Abstract
It is a central tenet of cochlear neurobiology that mammalian ears rely on a local, mechanical amplification process for their high sensitivity and sharp frequency selectivity. While there is general agreement that outer hair cells provide the amplification, two mechanisms have been proposed: stereociliary motility and somatic motility. The latter is driven by the motor protein prestin. Electrophysiological phenotyping of a prestin knockout mouse intimated that somatic motility is the amplifier. However, outer hair cells of knockout mice have significantly altered mechanical properties, which makes this mouse model unsatisfactory. Here we study a new mouse model without alteration to outer hair cell and organ of Corti mechanics or to mechano-electric transduction, but with diminished prestin function. These animals have knockout-like behavior, demonstrating that prestin-based electromotility is required for cochlear amplification.
In all discussions about amplification in the mammalian cochlea, it is assumed that outer hair cells (OHC) are the amplifiers and that inner hair cells (IHC) are passive detectors of the amplified vibratory signal (e.g., Dallos, 1992). This assumption has a long history that began with chemical ablation of OHCs and demonstration of significant effects on hearing threshold (Ryan and Dallos, 1975) and frequency selectivity (Dallos and Harris, 1978). Subsequently, the groundwork for two competing theories of cochlear amplification was established.
In 1985, Crawford and Fettiplace demonstrated voltage-dependent movement of the stereocilia in the turtle cochlea and Brownell et al. discovered somatic motility of mammalian OHCs. Both reports engendered follow-up, with somatic motility enjoying broader support as the principal mechanism of amplification in mammals. However, two recent publications (Chan and Hudspeth, 2005; Kennedy et al., 2005), and their numerous antecedents, appear to support the ciliary mechanism (Hudspeth, 1997).
The need for amplification is sought in the highly-damped nature of the cochlear partition, which, without some boost, would not permit sharply tuned, sensitive operation (Gold, 1948). Consequently, a process is required to counteract the damping by injecting mechanical energy on a cycle-by-cycle basis (Neely and Kim, 1983; de Boer, 1986). Because tuning curves obtained from single auditory nerve fibers are similar to those recorded at the basilar membrane (Narayan et al., 1998), this amplifier process must influence all elements of the coupled cochlear mechanical system. Thus, it is not sufficient for the amplifier to operate on the mechano-electric transducer (MET) channels alone, i.e., its operation must be reflected in the vibration of all components, including the basilar membrane. This requirement dictates that there be an adequate mechanical impedance match between the amplifier and its load. If the stiffness of a constituent mechanical element (such as the OHC) changes, so does its impedance and, consequently, a match is no longer obtained with the result that amplification will decrease.
OHC somatic electromotility is powered by the novel motor protein prestin (SLC26A5: Zheng et al., 2000). Consequently, it was assumed that development of a prestin knockout (KO) mouse would provide a definitive choice between the two extant theories of amplification. Indeed, OHCs isolated from the prestin-KO mouse were not motile and the animal produced an electrophysiological phenotype consistent with the lack of amplification (Liberman et al., 2002). Despite normal appearance of hair bundles (Wu et al., 2004) and expression of candidate ciliary-motor proteins (Liberman et al., 2002), there was insufficient evidence as to the integrity of the forward transduction mechanism. However, forward transduction was shown to be normal in subsequent experiments on the KO mouse model, supporting the dominant role of somatic motility in amplification (Cheatham et al., 2004; Jia and He, 2005). Although this body of work was suggestive, there were other confounding features that indicated a need for caution. Among these were the shorter length of OHCs (~60% of normal; Liberman et al., 2002; Cheatham et al., 2004, 2007), intimating the possibility of abnormal cochlear micromechanics in the KO. As suggested above, impedance matching of the amplifier to its cochlear load is an essential requirement for effective amplification. Thus a significant change in the mechanical load upon the putative ciliary amplifier could simulate the no-amplification phenotype. Previous studies of the KO did not examine mechanical integrity. Hence, the behavior of the prestin KO mouse cannot be unequivocally assigned to a lack of amplification via somatic motility. As a result, a different model is needed to assess the contribution of prestin to amplification. Results derived from such a model are presented here.
