 310 | 4.3 | 0.008 |
| 21. 18, −12,6 | 3.5 | −12, −30,0 | 3.4 | |||||
|
8. Left Cerebellum/Occipital (fusiform, cuneus)
BA 18, 19, 31, 37 |
188 | .000 | −27, −72, −30 | 1012 | 4.3 | 0.000 | ||
| −18, −69, −27 | 4.1 | |||||||
| −24, −78, −12 | 5.2 | |||||||
| 22. −33,60, −18 | 5.1 | −39, −63, −15 | 4.4 | |||||
| 23. −30, −45, −30 | 4.0 | −24, −81,24 | 3.2 | |||||
| −24, −90,15 | 3.1 | |||||||
| −27, −84,21 | 3.0 | |||||||
| 9. Right Cerebellum/Occipital
BA 19 |
14 | .001* | 21, −54, −42 | 361 | 4.1 | 0.003 | ||
| 30, −57, −42 | 4.1 | |||||||
| 24. 6, −66, −27 | 3.7 | 33, −78, −15 | 3.4 | |||||
| 25. 3, −84,3 | 3.6 | 15, −84, −24 | 3.3 | |||||
| 36, −51, −30 | 3.2 | |||||||
| 9, −69, −36 | 3.0 | |||||||
Number of voxels;
p<0.05 uncorrected, voxel level, included for complete description of activated cluster;
with small volume correction (5 mm).
Fifteen of the 16 cocaine abusers fulfilled criteria for current cocaine dependence (N=9) or cocaine early remission (N=6). One cocaine abuser, who admitted to weekly use of cocaine, did not meet current abuse or dependence criteria, but met DSM-IV criteria for past polysubstance abuse, which included crack cocaine. The nine abusers with current cocaine dependence reported using cocaine the night before the study; their urine was positive for cocaine, indicating that they had used cocaine within the previous 72 hours. We chose not to exclude subjects with recent cocaine use because specific regional changes in fMRI BOLD can be measured reliably even after acute cocaine infusion (9); moreover, all measures return to baseline by 2 hours post-infusion (9) due to cocaine’s short half-life in the brain (20 minutes, (10)). Nevertheless, drug urine status in the cocaine abusers as well as the age and smoking differences between the groups were accounted for in the analyses, as described in Results.
fMRI Activation Paradigm and State Motivation
Following training, subjects either responded (pressed a button) or refrained from responding during a trigger, depending on one of two preceding instruction stimuli (adapted from Thut et al. (11)). There were 9 pairs of press and no press trials within each of three identical conditions. These were distinguished only by blocked levels of monetary reward received for correct performance on this forced-choice task: high money (45 cents); low money (1 cent); and no money (0 cent). Each monetary condition/block was of 63 sec duration, preceded by a 35 sec fixation cross to preclude carry over effects. Every three (different) monetary blocks constituted a run for a total of 6 runs. To simulate real life incentive motivation, subjects received up to $50 for this task, seeing a numeral designating the reward contingencies before each monetary block and immediately after each trial. This was a relatively substantial amount of money as it doubled the subjects’ total earnings during the complete study day.
The task was presented via MRI compatible goggles. Reaction time (RT) and accuracy data were collected across all trials. Upon task completion, subjects were asked to rate their interest and excitement in the three monetary conditions on two visual analogue scales (range: 0 to 7, boring to interesting and dull to exciting, respectively). These ratings were averaged to represent self-reported task engagement. Monetary differentials (45 minus 0) were calculated for the RT, accuracy, and averaged rating: the first two were used as objective and the latter as subjective measures of state motivation. In this fMRI incentive task we did not establish a propensity to go (the ratio of go to no-go was 50%), and RT was therefore not considered a state measure of inhibitory control.
