Abstract
Mutations that increase the copy number of the pSC101 replicon have been used for construction of new cloning vectors. Replacement of glutamate at position 93 in RepA yields plasmids that replicate at medium (27 copies/cell) and high (∼240 copies/cell) copy numbers. Based on the crystal structure of RepE, a structurally similar replication initiator protein from the F factor, the pSC101 repA mutants are predicted to be defective in dimerization. The cloning vectors permit increased expression of gene products along with the advantages of pSC101-derivative plasmids, including stable maintenance and compatibility with ColE1 plasmids. The plasmids also allow blue/white screening for DNA inserts and impart resistance to ampicillin, chloramphenicol and kanamycin. The vectors were used in a genetic assay to suppress temperature-sensitive mutants of ffh, encoding the protein component of the E. coli signal recognition particle, by overproduction of 4.5S RNA. While expression of 4.5S RNA from a wild type pSC101-derivative plasmid was not sufficient for suppression, use of the new vectors did suppress the temperature-sensitive phenotype.
Keywords: copy number mutants, gene expression, plasmid incompatibility
1. Introduction
In recombinant DNA technology, plasmids form the foundation for gene cloning and expression. While plasmid systems are available for a variety of prokaryotic and eukarytotic hosts, most vectors are used in combination with E. coli K-12 strains. E. coli plasmid vectors have been modified in numerous ways to enable selection and screening for DNA inserts, for regulation of gene expression by inclusion of a variety of promoters, to facilitate protein purification by addition of epitope tags to gene products, and allow the choice of multiple antibiotic resistance markers (Lu, 2004). Despite the large variety of plasmid derivatives, most vectors have been constructed from only a few different replicons. Most plasmids used for recombinant DNA utilize the replication origin from the ColE1-like plasmids pMB1 (including pBR322 and pUC18/19-derivatives) (Hershfield et al., 1974) and p15A (Chang and Cohen, 1978), or pSC101 (Cohen et al., 1973).
In contrast to the anti-sense RNA replication control used by ColE1 plasmids (Cesareni et al., 1991; Polisky, 1988), pSC101 uses the RepA protein to initiate and regulate replication (Armstrong et al., 1984) through binding repeated sequences known as iterons at the ori (Chattoraj, 2000; Churchward et al., 1983; Vocke and Bastia, 1983). RepA also autoregulates its own expression by binding as a dimer to inverted repeats near the repA promoter (Linder et al., 1985). pSC101-derivative plasmids are also stably maintained owing to the presence of the par locus (Tucker et al., 1984). pSC101-derivative plasmids replicate at a relatively low copy number (<8 copies/cell) (Cabello et al., 1976; Hasunuma and Sekiguchi, 1977) and numerous cloning vectors based on this replicon have been reported (Hashimoto-Gotoh et al., 2000; Hasnain and Thomas, 1986; Hoang et al., 1999; Lerner and Inouye, 1990; Phillips, 1999; Stoker et al., 1982; Takeshita et al., 1987; Wang and Kushner, 1991).
Another useful property of the pSC101 replicon is that it is compatible with ColE1 plasmids, allowing multiple vectors to be propagated in the same cell. Although there frequently is a need to introduce multiple plasmids to the same cell, the options can be limited. In addition to pSC101 plasmids, different ColE1 replicons can also be compatible with one another, such as pMB1 and p15A-derivative plasmids. However, like pSC101, p15A vectors, including pACYC177 and pACYC184, are relatively low copy number plasmids (Hiszczynska-Sawicka and Kur, 1997), which can limit their utility. Cloning vectors based on other ColE1-like plasmids have been reported, but they replicate at either relatively low or very high copy numbers (Phillips et al., 2000). Despite these options for constructing strains of E. coli and other closely related bacteria transformed with multiple plasmids, we sought additional, well-characterized plasmids that can replicate at multiple copy numbers (low, medium and high) that are also compatible with all ColE1 vectors.
To characterize pSC101 replication, mutations that increase copy number have been found. These mutations, in general, resulted from changes at several amino acid positions in the RepA protein (Furuno et al., 2000; Xia et al., 1993; Xia et al., 1991). Although these mutations have proven useful for understanding plasmid replication control, they have not been developed for use in recombinant DNA. We, therefore, used a repA mutant altered at glutamate (Glu) 93, which results in a 4-5 fold increase in copy number (Xia et al., 1991), to construct a new series of cloning vectors that replicate at various copies/cell and that also offer the advantages of the well-characterized pSC101 replicon. We further reasoned that additional amino acid substitutions of Glu 93 might further disrupt the normal regulation of replication initiation by RepA yielding new classes of copy number mutants. Indeed, through this strategy we isolated a new pSC101 replicon mutant that replicates at high copy number (∼240 copies/cell) and also incorporated it into cloning vectors. We demonstrated the usefulness of these plasmids in an assay of genetic suppression of signal recognition particle mutants in E. coli. These vectors should be useful for any number of studies where stably maintained ColE1-compatible pSC101-derivative plasmids are required at elevated copy numbers.