RESULTS
Creation and properties of the 499 KI mouse
To further examine the possibility that prestin is the motor for the mammalian cochlear amplifier, we created a prestin knockin (KI) mouse in which two residues were replaced (V499G/Y501H; Fig. 1A; for simplicity, the altered molecule is subsequently referred to as “499” and the V499G/Y501H knockin mouse as “KI”) near the presumed junction between the last transmembrane domain and the intracellular C-terminus of prestin. Choice of these substitutions was based on transfected HEK cell studies (Zheng et al., 2005), showing that 499 mutant prestin is targeted to the plasma membrane but displays significantly diminished functional characteristics (i.e., non-linear capacitance or NLC). Because NLC reflects electromotility-related charge displacement in OHCs, it is commonly used to assess motor function (Ashmore, 1990; Santos-Sacchi, 1991).
Figure 1.
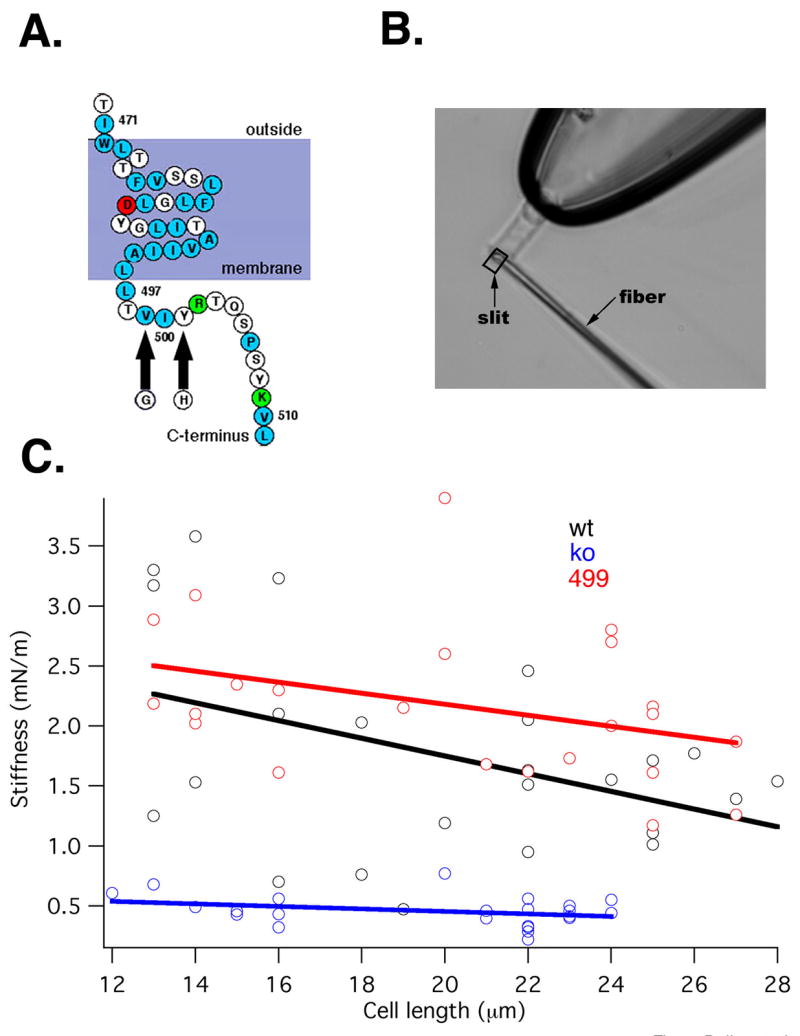
A. Partial amino-acid sequence of the predicted topology of the prestin molecule showing the last putative membrane-spanning helix and part of the C-terminus. The 499/501 mutation is indicated by arrows. 1.B. Image of a KO mouse OHC held by a suction pipette, along with a driven fiber positioned against the cell’s ciliated pole for the purpose of stiffness determination. The combined displacement of fiber and cell is monitored by a photodiode through a rectangular slit. 1.C. Plots of OHC somatic stiffness vs. cell length. Wild-type (black N=22), prestin KO (blue N=21) and 499 KI (red N=23; this color scheme will be followed in all subsequent plots) are shown. Also presented are linear regression lines fitted to the data.