Trait Evaluations
Tellegen’s multidimensional personality questionnaire (MPQ) (12) was available in 10 comparison subjects and 10 cocaine abusers (Table 1s). The MPQ achievement and self-control scales were used as trait measures of incentive motivation and inhibitory control, respectively.
fMRI Data Processing and Image Analysis
MRI scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. Blood oxygen level dependent (BOLD) responses were measured with a T2*-weighted single-shot gradient-echo EPI sequence (TE/TR=20/3500 ms, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64×64 matrix size, 90°-flip angle, 200kHz bandwidth with ramp sampling, 91 time points, 4 dummy scans). Padding was used to minimize motion, which was inside the accepted threshold of 1 mm maximum displacement (32% of the voxel size) and 1° rotation as determined immediately after each run (13). A T1-weighted 3D-MDEFT sequence (14) was used for structural imaging; all MRI images were inspected to rule out gross morphological brain abnormalities.
All time series were converted into SPM99 format (Wellcome Department of Cognitive Neurology, London UK). A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment, to correct for head motion. The realigned datasets were normalized to the Talairach frame with a 12-parameters affine transformation (15), using a voxel size of 3×3×3 mm3. An 8-mm full-width-half-maximum Gaussian kernel was used to smooth the data. A general linear model (16) and a box-car design convolved with a canonical hemodynamic response function were used to calculate the activation maps. The time series were band pass filtered with the hemodynamic response function as low pass filter and 1/750 sec cut-off frequency as high-pass filter.
Statistical Analyses
Goal 1
To identify brain areas activated specifically to monetary reward as compared to a neutral cue, a voxel based (whole brain) statistical analysis with 2 positive contrasts (45>0 and 1>0) was applied for each run separately for each subject (fixed effects analyses). Maps of BOLD signals for individual subjects were then averaged using a custom program written in IDL (Research Systems, Boulder, CO) across all 6 runs and included in a combined statistical analysis. For this random effects second level analysis, a repeated measures between subjects ANOVA was conducted by SPM (a mask of the general task activations, i.e., 45, 1, or 0 > a fixation baseline was used at p<0.05, voxels=0 for purposes of mask inclusiveness). Statistical thresholds were 0.005 uncorrected for the main effect of monetary reward (45¢ or 1¢ > 0¢) across all subjects (second order analysis) and 0.05 uncorrected for the between subjects monetary comparison (third order analysis and our first a priori hypothesis). Thus, the threshold was reduced from 0.005 to 0.05 due to the anticipated loss of power associated with the increased rigorousness of the analyses from second to third order (comparing money conditions between groups vs. studying each effect in isolation).
Similarly to our other fMRI studies (e.g., (17)), functional regions of interest (ROIs) with a relatively large volume of 729 mm3 (27 voxels) were then defined at the cluster centers of the regions that showed a significant monetary reward effect across all study subjects; within each region the estimated BOLD fMRI signal was calculated and expressed as a percentage of change for each monetary condition from baseline and then averaged to represent all significantly activated clusters. Clarification of anatomical specificity was corroborated with a co-planar stereotaxic atlas of the human brain (18). These ROIs were used to complement the SPM analyses; a two (group) by three (money) repeated measures ANOVA was performed for each of the clusters, and the main effects of money and diagnosis or the interaction were followed by independent (group differences) or paired (reward differences) t-tests. In addition, planned comparisons were performed to test our a priori first hypothesis that cocaine abusers would display reduced sensitivity to gradients of reward. Statistical significance for these ROI analyses was defined at p < 0.05. Note that in these ROI analyses, one comparison subject was removed due to loss of 70% of the BOLD fMRI data. All subsequent analyses are therefore reported for 12 comparison subjects.
Goal 2
Correlations were conducted specifically for sensitivity to monetary reward as compared to the neutral cue (45¢ > 0¢) between the selected three state motivation measures, separately for each study group.