2. Materials and methods
2.1. Generation of copy number mutants by site-directed mutagenesis
The original cop mutation isolated by Xia et al. (1991) along with four additional mutations that changed the Glu at position 93 of the RepA protein were generated by PCR mutagenesis. To facilitate construction of the different copy number mutants, a BglII restriction site was first introduced to repA corresponding to residue 93 in the pSC101-derivative plasmid pCL21 (Lerner and Inouye, 1990).
Primers repABglIIS: 5′-ACAGATCTTCCAGTGGA CAAACTATGCCAAGTTC-3′ and repABglIIAS: 5′-TGAGGAAGATCTCAAAGCCTTTAACCAAAGGATTCCTG-3′ (the BglII site is italicized) were used in an inverse PCR reaction (Stemmer and Morris, 1992) using the reaction conditions: 95°C for 1 m; 95°C for 30 s, 55°C for 30 s and 72°C for 2 m for 25 cycles; 72°C for 10 m. PCR products of the appropriate size (∼4.5 kb) were agarose gel purified and treated with DpnI at 37°C for 1 h. before digestion with BglII and self-ligation. The ligation reactions were used to transform DH5α and the resulting plasmids were purified using a QIAprep Spin Miniprep kit (Qiagen, Valencia, CA) and confirmed by DNA sequencing. The resulting construct was named pCL21-BglII and used in subsequent PCR mutagenesis reactions.
PCR was used to generate DNA fragments containing specific alterations that were used to replace the BglII-EagI fragment from pCL21-BglII. Using pCL21-BglII as a template, PCR reactions using the conditions: 95°C for 1 m; 95°C for 30 s, 55°C for 30 s and 72°C for 1 m for 30 cycles; 72°C for 5 m. The sense primer EagI-S, 5′-CTCGGCCGTCGCGGCGC-3′ (EagI site is italicized), was used in combination with the following anti-sense primers containing a BglII or BamHI (italicized) restriction site and the desired mutation (underlined):
BglII-E93K: 5′-CTGGAAGATCTTAAAGCCTTTAACCAAAGGATTCCTG-3′;
BglII-E93N: 5′-CTGGAAGATCTGAAAGCCTTTAACCAAAGGATTCCTG-3′; BamHI-E93R:
5′-CTGGAGGATCCGAAAGCCTTTAACCAAAGGATTCCTG-3′; BamHI-E93W: 5′-
CTGGAGGATCCAAAAGCCTTTAACCAAAGGATTCCTG-3′; BamHI-E93G: 5′-
CTGGAGGATCCCAAAGCCTTTAACCAAAGGATTCCTG-3′.
Following PCR, 1.3-kb fragments were gel purified from each reaction, digested with EagI and BglII or BamHI and ligated with pCL21-BglII digested with EagI and BglII. Plasmid DNA was isolated from transformants of each ligation reaction to determine the relative yield of each construct. Mutations were confirmed by DNA sequencing.
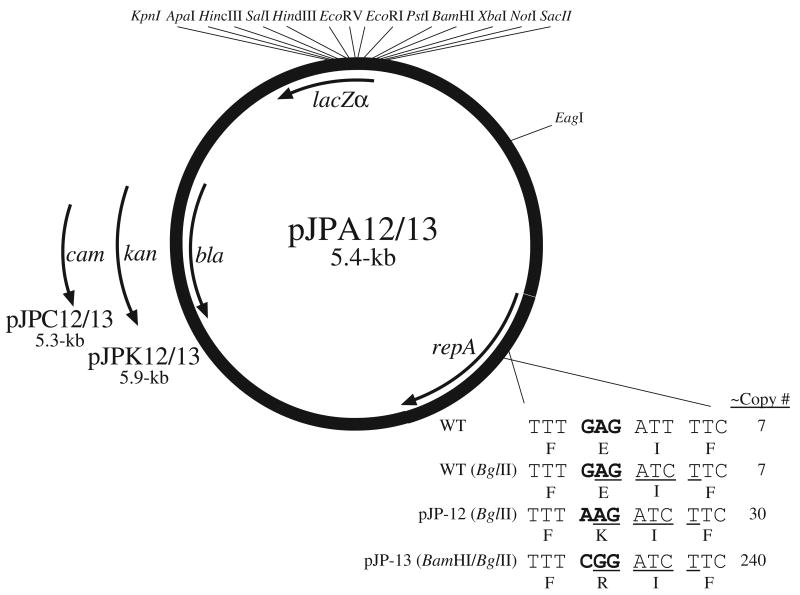
Two mutations (E93K and E93R) were used to construct new cloning vectors by cloning a ∼1.1-kb NdeI to SpeI fragment into the pSC101-derivative plasmid pWSK29 (Wang and Kushner, 1991) to generate pJPA12 and pJPA13 (Fig 1). BspHI fragments encoding resistance to chloramphenicol (CamR) and kanamcyin (KanR) were isolated from pDHC29 and pDHK29 (Phillips et al., 2000), respectively, and used to replace bla, encoding ampicillin resistance (AmpR) from pJPA12 and pJPA13, generating pJPC12 and pJPK13 (Fig 1).