Although 499 homozygous mice suffer progressive OHC loss in the basal cochlear turn, individual OHCs have normal lengths (WT: 15.4±0.62 mm, 499: 15.1±1.1 mm, N=30 for both, measured at ~500 μm from the base of the cochlea. Student’s t-test, p=0.15). We also measured OHC somatic stiffness in cells derived from KI, WT and KO mice. As noted above, to maintain a normal mechanical impedance match between the amplifier and its load, OHC stiffness should not deviate from its wild-type norm. As shown in Fig. 1.B, a calibrated glass fiber is positioned against the cuticular plate of an OHC with ~80% of its length extruded from a microchamber (see Methods). Measurements of axial stiffness plotted in Fig. 1C indicate that OHCs harvested from WT and 499 mice are equally stiff. We tested this similarity by using the “two regression lines” test (Tsutakawa and Hewett, 1978). Regressions representing the two populations (KI and WT) are not statistically different (p=0.27). In contrast, regressions of both WT and KI are significantly different from that of the KO. In fact, the somatic stiffness of OHCs derived from KO mice is between one-fourth and one-third that of WT controls. Thus, in contrast to the prestin KO, the structure and mechanical properties of the organ of Corti are wild-type like in 499 homozygotes. This finding yields two important conclusions. First, inasmuch as OHCs of KI mice are mechanically similar to those of WT, the KI is appropriate for assessing prestin-based amplifier function. Second, because OHCs derived from prestin KO mice show reduced stiffness, the KO is not a usable model to test the importance of prestin-based electromotility in cochlear amplification.
Since mutations in prestin often cause protein misfolding and interfere with proper membrane targeting (Zheng et al., 2005, He et al., 2006), we compared wild type and mutant prestin protein expression patterns in cochleae derived from WT and KI mice using immunofluorescence. For each genotype, one cochlea was treated with Triton X-100, thus permeabilizing the plasma membrane and allowing prestin antibodies to enter the cytoplasm. As shown in Fig. 2A, cochleae derived from WT and KI mice have similar prestin staining patterns (demonstrated with anti-C-mPres). The immunofluorescence is only observed when samples are pretreated with Triton X-100, suggesting that the C-terminus of the 499 mutant protein is located inside cells, just as wild type prestin. The typical “ring” staining pattern of OHCs observed in organ of Corti samples suggests that prestin and mutant prestin are restricted to the lateral membrane of OHCs. Similar results were also found when using anti-N-mPres. In addition, we investigated the oligomeric status (Zheng et al., 2006) of mutant prestin. Similar to WT, 499 prestin forms monomers and dimers, and the dimer bands disappear after pretreatment with the reducing reagent ethanedithiol (Fig. 2B). There is also no statistical difference between WT and 499-prestin in the amounts of monomer and dimer, or their ratio (Fig. 2C). In combination, these results suggest that 499 KI mice possess normal cochlear/OHC structural and mechanical properties.
Figure 2.

A. Plasma membrane targeting is examined by immunostaining in OHCs of WT and 499 prestin mice using confocal microscopy. Whole-mount preparations of the apical cochlear turns of WT and 499 mice are shown at P25. Immunofluorescent images derived from WT (first two images) and 499 homozygous mice (second two images) stained with anti-C-mPres. First and third images: treatment with triton X-100, second and fourth images: no treatment. Similar staining patterns were also observed using anti-N-mPres. 2.B. Prestin’s oligomeric status in WT and 499 cochleae examined by NEXT-PAGE/Western blot. EDT= ethanedithiol. 2.C. Intensities of monomer and dimer bands are compared between WT and 499 cochleae. There is no statistical difference between the amounts of monomer (t-test, p=0.11), dimer (p=0.99) or their ratio (p=0.59; N=3).
In vitro experiments
As shown in Fig. 3A,B, OHCs derived from 499 animals express significantly reduced nonlinear capacitance and electromotility compared to WT littermates. It also appears that the mutation induces a large positive-direction shift in both NLC and motility, with a concomitant reduction in response magnitude around the resting potential of OHCs. While the saturated motile and NLC responses cannot be reached in the 499 OHCs (due to membrane breakdown at very large depolarized membrane potentials), they appear to be small. This behavior recapitulates that seen with severe reduction of intracellular chloride (Rybalchenko and Santos-Sacchi, 2003). More importantly, at the putative in vivo membrane potential of −70 mV (Dallos, 1985; Russell and Sellick, 1983), the slope of the average motility function changes from 7.10 nm/mV (WT) to 0.53 nm/mV (499 KI). Thus, the gain of amplification based on somatic motility is on average 7.5% of normal. From the model proposed by Patuzzi (1996), the expected threshold shift based on the 7.5% gain is approximately 54.5 dB, which is indistinguishable from that exhibited by KO animals (Cheatham et al., 2004).
Figure 3. In vitro measurements of hair cell function.