Goal 3
First, correlations were conducted specifically for sensitivity to monetary reward as compared to the neutral cue (random effects 45¢ > 0¢) between the PFC ROIs with the selected three state motivation measures, and with the achievement and self-control MPQ scales, separately for each group. Second, similar ROI correlations were conducted for the lateral PFC and the OFC, in this case between the absolute BOLD responses to monetary reward (45¢ > baseline). Simple linear voxel based (whole brain) correlation analyses were used to validate these ROI correlations. The significance threshold for the first a priori analysis was set at p<0.05, uncorrected; for the second analysis it was p < 0.005, uncorrected. A small volume correction (19) was used for the a priori region of interest (PFC). Minimum cluster size was 100 contiguous voxels (2700 mm3) for both analyses, masked with general task activations. Here, a large volume was selected to protect against Type I error in these correlation analyses.
Results
Goal 1: Monetary Reward Neural Effect
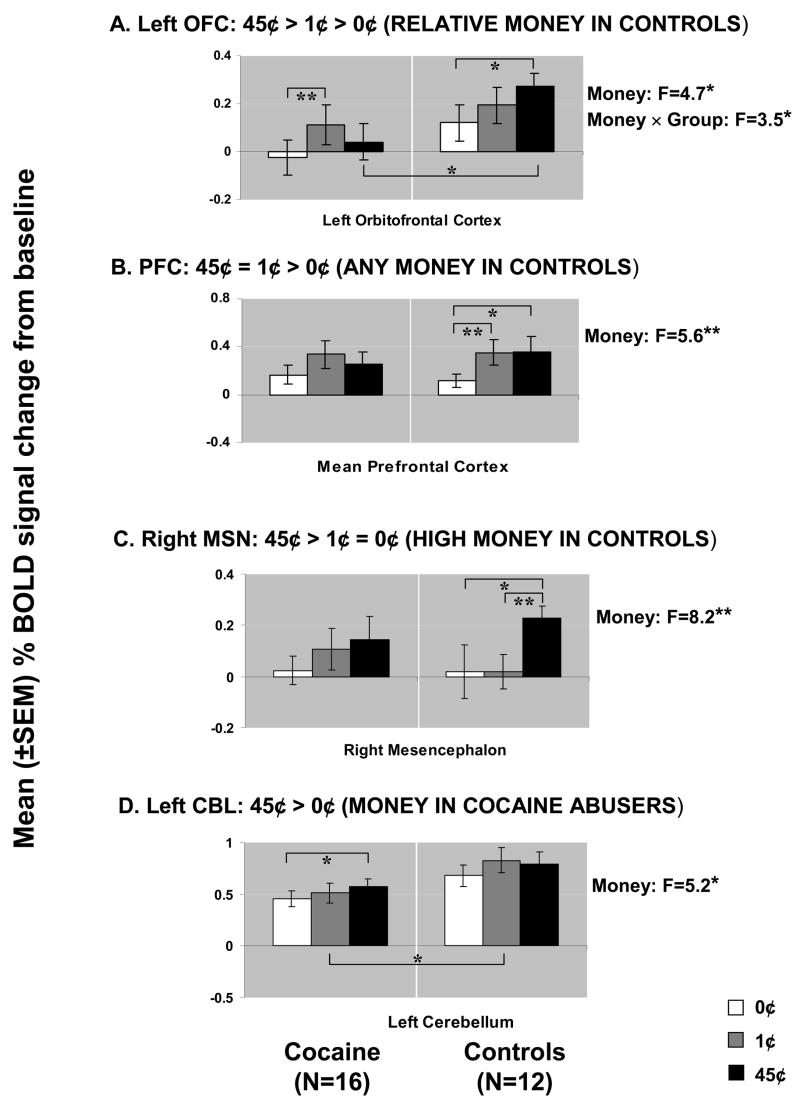
The SPM analyses of the monetary main effect (45¢ or 1¢ > 0¢) in all subjects revealed activations in 25 regions comprising 9 clusters which included the right and left lateral OFC, the lateral and ventromedial (including the anterior cingulate cortex) PFC, the mesencephalon, thalamus, and cerebellum (but also the occipital lobe), all bilaterally (Table 1A and Figure 1A). However, consistent with our first a priori hypothesis, direct group analyses revealed that the activations in the comparison subjects but not the cocaine abusers were driving these results (Table 1B and Figure 1B).