Fig. 1.
New cloning vectors constructed from pSC101 repA elevated copy number mutants. Shown are unique restriction enzyme recognition sites; lacZα, encoding the α peptide from β-galactosidase; bla (AmpR); kan (KanR); and cam, (CamR). The sizes of each plasmid in kb are also shown. The expanded region below repA shows the relevant sequences of the gene and product from wild type pSC101 along with the repA (BglII) mutation. Also shown are the two high copy number mutations. The restriction sites used to construct the vectors are underlined and the sequences encoding amino acid position 93 are shown in bold. The copy number of each replicon is also shown. The position of the EagI site used in constructing the vectors is shown, however, this site is not unique.
2.2. Copy number determination
The copy number of each of the copy number mutants was performed using qPCR similar to a method described previously (Whelan et al., 2003). Two genes, the plasmid encoded bla and ffh, located on the E. coli chromosome, were selected as targets for qPCR analysis. Copy number was determined by first measuring the absolute amounts of plasmid DNA by comparison with a standard curve. Next, the relative amounts of plasmid DNA were reported as a ratio of plasmid copies/chromosome.
A standard curve was generated to establish the relationship of the threshold cycle values (CT) with the absolute copy number of the target sequences. The plasmid pBAD-ffh (lab collection), a 5.5-kb, pBR322-derivative plasmid carrying both target genes (bla and ffh), was used to generate the standard curve. Serial dilutions of a known quantity of pBAD-ffh were subjected to qPCR using a BioRad MyiQ single color detection system (Bio-Rad, Hercules, CA). Reactions were performed using the DyNAmo™ HS SYBR® Green qPCR Kit (Finnzymes, Woburn MA) using the following conditions: 95°C for 10 s, 62°C for 10 s and 72°C for 10 s, for 40 cycles. The primers used for probing bla on the plasmids were:
blaQf : 5′CTACGATACGGGAGGGCTTA-3′; and
blaQr : 5′-ATAAATCTGGAGCCGGTGAG-3′ (Lee et al., 2006). The primers used for probing
ffh were: ffh-S : 5′-TAAACTCGGTAAGTTCCTGCGCGA-3′; and ffh-AS :
5′-AGCGCCGCGTTAA CAATATCAACC-3′.
pBAD-ffh was purified and its concentration measured using a NanoDrop ND-1000 Spectrophotometer. The concentrations were used to calculate the absolute number of copies of plasmid in the sample using the equation (Whelan et al., 2003):
Copies of plasmid = [6.02 × 1023 (copy/mol) × amount (g)] / [length (bp) × 660 (g/mol/bp)]
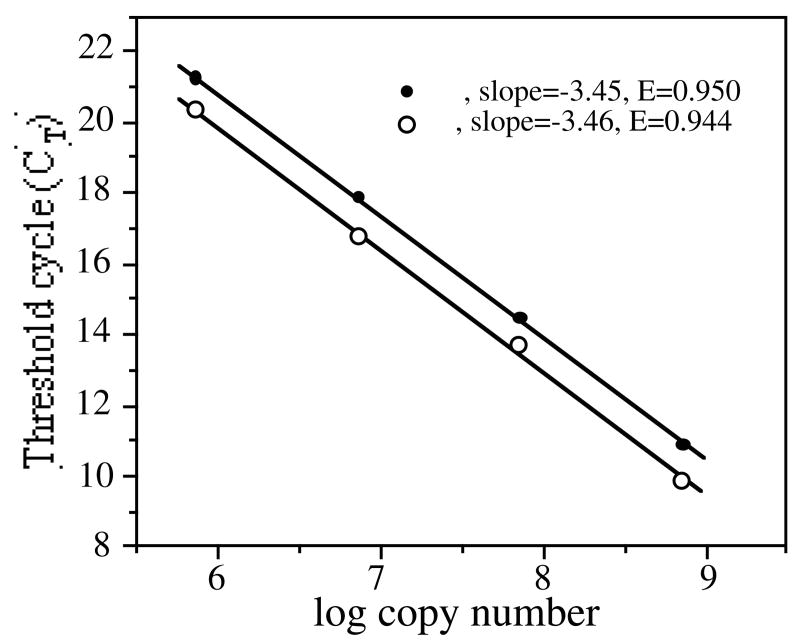
Plasmid DNA was serially diluted from 7.4 × 105 to 7.4 × 109 molecules/μl and the qPCR reactions were performed in triplicate. The CT values were calculated using the BioRad MyiQ software version 3.032 and were plotted against the log of the calculated total copies of plasmid in each sample (Fig 2). From these standard curves, the efficiency for each target (E in Fig 2) was calculated by the MyiQ software and was found to be near ideal.
Fig. 2.
Standard curves for bla and ffh. The curves were constructed by serially diluting a sample of pBAD-ffh, as described in Materials and methods. Each dilution was analyzed by qPCR probing for both bla and ffh. The threshold cycle values (CT), defined as the cycle number where exponential increase of PCR product ends, for each sample were plotted against the log of the copy number. E represents the efficiency of the PCR reaction corresponding to the number of cycles needed for duplication of signal and was calculated by the MyiQ software.