3.A. Average NLC (±S.D) for WT and 499 OHCs (499 NLC N=8; WT NLC N=11). 3.B. Average motility (cell-length change) ±S.D. (WT, N=11; 499, N=9). Cell contraction is plotted up. Linear capacitance values, measured as the asymptotes of the NLC curves at large positive membrane potential for WT and large negative voltage for 499 mice, are similar. (Clin±SD for WT: 7.8±0.9 pF and 499: 7.80±0.66 pF and t-test: p=0.72). Thus, cell-membrane dimensions in the two genotypes, reflected in linear capacitance, are comparable. 3.C. Representative transducer currents in response to 100 Hz sinusoidal displacements of the basilar membrane from one WT and one 499 OHC in the hemicochlea. Amplitude of BM displacement varied between approximately ±120 nm in equal steps. Inward current is plotted down, holding potential is −70 mV. 3.D. Average (±S.D., N=5 WT; N=5 KI) change in transducer current magnitude in response to sinusoidal displacements of the BM. Inward current is plotted down, holding potential is −70 mV. 3.E. Representative transducer currents in response to 20 msec DC displacement of the TM toward scala tympani for one apical WT OHC and a corresponding 499 cell.
In order to assess the contribution of prestin-based somatic electromotility to cochlear amplification, it is essential to demonstrate that MET channel function, the basis of ciliary motility, is intact. Accordingly, we demonstrate that MET currents are wild-type like, strongly suggesting that forward transduction is unaltered in these animals. In Fig. 3C two individual examples are shown for current traces measured in response to increasing sinusoidal displacements of the basilar membrane. Average (±S.D) change in transducer current is depicted in Fig. 3D (N=5 for each genotype). The difference between the average behaviors was tested with ANOVA and found to be insignificant (F=1.62, p=0.24). Finally, Fig. 3E demonstrates that fast adaptation, presumably intimately tied to stereociliary amplification (e.g., LeMasurier and Gillespie, 2005), is also indistinguishable from that seen in wild-type mice. For example, low-level average fast adaptation time constants are 0.120±0.011 msec for WT (n=5) and 0.120±0.006 msec for 499 (n=5) (t-test: p=0.62). All the in vitro data shown thus far indicate that, in contrast to prestin null mice, the 499 KI mice provide an ideal model for testing the hypothesis that prestin is required for cochlear amplification.
To reiterate, in 499 mice an altered form of prestin is present in the OHC lateral membrane and the dimensions of OHCs as well as their stiffness characteristics and MET-functions are normal. However, the cells yield greatly reduced electromotile responses. If prestin-based electromotility is required for cochlear amplification, one should see a phenotype similar to that obtained from the KO (Liberman et al., 2002; Cheatham et al., 2004; 2007), which in turn recapitulates the no-OHC behavior (Ryan and Dallos, 1975; Dallos and Harris, 1978). This is examined by studying compound action potentials in vivo.
In vivo experiments
Recordings of various indices of cochlear performance are shown in Fig. 4. Included are compound action potential (CAP) threshold functions (Dallos et al., 1978; Johnstone et al., 1979) and CAP simultaneous masking tuning curves (Dallos and Cheatham, 1976). The first measure provides a description of hearing sensitivity, i.e., the CAP threshold curves reflect the animals’ audiogram. The second index, CAP masking tuning curve, provides a measure of the threshold characteristics of a small group of auditory nerve fibers with similar characteristic frequencies, i.e., they provide an indication of frequency selectivity at the “output” of the cochlea. Fig. 4A shows CAP thresholds for 499 (average±S.D., N=7) and corresponding WT (average±S.D., N=7) mice. CAP threshold is defined as the sound pressure level at the eardrum that is required to produce a criterion magnitude CAP at a given frequency. The 499 animals show a large threshold shift compared to WT. As seen previously in KOs (Cheatham et al., 2004), mice lacking functional prestin exhibit a large change in sensitivity. In Fig. 4B the average difference between individual 499 threshold curves and the average WT threshold curve is given. Included for comparison is the average CAP threshold difference for prestin KO mice (N=8) and their WT controls (N=10). We note that the threshold shift changes from ~30 dB at low frequencies to ~55 dB at 27 kHz. The decrease at the highest frequencies is due to age-related hair cell loss, which produces high-frequency threshold shift even in WT animals (Fig. 4A; shaded region). The apparent reversal at the lowest frequencies, where thresholds are determined by the tail-sensitivities of high frequency fibers, is unexplained. ANOVA to test for differences between 499 and KO was performed. The resultant F-ratio is 0.29 and p=0.59. It is apparent that 499 KI and prestin KO threshold shifts are not statistically different. In addition, click auditory brainstem responses corroborate the CAP measurements (data not shown). Finally, in Fig. 4C CAP average (±S.D.) masking tuning curves are shown for 499 KI (N=7) and WT (N=5) mice. As in KO animals (Cheatham et al., 2004; 2007), tuning is absent in the 499 mice.