Figure 1.
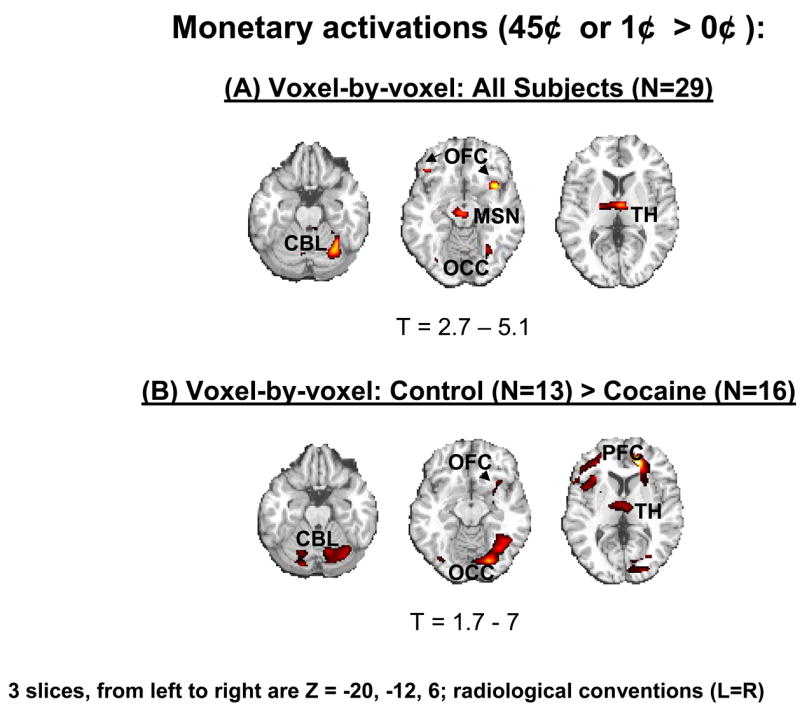
SPM results of the monetary reward effect (45¢ or 1¢ > 0¢) in all subjects (A: comparison subjects, N=13, and cocaine abusers, N=16) and in direct group comparisons (B: comparison subjects > cocaine abusers). Statistical thresholds were p<0.005 uncorrected for A and p<0.05 uncorrected for B (third order analyses and a priori hypothesis), minimum cluster size was 5 contiguous voxels (135 mm3). CBL is cerebellum, OFC is orbitofrontal cortex, MSN is mesencephalon, OCC is occipital cortex, TH is thalamus, and PFC is prefrontal cortex.
The complementary ROI analyses revealed a significant monetary main effect in six of these clusters (Table 1, clusters in boldface, and Figure 2). Further, a money by group interaction was significant in the left OFC (Figure 2A); indeed, an overall test of coincidence of the study groups’ regression lines was statistically significant (F=3.49(2,80), p < 0.05), indicating different lines of best fit (from lowest to highest monetary reward) in this ROI as a function of group. All other significant results are marked in Figure 2 and further described in Discussion.
Figure 2.
Average BOLD signals in the ROIs (see Figure 1A and Table 1, regions in boldface) located at the left orbitofrontal cortex (A: OFC), prefrontal cortex (B: PFC, mean signal), right mesencephalon (C: MSN), and left cerebellum (D: CBL) as a function of monetary reward (white = 0¢; gray = 1¢; black = 45¢) and diagnostic group (left: 16 cocaine abusers; right: 12 comparison subjects, ss). Bar graphs represent mean % signal change from baseline ± SEM. ANOVA F results are presented on the right: df = 2, 25 (Money) or 1, 26 (Group). Results of significant t-tests are marked inside the figures: df = 11 (comparison subjects), 15 (cocaine), 26 (group differences); all significant t > |2.1|; *p<0.05; **p<0.01.