To use the standard curve for copy number determination, each pSC101-derivative plasmid, as well as pBR322, was introduced to DH5α and transformants were grown to mid-log phase. Total DNA was extracted using a MasterPure Complete DNA & RNA Purification kit (Epicentre, Madison WI). The samples were then run in a standard qPCR reaction in triplicate probing separately for both bla (plasmid encoded) and ffh (chromosomal encoded) to determine CT values.
All CT values were then converted to total copy number using the respective standard curve for each probe. The plasmid copy number per chromosome was then determined as the ratio of the copy number of bla, representing the total copy number of plasmids, to the copy number of ffh, representing the total copy number of the chromosome. The procedure was repeated with DNA extracted from two independent samples.
2.3. Determination of plasmid stability and compatibility
A control plasmid was constructed by replacing the bla gene from pWSK29 with a BspHI fragment encoding cam from pDHC30 (Phillips et al., 2000). To test plasmid stability, pJPC11, pJPC12 and pWSK29-cam were used to transform NEB5α and the cells grown in LB overnight in the absence of antibiotic selection. One overnight culture was considered the result of 10 generations of growth. Each overnight culture was diluted by 1000 (5 μl into 5 ml) and grown again for a total of 80 generations. Cultures were diluted after every 20 generations of growth and plated onto LB plates both with and without Cam. Colonies were counted after an overnight incubation. Stability was reported as the number of colonies growing on the antibiotic plates/the total number of colonies on the non-selective plates.
Compatibility with ColE1 replicon plasmids was tested by transforming pJPC12, pJPC13 and pWSK29-cam into DH5α containing pBR322. The strains were grown for multiple generations, as described above. For this study, Amp was included in the growth medium to select for pBR322. Cultures were diluted after every 20 generations of growth and plated onto LB + Amp plates both with and without Cam. Plasmid compatibility was reported as the number of colonies growing on the Amp + Cam plates/the total number of colonies on the Amp plates.
2.4. Suppression of temperature sensitive Ffh
To test for suppression of the temperature sensitivity of an ffh mutant, plasmids pWSK30ffs, pJPA12ffs and pJPA13ffs were constructed by cloning a 0.6–kb EcoRI-BamHI fragment from pSB832 (Park et al., 2002) into each plasmid digested with the same enzymes. Plasmids were used to transform the strains SKP1101 (ffhTS) and SKP1102 (ffh+) (Park et al., 2002) and the reactions were plated at 30°C and 42°C overnight. After incubation for 24 hours, colonies were counted. Suppression of the temperature sensitivity of SKP1101 was calculated by dividing the number of colonies on the 42°C plates by the number on the 30°C plates. Also, colonies were streaked from the 30°C plates at 30°C and 42°C to confirm suppression of the TS phenotype.
3. Results and discussion
3.1. Isolation of copy number mutants
Previous studies revealed that a change of Glu to Lys in the RepA initiator protein resulted in a 4-5 fold increase in plasmid copy number of pSC101 (Xia et al., 1991). We reasoned that replacement of Glu93 with other amino acids could result in further changes to the copy number of the pSC101 replicon and that these new repA mutations could be used to construct useful cloning vectors. We used PCR mutagenesis to generate multiple repA mutants by changing the triplet GAG (encoding Glu93) to CGG (Arg), AAG (Lys), TGG (Trp), CAG (Gln) and GGG (Gly). These amino acids represent a diversity of side chain sizes and are encoded by bases that are compatible with the BglII restriction site engineered into repA of pCL21-BglII (Table 1). Mutants were constructed as described in Materials and methods and all resulted in elevated plasmid DNA yields in comparison to the wild-type repA parent, pCL21-BglII (data not shown).
Table 1.
The plasmids and strains constructed and used in this work
| Plasmid or E. coli Strain | Relevant Genotype | Source or Reference | |
|---|---|---|---|
| Plasmids | |||
| pWSK30 | repA+ | (Wang and Kushner, 1991) | |
| pCL21 | repA+ | (Lerner and Inouye, 1990) | |
| pCL21-BglII | repA-BglII (silent mutation) | This work | |
| pBR322 | bla | Lab collection | |
| pBADffh | bla, ffh | Lab collection | |
| pJPA12 | repAE93K, bla | This work | |
| pJPA13 | repAE93R, bla | This work | |
| pJPC12 | repAE93K, cam | This work | |
| pJPC13 | repAE93R, cam | This work | |
| pJPK12 | repAE93K, kan | This work | |
| pJPK13 | repAE93R, kan | This work | |
| pWSKffs | repA+ffs+ | This work | |
| pJPA11ffs | repA-62, ffs+ bla | This work | |
| pJPA12ffs | repAE93K, ffs+ bla | This work | |
| pJPA13ffs | repAE93R, ffs+ bla | This work | |
| Strains | |||
| NEB5α |
fhuA2Δ(argF-lacZ)U169 phoA
glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 (general cloning host) |
New England Biolabs | |
| SKP1101 | ffhTS, cam | (Park et al., 2002) | |
| SKP1102 | ffh+, cam | (Park et al., 2002) | |
Quantification of the plasmid DNA from the different repA mutants revealed two classes of high copy number mutants. As reported, repAE93K yielded a modest increase in copy number (Xia et al., 1991) (Table 1). We observed a similar increase in plasmid copy number with the repAE93G mutation. When the repAE93R, repAE93W or repAE93N mutants were characterized, even higher plasmid DNA yields were observed. We chose to continue to further characterize the repAE93K and repAE93R variants, which represented both classes of increased copy number mutants (Table 2).