Figure 4. In vivo results showing CAP data obtained from a round-window electrode.
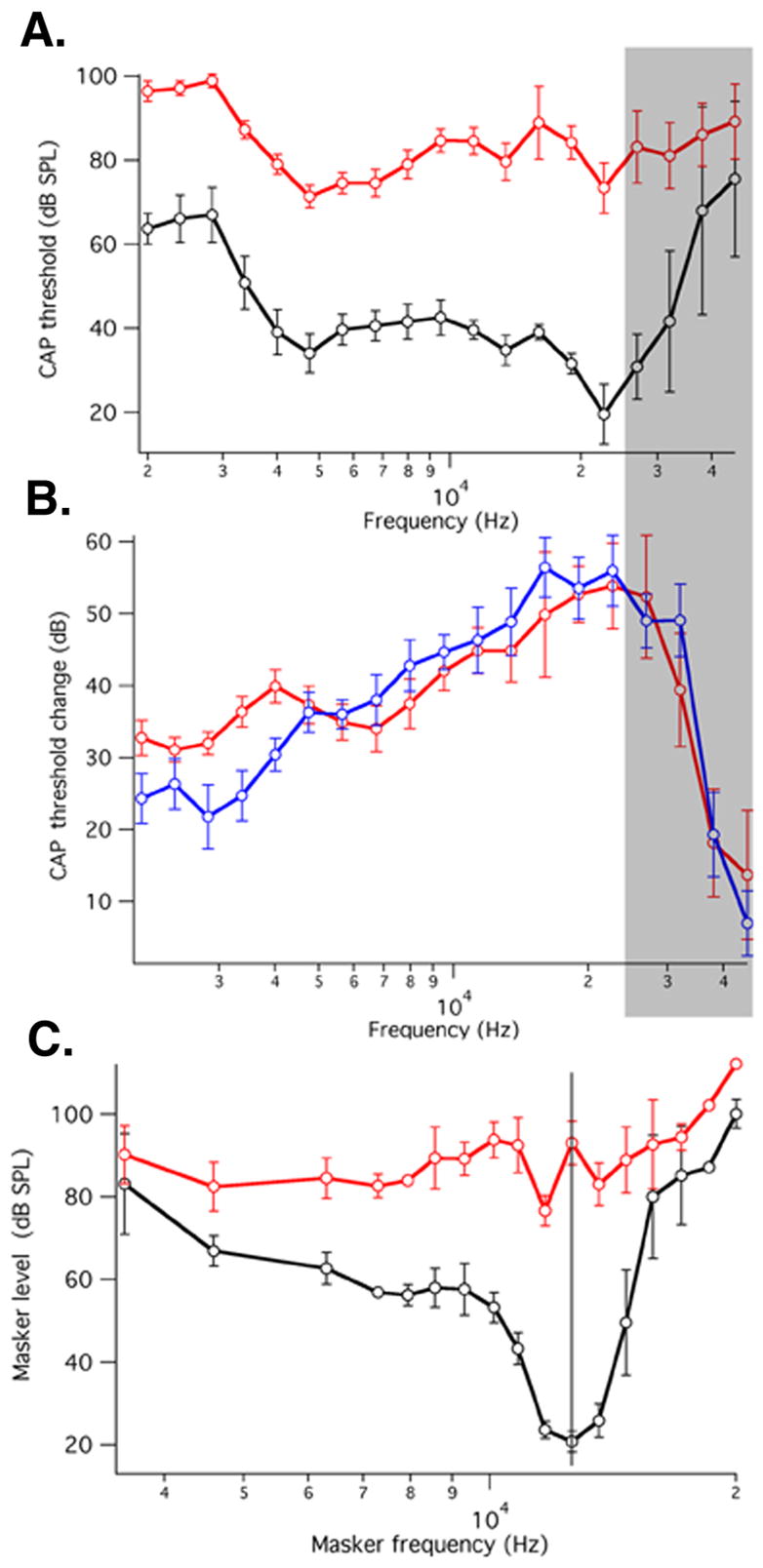
4.A. Average (±S.D. N=6 WT; N=7 KI) CAP thresholds (sound pressure level required to measure10 μV CAP) as a function of stimulus frequency for 499 KI and WT mice. 4.B. Average differences (±S.D) between individual 499 KI thresholds and the corresponding average WT threshold (red). Average differences (±S.D) between individual prestin KO thresholds and the corresponding average WT threshold (blue). The KO mice used for comparison were described in Cheatham et al. (2007). Shaded region approximately corresponds to extent of age-related hair-cell loss. 4.C. Average (±S.D) CAP masking tuning curves for 499 KI (N=7) and WT mice (N=5). Probe tone frequency is 12 kHz. Inasmuch as our tuning-curve collection platform uses slightly different masker frequencies for each tuning curve, an average frequency scale was created. As a consequence, the average WT tuning curve appears to be more shallowly tuned than those published previously (Dallos and Cheatham, 1976; Cheatham et al., 2004; 2007).