We examined the effect of age, urine status, and cigarette smoking by conducting correlations or t-tests with all 9 clusters’ responses to absolute or relative monetary reward (45¢, 1¢, or 0¢ > baseline, 45¢>0¢, and 1¢>0¢) as the dependent variables (3 covariates × 9 ROIs × 5 reward conditions = 135 analyses); even with a lenient Bonferroni correction (p<0.01), there were no significant correlations between age and any of these ROIs, nor did monetary responses in these ROIs differ as a function of urine status or cigarette smoking history, in each of the study groups or in the complete sample.
Goal 2: State Motivation
All three behavioral measures of state motivation (45¢ > 0¢) were significantly intercorrelated in the comparison subjects (variables 1–3 in Table 2, lower half) but not in the cocaine abusers (Table 2, upper half). In the cocaine subjects there was instead a correlation between the differential RT with MPQ self-control, such that the faster the RT to the higher monetary reward, the more the self-reported trait control. Again, age, urine status, or cigarette smoking did not affect these results.
Table 2.
Correlations between selected dependent study variables. Differential (change) scores were calculated between the high monetary reward and the neutral cue (45¢ - 0¢) for all three state motivation (variables 1–3) and also for the BOLD responses in the four frontolimbic regions (variables 6–9) separately for cocaine abusers and comparison subjects. Values are also provided for trait (MPQ) motivation and control (variables 4–5). Values are mean ± SD (or ± SEM for the BOLD responses) and Pearson r for correlations between the selected nine variables. For group differences in the continuous variables independent t-tests were used. Correlations for comparison subjects are in the lower half and for cocaine abusers in the upper half (bordered and italic) of the correlation matrix (if non significant, correlations are shown only if the same correlations are significant for the other group).

|
Goal 3: Brain-Behavioral Associations
The differential signal change (45¢ > 0¢) in the lateral PFC correlated significantly with motivation at both the state (differential RT) and trait (MPQ achievement) levels and also with trait self-control but only in the cocaine abusers (variable 8 with variables 1, 4–5, Table 2, upper half). Voxel based correlations in the cocaine abusers confirmed the involvement of the lateral PFC in RT (Table 2sA), achievement (Table 2sB), and self-control (Table 2sC and Figure 3A, inserted map). Selected drug use variables (Table 1s) did not correlate with the differential BOLD response in the lateral PFC or with MPQ self-control; further, this correlation (Figure 3A) remained unchanged after controlling with partial correlations for age, urine status, and smoking history (see Figure 1s).
Figure 3.
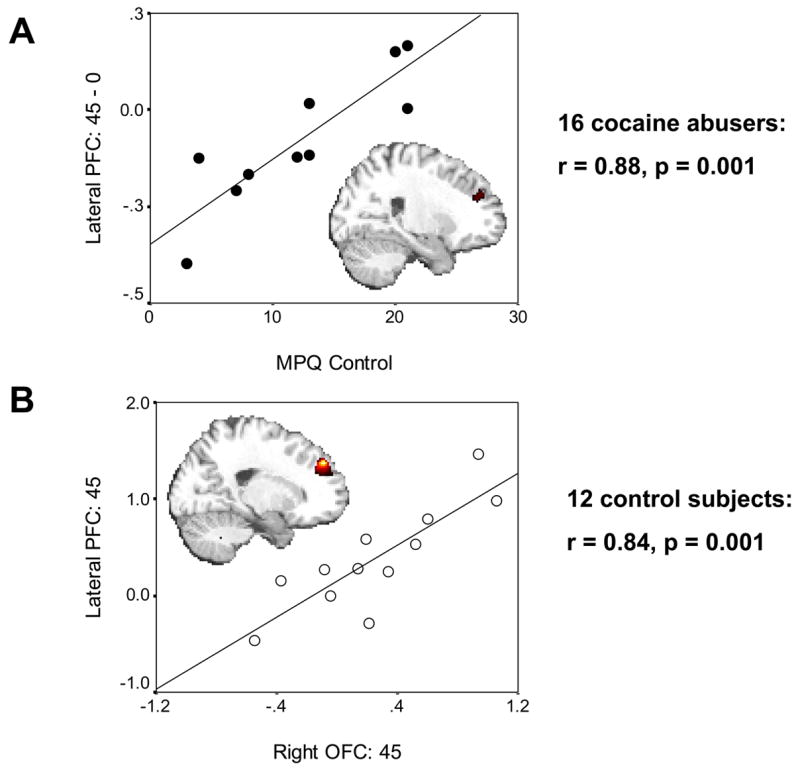
A: Correlation between the lateral PFC and inhibitory control in 16 cocaine abusers. Scatterplot shows association between the BOLD signal change for monetary reward as compared to the neutral cue (45¢ > 0¢) in the lateral PFC (x=33, y=36, z=15) with MPQ self-control (r=0.88, p = 0.001); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (Table 2sC). Thresholded at p < 0.05 uncorrected. B: Correlation between the lateral PFC and OFC in 12 comparison subjects. Scatterplot shows association between the BOLD signal change for monetary reward as compared to baseline (45¢ > baseline) in the lateral PFC (x=−21, y=48, z=36) with same responses in the OFC (x=42, y=33, z=−12) (r=0.84, p = 0.001); the inserted statistical map of brain activation depicts the cluster location corresponding to this correlation (Table 2sD). Thresholded at p < 0.005 uncorrected. Minimum cluster size 100 contiguous voxels, 2700 mm3, for both.