Table 2.
Copy numbers of plasmids as determined by qPCR
| Plasmid | repA Genotype | Copy Number* |
|---|---|---|
| pWSK30 | repA+ | 6.7±0.2 |
| pJPA12 | repAE93K | 27.0±1.5 |
| pJPA13 | repAE93R | 232.7±9.8 |
| pBR322 | NA | 16.5±0.6 |
Copy numbers were calculated as described in Materials and methods. NA, not applicable.
3.2. Plasmid copy number determination
Quantitative PCR (qPCR) was used to measure the copy number of the repAE93K and repAE93R high copy mutants, using a method reported by Whelan et al. (Whelan et al., 2003). To enable the use of primers specific for the plasmid encoded bla gene (Whelan et al., 2003), we cloned the two repA mutant alleles into pWSK29, a low copy number, pSC101-derivative plasmid (Wang and Kushner, 1991), as described in Materials and methods.
A standard curve was first generated to determine the relationship between threshold cycle value (CT) and copy number of bla and ffh carried on the same recombinant plasmid. As shown in Fig 2, the standard curves for both sequences were generated with near ideal efficiency with values of 95% and 94.4% respectively. Efficiency refers to the increase of signal per cycle at the signal threshold used to calculate the CT values. An ideal efficiency is achieved when all of the double stranded templates are denatured, each strand anneals to a primer, and the polymerase replicates the each template. Under these ideal conditions the amount of signal should double with each cycle of the PCR or have an efficiency (E) of 1.0. Also, the standard curves showed consistency over the range of dilutions, with a linear regression of greater than 0.99. These results indicated that the standard curve could be used to obtain an accurate determination of plasmid copy number.
The copy number of each pSC101-derivtive plasmid and a control plasmid was determined by first using qPCR to measure the CT values of both bla and ffh from samples of total DNA isolated directly from E. coli transformants. The absolute copy number was then found for each target gene from the standard curve. The results are reported as plasmid copy number per chromosome, since a single copy of bla is on the plasmid and a single copy of ffh is on the chromosome.
The copy number measurements are summarized in Table 2. As a control for the qPCR method, we also measured the copy number of the ColE1 plasmid pBR322 and of the pSC101-derivative pWSK29. The qPCR method yielded a copy number of 16.5 plasmids/chromosome for pBR322, consistent with a range of 15-20 plasmids per chromosome (Lee et al., 2006; Lin-Chao and Bremer, 1986). In addition, pWSK29 was found to have a copy number of 6.7 plasmids/chromosome, closely matching previous measurements for pSC101 of ∼6 plasmids/chromosome (Cabello et al., 1976; Hasunuma and Sekiguchi, 1977). Measurements of the high copy number mutants showed the repAE93K mutation resulted in a copy number of 27 plasmids/chromosome. We categorized this mutant as a medium copy number plasmid. This value is consistent with the published report the E93K substitution increased the copy number of pSC101 4-5 fold (Xia et al., 1991). In contrast, the E93R replacement yielded a plasmid that replicated to 237 copies/chromosome and was designated as a high copy number plasmid.
3.3 Model for copy number control in repA mutants
Since the crystal structure of RepA is not available, we used the three-dimensional structure of the highly similar RepE initiator protein from the F factor to understand how substitutions at E93 resulted in elevated plasmid high copy number. In addition to being similar to pSC101 RepA, RepE is highly similar to initiator proteins from other replicons, including plasmids R6K, pCU01, pPS10 and bacteriophage P1 (Sharma et al., 2004). For example, all of the proteins contact independent half sites of single iterons by specific α-helices and use a series of β-sheet structures near the amino terminus for dimerization (Sharma et al., 2004). Fig 3 shows that the Glu side chain from RepE, which is in the identical location in RepA, is located in one of these β-sheets comprising the dimerization domain (β2b in Fig 3).
Fig. 3.