DISCUSSION
Outer hair cell mediated mechanical amplification is a signal feature of the mammalian cochlea (e.g., Dallos, 1992). However, in spite of extensive research, there is no general agreement as to the mechanism of amplification. In non-mammalian vertebrates amplification also exists and is based on stereociliary motility, powered by the reciprocal action of MET channels (e.g., Hudspeth, 1997). Prestin-based somatic OHC motility is a mammalian innovation. In theory, either mechanism could provide amplification in the mammal; which one does and in what manner is a central question of cochlear neurobiology.
If cochlear amplification is explained by either ciliary or somatic processes, i.e., if a simple dichotomy of independent mechanisms is assumed, and one wishes to select between the two, the present results rule out ciliary amplification. Accordingly, prestin KI mice display their characteristic phenotype because they lack an amplifier and not because the micromechanical load on the ciliary amplifier is altered. Support for this statement comes from results reported here showing normal OHC length and stiffness, as well as normal MET function. Thus, one may surmise that, in spite of mechanical changes in OHCs lacking prestin, the KO mouse, studied previously, lacks sensitivity and frequency selectivity because amplification is absent and not because the mechanical properties of its OHCs are altered. Our recent study, using a different prestin KI model, is in line with this conclusion (Gao et al., 2007).
Alternatively, there are numerous instances in the literature suggesting cooperativity between the two putative amplifier processes (e.g., Fettiplace, 2006). In its most advanced form, this model envisions ciliary motility to be the amplifier with somatic motility adjusting its operating point. The adjustment presumably minimizes the DC component of the OHC receptor potential by shifting the operating point of the mechano-electric transducer via somatic-motility feedback. Supporting this schema is the observation that intracellular receptor potentials from OHCs in the high-frequency region of the cochlea reveal symmetrical AC responses with virtually no DC component up to high sound levels (Russell and Sellick, 1983; Cody and Russell, 1987). One problem with this supporting argument is that using an alternative approach to intracellular recording from OHCs in vivo, well-developed DC responses are measured from the entire apical half of the cochlea, including regions where cochlear amplification is clearly operative (Dallos, 1985; Cheatham and Dallos, 1993). Hence, the existence, or lack of a DC receptor potential throughout the cochlea is not conclusively established. Another potential problem with the notion that DC somatic length changes serve as mechanical inputs to the MET thereby controlling its operating point, is the universal presence of adaptation in all hair cell systems studied (e.g., Eatock, 2000). Functionally, adaptation of the MET apparatus is a nonlinear high-pass filter, particularly effective at low input levels where amplification is maximal. As a high-pass filter, adaptation reduces the effectiveness of DC feedback from somatic motility to the MET transducer, i.e., the putative ciliary amplifier.
Assuming separate functions for the two processes, we ask what role might be played by feedback mechanisms associated with the MET channels. The latter, aside from their forward transducer role, function as amplifiers in hair cell systems of lower vertebrates (Hudspeth, 1997; Manley, 2001). It has been demonstrated that ciliary processes, aside from amplifying, may also tune the transducer current to cell-specific frequencies (Martin and Hudspeth, 1999). In fact, in all models of ciliary feedback, tuning and amplification are intimately associated. It is possible that in mammals the reciprocal behavior of MET channels is principally represented by frequency tuning of the OHC transducer current, with the amplificatory behavior effectively squelched by the significant mechanical load. The latter is manifest by the need to displace the basilar membrane–tectorial membrane complex by forces generated in the MET channels. This putative inability, however, should not preclude tuning. One can envision a scheme whereby a division of labor is established between the ciliary (MET) process and prestin. The former tunes the transducer current so that in any hair cell only a relatively narrow, tonotopically arranged, frequency band produces AC voltage gradients, thereby activating prestin motors, which are fully responsible for amplification. Although this dichotomy between tuning and amplification has been intimated before (Robles and Ruggero, 2001), its clearest expression is given by Ricci (2003): “It is likely that OHC motility provides the mechanical positive feedback but that this feedback is tuned by some other component, namely the sensory hair bundle.” Recent demonstrations that voltage-induced bundle motion is, either fully (Jia and He, 2005) or at least in significant part (Kennedy et al., 2006), due to somatic motility, are supportive of this possibility. Longitudinally graded OHC transducer currents and time constants for fast adaptation also support the possibility of a location-specific cilia-based tuning mechanism in the organ of Corti (He et al., 2004; Ricci et al., 2005). It has also been shown that the reduction in driving force to somatic motility at high frequencies, due to attenuation of the AC receptor potential by filtering at the OHC’s basolateral membrane, can be overcome in various ways (for a summary see Dallos et al., 2006). We note that this alleged reduction is often cited as a critical impediment to somatic motility being the amplifier. Finally, prestin’s dominant role in amplification is supported by recent in vivo experiments (Santos-Sacchi et al., 2006).