In the comparison subjects there was a significant correlation between the lateral PFC and the right OFC (45¢ > baseline for both) in both voxel based (whole brain) (Table 2sD and Figure 3B, inserted map) and ROI (Figure 3B, linear regression) analyses. These correlations were not significant in the cocaine abusers.
Discussion
Here we report for the first time a compromised neuronal sensitivity to monetary reward in cocaine abusers. We further report novel correlations between PFC sensitivity to money and motivation and self-control in the cocaine abusers but not comparison subjects, who instead demonstrated an association between reward induced change in performance and self-reported task engagement, and an intact association between the lateral PFC and OFC signals to money.
Goal 1. Reduced Complexity of Neuronal Responses to a Non Drug Reward in Addiction
Replicating and extending previous findings in healthy subjects (e.g., (20)), sustained monetary reward was associated with a robust and complex neuronal activation pattern in the comparison subjects (Figures 1–2): there was a trend for the left OFC to respond in a graded fashion (45¢ > 1¢ > 0¢) (Figure 2A), the lateral and medial PFC responded instead to the two conditions of monetary value equally (45¢ = 1¢ > 0¢) (Figure 2B), while the mesencephalon displayed a third pattern of sensitivity to the highest available reward only (45¢ > 1¢ = 0¢) (Figure 2C). In general, these results are consistent with role of the a) OFC in relative reward processing in the primate (21) and in healthy human subjects ((20), (22–25)); b) PFC in the control of attention (8) possibly irrespective of reward magnitude (26); and c) mesencephalon in an all-or-nothing reward processing in the primate (27) and in healthy human subjects (20).
The cocaine addicted subjects did not display this complex pattern of activation to monetary reward, demonstrating either reduced regional BOLD signal in the between group analyses or less sensitivity to differences between the monetary conditions in the within group analyses (Table 1B, Figure 1B, and Figure 2 left). Attenuated mesocorticolimbic neural activations to monetary reward have been previously reported in adolescence (28) and Parkinson’s disease (29). Our study extends these results to drug addicted individuals. The importance of this finding lies in the conditioning between monetary availability and drug procurement. It is therefore possible that for the drug addicted individual, only more immediate drug-related cues (e.g., pictures or a video, see (3)) or the drug itself could have activated this circuit at a comparable level with that induced by a non drug-related reward in the non drug addicted individual.
A relative exception was the left cerebellum, where only the cocaine abusers displayed a significant monetary effect (45¢ > 0¢; note however that the between group analysis still showed larger reward-related activations in the comparison subjects) (Figure 2D). This within subjects result is consistent with reports of compensatory mechanisms in the cerebellum in psychopathology, e.g., over reliance on the cerebellum by cocaine abusers during a working memory task (30) and by Parkinson’s patients during a rewarded task (29).