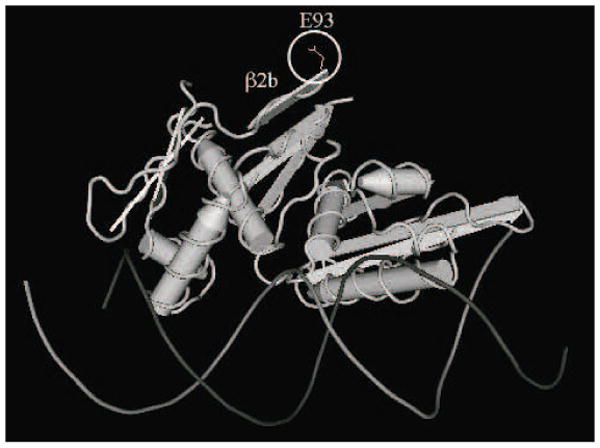
Structure of RepE initiator protein from F factor, a protein structurally similar to RepA of pSC101. The Glu at position 93 (E93) of RepE is identical to RepA. The side chain of E93 is circled and is located in β2b, a β-sheet important for protein dimerization. The structure of RepE (Diaz-Lopez et al., 2003) was obtained from PDB file 1REP and imaged with the program Cn3D. Helical regions are represented as cylinders and β-sheets as ribbons. The initiator protein is shown contacting DNA.
Initial characterization of the high copy number mutant E93K originally isolated by Xia, et al. (1991) concluded that the mutant RepA protein was found at a higher protein concentration and had an increased affinity for repeated sequences located at replication origin (Xia et al., 1993). Given the understanding of pSC101 replication control (Chattoraj, 2000), it is likely that mutations altering Glu93 would reduce the efficiency of initiator protein to form dimers. Since RepA autoregulates its own synthesis by preferentially binding to sites near the repA promoter as a dimer, repAE93 mutants express higher levels of the monomeric form of the protein that are more active in initiating replication.
In isolating the original repAE93K mutant, Xia et al. (1991)(Xia et al., 1991) selected a spontaneous mutant that was able to grow on elevated levels of antibiotic. This copy number mutant resulted from a commonly found G to A transition (GluGAG to LysAAG). The repAE93R mutation, however, results from two base pair changes, including a rarer G to C tranversion (GluGAG to ArgCGG). Likewise, the other high copy mutants described in this study would have been difficult to isolate by conventional random mutagenesis since they require either a double mutation (GluGAG to TrpTGG) or a single transversion (GluGAG to GlnCAG).
3.4. Plasmid stability and compatibility
To determine if the increased copy number plasmids retain the stability properties of pSC101, we measured the frequency of loss of each plasmid after growth for multiple generations in the absence of antibiotic selection. As shown in Table 3, essentially 100% of the cells transformed with the low (pWSK29) or medium- (pJPA12) copy number plasmids retained the plasmids even after 80 generations without Amp. The high copy number plasmid pJPA13 exhibited decreased stability, however, with nearly half of the cells having lost the plasmid after 60 generations without selection. Nearly all of the cells retained the plasmid after 20 generations, however. The decreased stability of JP13 could be due to a combination of the increased metabolic load placed on E. coli imposed by the high copy number plasmid and disrupted dispersion of plasmid clusters in the cell (Weitao et al., 2000). Continual selective pressure by antibiotics is necessary to ensure this replicon is maintained in the culture.
Table 3.
Stability of pSC101 vectors in the absence of antibiotic selection
| Plasmid | Plasmid stability* | ||||
|---|---|---|---|---|---|
| Number of generations | |||||
| 1 | 20 | 40 | 60 | 80 | |
| pWSK30 | 1.0 | 0.97 | 1.0 | 1.0 | 1.0 |
| pJPA12 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| pJPA13 | 1.0 | 0.97 | 0.63 | 0.45 | 0.45 |
Plasmid stability reported as number of colonies appearing on LB + Amp/number of colonies on LB alone after growth for the indicated number of generations without antibiotic.
To determine if the high copy number plasmid maintain their compatibility with ColE1 plasmids, E. coli was transformed with pBR322 (ColE1 replicon) and pJPC12, pJPC13, or pWSK29-cam, a CamR derivative of pWSK29 (Wang and Kushner, 1991) constructed for this study. Cells were grown in the presence of Amp to select for pBR322 for up to 80 generations. Following growth, loss of each plasmid was determined by testing sensitivity to Amp (pBR322) or Cam. As shown in Table 4, pJPC12 and the control pWSK29-cam were fully maintained, even after 80 generations, while increased loss of pJPC13 was observed. Since the pJPC13 was lost from the culture at a similar rate as shown in Table 3, we conclude that plasmid loss was due to increased instability of the high copy number plasmid and not altered incompatibility with ColE1 plasmids.
Table 4.
Compatibility of high copy pSC101 vectors with the ColE1 replicon pBR322
| Plasmid | Plasmid stability* | ||||
|---|---|---|---|---|---|
| Number of generations | |||||
| 1 | 20 | 40 | 60 | 80 | |
| pWSK29-cam | 1.0 | 0.98 | 0.98 | 0.97 | 0.89 |
| pJPC12 | 1.0 | 1.0 | 0.94 | 0.93 | 0.91 |
| pJPC13 | 1.0 | 0.74 | 0.68 | 0.65 | 0.56 |
Plasmid stability reported as number of colonies appearing on LB + Cam/ number of colonies on LB + Amp (pBR322 selection) after growth for the indicated number of generations without antibiotics.