If prestin developed in order to produce a DC adjustment to the operating point of the MET channel, as theories of cooperativity suggest, one might question the use of a novel protein for this purpose. What sets prestin apart from other biological motors is its direct voltage-to-displacement conversion process and its speed. The protein is capable of producing force at rates exceeding 70 kHz (Frank et al., 1999). Hence, one asks what might be the evolutionary advantage of converting a sulfate transporter into a high-speed motor, and then using it only at DC when conventional enzymatic motors could have fulfilled this role. It appears to us that assigning amplification to prestin and perhaps pre-filtering to the MET channel is a reasonable means of achieving OHC function at any stimulus frequency in mammals. Nevertheless, neither the prestin KO nor any of the KI models studied so far is capable of discriminating between models in which prestin is the sole amplifier and in which prestin-based DC motility adjusts the MET operating point. This is due to the simple fact that as the “gain” of prestin is turned down, both amplification and operating point adjustment are reduced. What the electrophysiological results from prestin-mutant animals unequivocally show is that the normal molecule is essential for cochlear amplification. Without demonstrating normal mechanical properties and intact MET function, as done in the present work, this conclusion could not have been reached.
METHODS
See Online Supplemental Materials
Supplementary Material
Acknowledgments
We thank T. Nicol for advice on statistics and J. Siegel and Y. Yu for technical assistance. R. Edge and K. Miller participated in some immunocytochemistry and K. Naik in some in vivo experiments. This work is supported in part by ALSAC, The Hugh Knowles Center and by NIH grants DC00089 (to P. D.), DC06471, CA023944, and CA21765 (to J. Zuo), DC 004696 (to D.Z.Z.H.), DC006412 (to J. Zheng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashmore JF. Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- Brownell W, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Chan D, Hudspeth AJ. Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005;8:149–155. doi: 10.1038/nn1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Dallos P. Longitudinal comparisons of IHC AC and DC receptor potentials recorded from the guinea-pig cochlea. Hear Res. 1993;68:107–114. doi: 10.1016/0378-5955(93)90069-d. [DOI] [PubMed] [Google Scholar]
- Cheatham MA, Huynh KH, Gao J, Zuo J, Dallos P. Cochlear function in Prestin knockout mice. J Physiol (London) 2004;560:821–830. doi: 10.1113/jphysiol.2004.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Huynh K, Zheng J, Du GG, Edge RM, Anderson CT, Ryan AF, Zuo J, Dallos P. Evaluation of a prestin mouse model derived from the 129S1 strain, Audiol. and Neurotol. 2007;12:378–390. doi: 10.1159/000106481. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. The responses of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol (London) 1987;383:551–569. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol (London) 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neuroscience. 1985;5:1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Cheatham MA. Compound action potential (AP) tuning curves. J Acoust Soc Amer. 1976;59:591–597. doi: 10.1121/1.380903. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris DM. Properties of auditory nerve responses in the absence of outer hair cells. J Neurophysiol . 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris DM, Özdamar Ö, Ryan A. Behavioral, compound action potential, and single unit thresholds: Relationship in normal and abnormal ears. J Acoust Soc Amer. 1978;64:151–157. doi: 10.1121/1.381980. [DOI] [PubMed] [Google Scholar]
- Dallos P, Zheng J, Cheatham MA. Prestin and the cochlear amplifier. J Physiol (London) 2006;576:37–42. doi: 10.1113/jphysiol.2006.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer E. Mechanics of the cochlea: Modeling efforts. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. Springer; New York: 1986. pp. 258–317. [Google Scholar]