Goal 2. Impaired Drive Perception in Addiction
Our second major finding concerns intercorrelations between all three state (task-related) behavioral measures of motivation in the comparison subjects but not cocaine abusers (Table 2, variables 1–3). Thus, in the former group only, the faster and more accurate the responses for the high monetary condition compared to the neutral cue, the higher was the self-reported engagement in the task. In contrast, the cocaine subjects’ reports of task engagement were disconnected from their actual task performance (speed or accuracy). This disconnect between the objective and subjective measures of state motivation in the cocaine abusers may reflect not only a discrepancy between actual behavior and explicit knowledge of rules of behavior (31) or reward and punishment outcome (32), but indeed a disruption in the ability to perceive inner motivational drives. This disruption may contribute to long-term self-control deficits as further suggested by our results (Table 2, variables 1 and 5).
Goal 3. The Lateral PFC in Trait Self-Control in Drug Addiction
In the cocaine abusers we observed significant correlations between the lateral PFC and state (differential RT) and trait motivation (MPQ achievement) and with trait self-control (MPQ control, Figure 3A). In particular, the latter correlation suggests that hyposensitivity to reward in the PFC mediates the reduced self-control reported by the cocaine abusers (Table 2, a significant between group difference in variable 5). This result is consistent with the role of the PFC in control of behavior as previously reviewed (1, 33) and with prior research in our laboratory pointing to an association between the PFC and inhibitory control in drug addiction (34–36). Our current results for the first time highlight the role of neural sensitivity to reward in trait inhibitory control.
The Underlying Mechanism: Disruption of Frontal Neuronal Networks
The mechanism underlying impaired perception of motivational drives and disrupted inhibitory control in drug addiction may involve a breakdown in frontal corticolimbic neuronal network communications. Thus, while in the comparison subjects the OFC tended to respond in a monotonically positive pattern to reward (Figure 2A right) and its responses to the high monetary reward were significantly associated with parallel responses in the lateral PFC (Figure 3B), both these patterns were lacking in the drug addicted subjects. It is therefore possible that in drug addiction a disrupted sensitivity to gradients in monetary value in the OFC contributes to the disrupted functioning of the lateral PFC, creating a communication breakdown that augments the cognitive-behavioral and emotional difficulties in these individuals. Indeed, changes in frontal white matter integrity (37) and their association with impulsivity (38) were recently reported in cocaine dependent subjects.
Limitations
Causal attributions should not be made without the replication of the correlational BOLD-behavior results using an experimental design (e.g., manipulation of value of reward, motivation, and inhibitory control in the same task). Also, these results need to be replicated in larger sample sizes and with more homogeneous groups of drug addicted individuals (e.g., all current vs. all detoxified cocaine abusers). In this regard, it is important to recall the age and abstinence differences between the two study groups; age (29) and abstinence from cigarette smoking (39) or cocaine (1, 40) could decrease neural sensitivity to reward. However, analyses revealed that in the current study these possibly confounding factors were not related to the BOLD or behavioral fMRI dependent variables or to their associations (e.g., Figure 1s). In addition, the experimental design did not allow for investigation of different epochs of reward processing (for example, anticipation vs. consummation, see (28)), and future studies could investigate whether the observed between-group differences are specific to distinct phases of reward processing. Finally, this study cannot distinguish whether the disrupted patterns of activation to monetary reward in the cocaine abusers reflect the chronic use of drugs or whether they antedated drug utilization and may have constituted a vulnerability factor for addiction.
Conclusions
We attribute the deficits in the subjective perception of motivational drives in the cocaine abusers to reduced OFC responsivity to gradients in a non drug-related reward and its effect on control of behavior by the lateral PFC. These abnormalities may contribute to the ascribed motivational impairments and deficits in controlling drug taking behavior in drug addicted individuals.