3.5. Construction of new cloning vectors
The mutant repA alleles were used for construction of new cloning vectors (Fig 1). As described in Materials and methods, derivatives of the AmpR plasmids pJPA12 (medium copy) and pJPA13 (high copy) were constructed. The resulting plasmids impart resistance to CamR (pJPC12/13) and KanR (pJPK12/13). The plasmids allow inserts to be identified by blue/white screening on 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal). They further offer the high stability and ColE1 compatibility characteristic of pSC101 plasmids.
3.6. Use of new elevated copy number cloning vectors for suppression analysis
Previous studies have shown that the temperature sensitivity of the ffh mutant SKP1101 can be suppressed by overproduction of 4.5S RNA, the interactive partner of Ffh in forming the signal recognition particle (Park et al., 2002). To better understand the SRP in E. coli, we were in need of a ColE1-compatible plasmid that would express 4.5S RNA in sufficient levels to suppress the ffhTs mutant. Previous attempts to use wild type pSC101 plasmids for suppression failed due to the insufficient levels of 4.5S RNA.
To test the high copy pSC101-derivative plasmids we inserted ffs, the structural gene for 4.5S RNA, into pWSK30, pJPA12 and pJPA13. The plasmids were used to transform SKP1101 and the ffh+ control strain SKP1102 (Park et al., 2002) and suppression was measured by comparing the efficiency of plating at 30°C and the non-permissive temperature of 42°C. The results are summarized in Table 5. All of the copy number mutants suppressed the temperature-sensitive phenotype (plating efficiencies were greater than 0.88). Consistent with our previous results, pWSK30ffs failed to suppress the temperature-sensitivity of SKP1101 (plating efficiency of less than 0.01). There was no significant difference in plating efficiencies between pJPA12 and pJPA13-derivative plasmids, as shown by the SKP1102 (ffh+) transformants (Table 5). The pJPA12 and 13-derivative plasmids expressing 4.5S RNA will be useful to study the interaction between RNA and the Ffh protein.
Table 5.
Efficiency of plating of S1101 and S1102 transformed with the differing copy number plasmids carrying ffs
| Plasmid | SKP1101 (ffhts) | SKP1102 (ffh+) |
|---|---|---|
| pWSKffs | <0.03±0.02 | 1.21±0.15 |
| pJPA12ffs | 0.89±0.08 | 0.95±0.14 |
| pJPA13ffs | 0.89±0.07 | 0.85±0.05 |
| pJPA13 | <0.01±0.01 | 0.94±0.03 |
The table shows the efficiency of plating shown as number of colony forming units at 42°C/number of colony forming units at 30°.
In summary, new cloning vectors based on elevated copy number mutants of the pSC101 replicon have been constructed. These plasmids will facilitate expression studies where ColE1-compatible plasmids that replicate at medium and high copy numbers are required. These new vectors offer the advantage of pSC101 replicon plasmids, while overcoming the limitations of relatively low copy number plasmids.
Acknowledgments
The authors thank Dhruba Chattoraj for his helpful comments. This work was supported by grant R01 GM069628 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong KA, Acosta R, Lender E, Machida Y, Pancotto M, McCormick M, Ohtsubo H, Ohtsubo E. A 37×103 molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984;175:331–347. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Cabello F, Timmis K, Cohen SN. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976;259:285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Cesareni G, Helmer-Citterich M, Castagnoli L. Control of ColE1 plasmid replication by antisense RNA. Trends Genet. 1991;7:230–235. doi: 10.1016/0168-9525(91)90370-6. [DOI] [PubMed] [Google Scholar]
- Chang AC, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj DK. Control of plasmid DNA replication by iterons: no longer paradoxical. Mol Microbiol. 2000;37:467–476. doi: 10.1046/j.1365-2958.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Churchward G, Linder P, Caro L. The nucleotide sequence of replication and maintenance functions encoded by plasmid pSC101. Nucleic Acids Res. 1983;11:5645–5659. doi: 10.1093/nar/11.16.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SN, Chang AC, Boyer HW, Helling RB. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci USA. 1973;70:3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Lopez T, Lages-Gonzalo M, Serrano-Lopez A, Alfonso C, Rivas G, Diaz-Orejas D, Girldo R. Structural changes in Rep, plasmid repliction initiator, upon binding to origin DNA. J Biol Chem. 2003;278:18606–18616. doi: 10.1074/jbc.M212024200. [DOI] [PubMed] [Google Scholar]
