Abstract
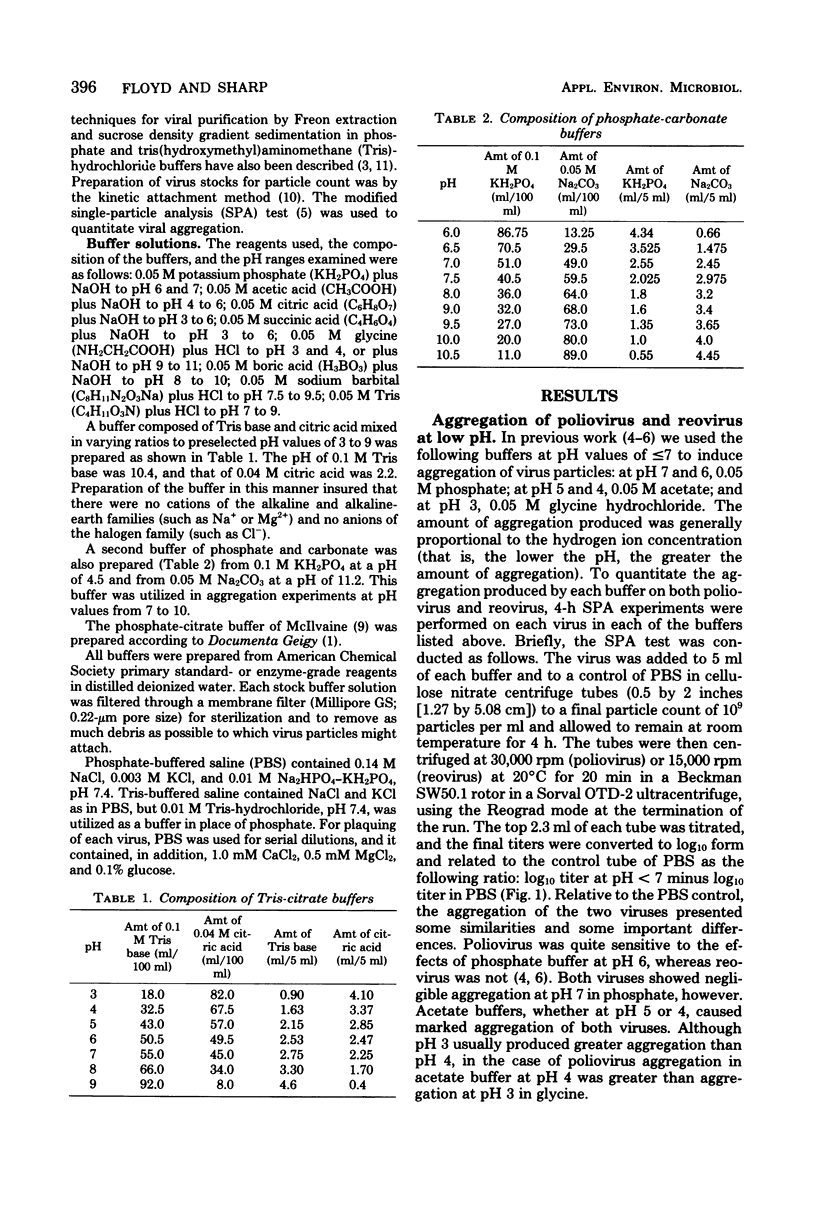
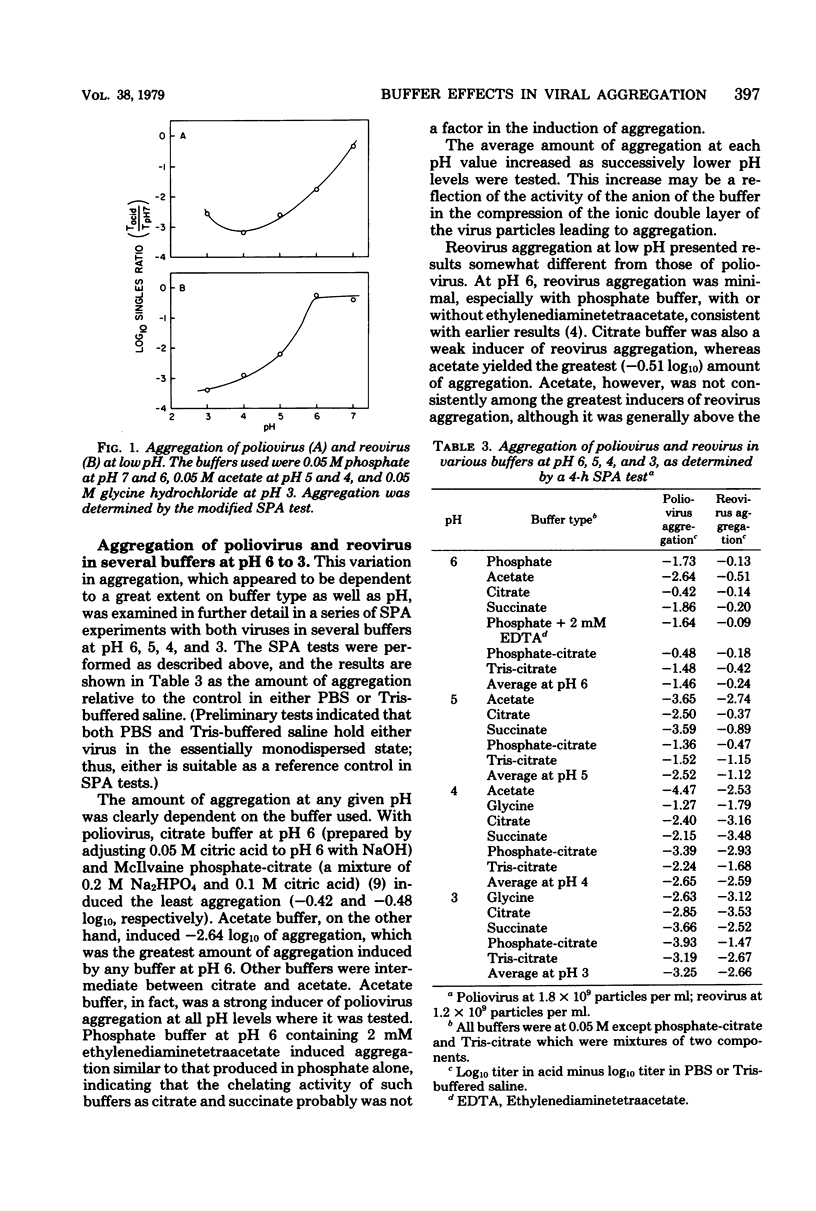
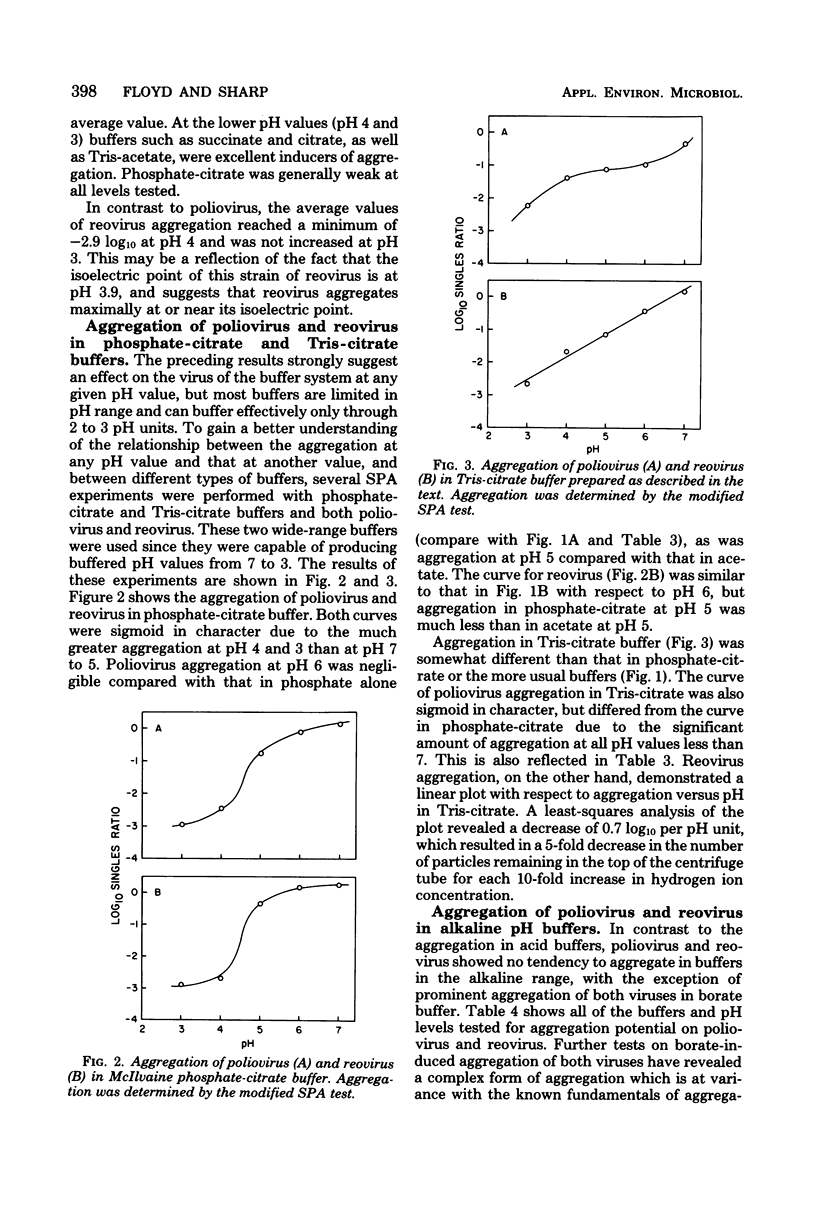
The effects of the buffer employed in maintaining a given pH value were tested on the aggregation of two viruses, poliovirus and reovirus. Poliovirus was found to aggregate at pH values of 6 and below, but not at pH 7 or above, except in borate buffer. Reovirus aggregated at pH 4 and below, but was found to aggregate only in acetate or tris(hydroxymethyl)aminomethane-citrate buffers at pH 5. Other buffers tested for aggregation of reovirus at pH 5 (succinate, citrate, and phosphate-citrate) induced little aggregation. No significant aggregation was found for reovirus at pH 6 and above. For both viruses, the most effective aggregation was induced by buffers having a substantial monovalently charged anionic component, such as acetate at pH 5 and 6 or citrate at pH 3. Cationic buffers at low pH, such as glycine, were generally weaker in aggregating ability than anionic buffers at the same pH. These results, when correlated with the isoelectric point of the viruses (poliovirus at pH 8.2; reovirus at pH 3.9) indicated that both viruses aggregated strongly when their overall charge was positive, but only under certain circumstances when their overall charge was negative. Although reovirus aggregated massively at its isoelectric point, poliovirus remained dispersed at its isoelectric point. The conclusion can be drawn that those pH and buffer conditions which induced aggregation of one virus do not necessarily induce it in another.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunlap R. C., Brown E. R., Barry D. W. Determination of the viral particle content of influenza vaccines by electron microxcopy. J Biol Stand. 1975;3(3):281–289. doi: 10.1016/0092-1157(75)90032-3. [DOI] [PubMed] [Google Scholar]
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: quantitation and kinetics of the aggregation of poliovirus and reovirus. Appl Environ Microbiol. 1978 Jun;35(6):1079–1083. doi: 10.1128/aem.35.6.1079-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R. S., Ackermann W. W. Evidence for conformational states of poliovirions: effects of cations on reactivity of poliovirions to guanidine. Proc Soc Exp Biol Med. 1975 Apr;148(4):1070–1074. doi: 10.3181/00379727-148-38690. [DOI] [PubMed] [Google Scholar]
- Fujioka R. S., Ackermann W. W. The inhibitory effects of MgCl2 on the inactivation kinetics of poliovirus by urea. Proc Soc Exp Biol Med. 1975 Apr;148(4):1063–1069. doi: 10.3181/00379727-148-38689. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Stabilization of poliovirus by cations. Tex Rep Biol Med. 1961;19:683–700. [PubMed] [Google Scholar]
- WALLIS C., MENICK J. L. Cationic stabilization--a new property of enteroviruses. Virology. 1962 Apr;16:504–506. doi: 10.1016/0042-6822(62)90234-9. [DOI] [PubMed] [Google Scholar]
- WALLIS C., SMITH K. O., MELNICH J. L. REOVIRUS ACTIVATION BY HEATING AND INACTIVATION BY COOLING IN MGC12 SOLUTIONS. Virology. 1964 Apr;22:608–619. doi: 10.1016/0042-6822(64)90083-2. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Different effects of MgC1-2 and MgSO-4 on the thermostability of viruses. Virology. 1965 Aug;26(4):694–699. doi: 10.1016/0042-6822(65)90332-6. [DOI] [PubMed] [Google Scholar]