Recommendations
Our results of impaired reward processing and perception of inner drives in the cocaine abusers provide a possible neuropsychological explanation for the deterioration over time in the effectiveness of insight oriented dynamically driven psychotherapies in drug addicted individuals (41). Using interventions aimed at helping drug abusers to recognize external situations that produce stress, craving, or the risk of relapse, and teaching them cognitive-behavioral skills to counteract these situations, may prove beneficial. In particular, therapeutic skill development could include cognitive strategies targeted at strengthening PFC control of behavior, especially under salient emotional situations.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to NDV: DA06891-06; to DT: R03 DA 017070-01, and to RZG: 1K23 DA15517-01); Laboratory Directed Research and Development from U.S. Department of Energy (OBER), NARSAD Young Investigator Award and Stony Brook/Brookhaven National Laboratory seed grant (79/1025459) (to RZG); National Institute on Alcohol Abuse and Alcoholism (AA/ODO9481-04 to NDV), and General Clinical Research Center (5-MO1-RR-10710).
Footnotes
Previous presentation. Presented in part as posters at the Annual Meetings of the Cognitive Neuroscience Society (New York, April 2005) and the American College on Neuropsychopharmacology (Hawaii, December 2005).
References
- 1.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rollnick S, Heather N, Bell A. Negotiating behaviour change in medical settings: the development of brief Motivational Interviewing. Journal of Mental Health. 1992;1:25–37. [Google Scholar]
- 3.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25(15):3932–9. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 6.Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10(3):263–71. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- 7.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 8.Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16(3):463–78. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 9.Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, Foley M, Hyman SE, Rosen B, Weisskoff R. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18(7):724–34. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Volkow N, Ding Y, Fowler J, Wang G, Logan J, Gatley J, Dewey S, Ashby C, Liebermann J, Hitzemann R, Wolf A. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Archives of General Psychiatry. 1995:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 11.Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8(5):1225–8. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- 12.Tellegen A, Waller NG. Exploring personality through test construction: development of the multidimensional personality questionnaire. In: Briggs SR, Cheek JM, editors. Personality measures: development and evaluation. Vol. 1. Greenwich: JAI Press; 1997. [Google Scholar]
- 13.Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20(2):1411–8. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34(3):308–12. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 15.Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6(4):344–52. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- 16.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- 17.Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage. 2004;23(4):1414–21. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- 19.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–7. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 22.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 23.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 24.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 25.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M. The appropriateness of behavioral responses coded in post-trial activity of primate prefrontal units. Neurosci Lett. 1989;101(1):113–7. doi: 10.1016/0304-3940(89)90450-3. [DOI] [PubMed] [Google Scholar]
- 27.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 28.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goerendt IK, Lawrence AD, Brooks DJ. Reward processing in health and Parkinson’s disease: neural organization and reorganization. Cereb Cortex. 2004;14(1):73–80. doi: 10.1093/cercor/bhg105. [DOI] [PubMed] [Google Scholar]
- 30.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123 ( Pt 11):2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 32.Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304(5674):1167–70. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein RZ, Volkow ND, Chang L, Wang GJ, Fowler JS, Depue RA, Gur RC. The orbitofrontal cortex in methamphetamine addiction: involvement in fear. Neuroreport. 2002;13(17):2253–7. doi: 10.1097/01.wnr0000044215.09266.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein RZ, Alia-Klein N, Leskovjan AC, Fowler JS, Wang GJ, Gur RC, Hitzemann R, Volkow ND. Anger and depression in cocaine addiction: association with the orbitofrontal cortex. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2004.10.002. in press. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12(11):2595–9. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51(11):890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 38.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced Anterior Corpus Callosum White Matter Integrity is Related to Increased Impulsivity and Reduced Discriminability in Cocaine-Dependent Subjects: Diffusion Tensor Imaging. Neuropsychopharmacology. 2005;30(3):610–7. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 39.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155(8):1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 41.McCambridge J, Strang J. Deterioration over time in effect of Motivational Interviewing in reducing drug consumption and related risk among young people. Addiction. 2005;100(4):470–8. doi: 10.1111/j.1360-0443.2005.01013.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.