- Furuno S, Watanabe-Murakami Y, Takebe-Suzuki N, Yamaguchi K. Negative control of plasmid pSC101 replication by increased concentrations of both initiator protein and iterons. J Gen Appl Microbiol. 2000;46:29–37. doi: 10.2323/jgam.46.29. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T, Yamaguchi M, Yasojima K, Tsujimura A, Wakabayashi Y, Watanabe Y. A set of temperature sensitive-replication/-segregation and temperature resistant plasmid vectors with different copy numbers and in an isogenic background (chloramphenicol, kanamycin, lacZ, repA, par, polA) Gene. 2000;241:185–191. doi: 10.1016/s0378-1119(99)00434-5. [DOI] [PubMed] [Google Scholar]
- Hasnain S, Thomas CM. Construction of a novel gene bank of Bacillus subtilis using a low copy number vector in Escherichia coli. J Gen Microbiol. 1986;132:1863–1874. doi: 10.1099/00221287-132-7-1863. [DOI] [PubMed] [Google Scholar]
- Hasunuma K, Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: Requirement for dnaA function. Mol Gen Genet. 1977;154:225–223. doi: 10.1007/BF00571277. [DOI] [PubMed] [Google Scholar]
- Hershfield V, Boyer HW, Yanofsky C, Lovett MA, Helinski DR. Plasmid ColE1 as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci USA. 1974;71:3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiszczynska-Sawicka E, Kur J. Effect of Escherichia coli IHF mutations on plasmid p15A copy number. Plasmid. 1997;38:174–179. doi: 10.1006/plas.1997.1307. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Ma Y, Stern RJ, McNeil MR, Schweizer HP. Construction and use of low-copy number T7 expression vectors for purification of problem proteins: purification of Mycobacterium tuberculosis RmlD and Pseudomonas aeruginosa LasI and RhlI proteins, and functional analysis of purified RhlI. Gene. 1999;237:361–371. doi: 10.1016/s0378-1119(99)00331-5. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 2006;123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lerner CG, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;18:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Chao S, Bremer H. Effect of the bacterial growth rate on replication control of plasmid pBR322 in Escherichia coli. Mol Gen Genet. 1986;203:143–149. doi: 10.1007/BF00330395. [DOI] [PubMed] [Google Scholar]
- Linder P, Churchward G, Xia GX, Yu YY, Caro L. An essential replication gene, repA, of plasmid pSC101 is autoregulated. J Mol Biol. 1985;181:383–393. doi: 10.1016/0022-2836(85)90227-x. [DOI] [PubMed] [Google Scholar]
- Lu Q. Plasmid vectors for gene cloning and expression. In: F B, Phillips GJ, editors. Plasmid Biology. ASM Press; Washington D.C.: 2004. [Google Scholar]
- Park SK, Jiang F, Dalbey RE, Phillips GJ. Functional analysis of the signal recognition particle in Escherichia coli by characterization of a temperature-sensitive ffh mutant. J Bacteriol. 2002;184:2642–2653. doi: 10.1128/JB.184.10.2642-2653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GJ. New cloning vectors with temperature-sensitive replication. Plasmid. 1999;41:78–81. doi: 10.1006/plas.1998.1380. [DOI] [PubMed] [Google Scholar]
- Phillips GJ, Park SK, Huber D. High copy number plasmids compatible with commonly used cloning vectors. Biotechniques. 2000;28:400–406. doi: 10.2144/00283bm02. [DOI] [PubMed] [Google Scholar]
- Polisky B. ColE1 replication control circuitry: sense from antisense. Cell. 1988;23:929–932. doi: 10.1016/0092-8674(88)90235-8. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sathyanarayana K, Bird JG, Hoskins JR, Lee B, Wickner S. Plasmid P1 Rep is homologous to the F plasmid RepE class of initiators. J Biol Chem. 2004;279:6027–6034. doi: 10.1074/jbc.M310917200. [DOI] [PubMed] [Google Scholar]
- Stemmer WP, Morris SK. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis. Biotechniques. 1992;13:214–220. [PubMed] [Google Scholar]
- Stoker NG, Fairweather NF, Spratt BG. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987:63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Tucker WT, Miller CA, Cohen SN. Structural and functional analysis of the par region of the pSC101 plasmid. Cell. 1984;38:191–201. doi: 10.1016/0092-8674(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Vocke C, Bastia D. DNA-protein interaction at the origin of DNA replication of the plasmid pSC101. Cell. 1983;35:495–502. doi: 10.1016/0092-8674(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- Weitao T, Dasgupta S, Nordström K. Plasmid R1 is present as clusters in the cells of Escherichia coli. Plasmid. 2000;43:200–204. doi: 10.1006/plas.1999.1457. [DOI] [PubMed] [Google Scholar]
- Whelan JA, Russel NB, Whelan MA. A method for the absolute quantification of cDNA using real time PCR. J Immunol Meth. 2003;278:261–269. doi: 10.1016/s0022-1759(03)00223-0. [DOI] [PubMed] [Google Scholar]
- Xia G, Manen D, Yu Y, Caro L. In vivo and in vitro studies of a copy number mutation of the RepA replication protein of plasmid pSC101. J Bacteriol. 1993;175:4165–4175. doi: 10.1128/jb.175.13.4165-4175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia GX, Manen D, Goebel T, Linder P, Churchward G, Caro L. A copy-number mutant of plasmid pSC101. Mol Microbiol. 1991;5:631–640. doi: 10.1111/j.1365-2958.1991.tb00734.x. [DOI] [PubMed] [Google Scholar]