- Eatock RA. Adaptation in hair cells. Ann Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Frank G, Hemmert W, Gummer AW. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci USA. 1999;96:4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang X, Wu X, Aguiñaga S, Huynh K, Matsuda K, Jia S, Patel M, Zheng J, Cheatham MA, He DZZ, Dallos P, Zuo J. Prestin-based outer hair cell electromotility in knockin mice does not appear to adjust the operating point of a cilia-based amplifier, Proc. Natl Acad Sci USA. 2007;104:12542–47. doi: 10.1073/pnas.0700356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold T. Hearing II: the physical basis of the action of the cochlea. Proc R Soc B. 1948;135:492–498. [Google Scholar]
- He DZZ, Jia S, Dallos P. Mechanoelectrical transduction of adult outer hair cells studied in the hemicochlea. Nature. 2004;429:766–770. doi: 10.1038/nature02591. [DOI] [PubMed] [Google Scholar]
- He DZZ, Zheng J, Kalinec F, Kakehata S, Santos-Sacchi J. Tuning in the amazing outer hair cell: Membrane wizardry with a twist and shout. J Membrane Biol. 2006;209:119–134. doi: 10.1007/s00232-005-0833-9. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–950. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Jia S, He DZZ. Motility-associated hair-bundle motion in mammalian outer hair cells. Nat Neurosci. 2005;8:1028–1034. doi: 10.1038/nn1509. [DOI] [PubMed] [Google Scholar]
- Johnstone JR, Alder VA, Johnstone BM. Cochlear action potential threshold and single unit thresholds. J Acoust Soc Amer. 1979;65:254–257. doi: 10.1121/1.382244. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Evans MG, Crawford AC, Fettiplace R. Depolarization of cochlear outer hair cells evokes active hair bundle motion by two mechanisms. J Neurosci. 2006;26:2757–2766. doi: 10.1523/JNEUROSCI.3808-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasurier M, Gillespie PG. Hair cell mechanotransduction and cochlear amplification. Neuron. 2005;48:403–415. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao JG, He DZZ, Wu XD, Jia SP, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Manley GA. Evidence for an active process and a cochlear amplifier in nonmammals. J Neurophysiol. 2001;86:541–549. doi: 10.1152/jn.2001.86.2.541. [DOI] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ. Active hair bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA. 1999;97:12026–12031. doi: 10.1073/pnas.96.25.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Kim DO. An active cochlear model showing sharp tuning and high sensitivity. Hear Res. 1983;9:123–130. doi: 10.1016/0378-5955(83)90022-9. [DOI] [PubMed] [Google Scholar]
- Oliver D, He DZZ, Klöcker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage-sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Patuzzi R. Cochlear micromechanics and macromechanics. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. Springer; New York: 1996. pp. 186–257. [Google Scholar]
- Ricci A. Active hair bundle movements and the cochlear amplifier. J Amer Acad Audiology. 2003;14:325–338. [PubMed] [Google Scholar]
- Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R. The transduction channel filter in auditory hair cells. J Neurosci. 2005;25:7831–7839. doi: 10.1523/JNEUROSCI.1127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol (London) 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Dallos P. Absence of cochlear outer hair cells: Effect on behavioural auditory threshold. Nature. 1975;253:44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. Cl-flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea pig. J Physiol (London) 2003;547:873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Song L, Zheng JF, Nuttall AL. Control of mammalian cochlear amplification by chloride ions. J Neurosci. 2006;26:3992–3998. doi: 10.1523/JNEUROSCI.4548-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci USA. 2007;104:7693–7698. doi: 10.1073/pnas.0608583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutakawa RK, Hewett JE. Comparison of 2 regression lines over a finite interval. Biometrics. 1978;34:391–398. [Google Scholar]
- Wu XD, Gao JG, Guo YK, Zuo J. Hearing threshold elevation precedes hair-cell loss in prestin knockout m ice. Mol Brain Res. 2004;126:30–37. doi: 10.1016/j.molbrainres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZZ, Long K, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Matsuda K, Orem A, Aguiñaga S, Deák L, Navarrete E, Madison LD, Dallos P. The C-terminus of prestin influences nonlinear capacitance and plasma membrane targeting. J Cell Science. 2005;118:2987–2996. doi: 10.1242/jcs.02431. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham MA. Analysis of the oligomeric structure of the motor protein prestin. J Biol Chem. 2006;281:19916–19924. doi: 10.1074/jbc.M513854200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


