Abstract
Epidemiological studies have indicated that increased consumption of cruciferous vegetables is associated with a statistically significant reduction in the risk for cancers. The major bioactive agent in these vegetables is a class of sulfur-containing glycosides called glucosinolates. Isothiocyanates, derivatives of glucosinolates, have been shown to possess anticancer properties in a variety of tumor cell lines. In this study, we evaluated the antigrowth, cell cycle modulation and proapoptotic effects of isothiocyanate iberin in human neuroblastoma cells. Treatment of neuroblastoma cells with iberin resulted in a dose- and time-dependent inhibition of growth, increased cytotoxicity, and G1 or G2 cell cycle arrest depending upon dose and cell type. The iberin-induced cell cycle arrest in neuroblastoma cells was associated with inhibition of expression of cyclin-dependent kinase Cdk2, Cdk4, and Cdk6 proteins. Fluorescence microscopic analysis of DNA-staining patterns with DAPI revealed an increase in apoptotic cell death in iberin treated cells as compared with control cells. FLICA staining showed that iberin-induced apoptosis and this apoptotic induction was found to be associated with activation of caspase-9, caspase-3, and PARP. These findings suggest the novel anticancer efficacy of iberin is mediated via induction of cell cycle arrest and apoptosis in human neuroblastoma cells and has strong potential for development as a therapeutic agent against cancer.
Keywords: Isothiocyanate, Iberin, Neuroblastoma, Apoptosis, Cell cycle, Chemoprevention
Introduction
The increased incidence of cancer in the general population has led investigators to search for compounds having an efficient suppressive effect against cancer. Considerable epidemiological evidence shows that diets high in vegetable and fiber lead to low cancer risks and confer protection from various forms of cancer (1–3). Consumption of cruciferous vegetables especially the genus Brassica (broccoli, cabbage, Brussels sprouts, kale, cauliflower etc) has been reported to reduce the risk of human cancer (3–6). Cruciferous vegetables contain a group of sulfur containing secondary metabolites termed glucosinolates and the chemopreventive benefit of these vegetables is attributed to their relative high glucosinolate contents (7–9).
Isothiocyanates are hydrolysates derived from glucosinolates and have recently been of intense interest for their anticarcinogenic activities and potential use in the chemoprevention of cancer. Several mechanisms for the activities of isothiocyanates in cancer chemoprevention have been proposed including inhibition of phase I carcinogen activating enzymes, induction of phase II carcinogen detoxification enzymes and induction of cell cycle arrest and apoptosis (10–13). Previous studies indicate that natural isothiocyanates such as sulforaphane and phenylethyl isothiocyanate possess strong antitumor activities in vitro and in vivo (14–19). Iberin, a sulfoxide analogue of sulforaphane, is a naturally occurring member of isothiocyanate family of cancer chemopreventive agents. There are few studies on iberin in comparison to sulforaphane. Iberin increased glutathione S-transferase and quinone reductase activities in the urinary bladder of the rats demonstrating protective effects against chemical carcinogenesis (20). Iberin also upregulated thioredoxin reductase1 expression in human MCF cells suggesting a role in maintenance of redox in cell homeostasis (21). However, the anticancer effects of iberin on the tumor cells have not been investigated in detail.
Neuroblastoma is an aggressive childhood cancer of the peripheral nervous system arising from neural crest sympathoadrenal progenitor cells (22). Despite recent advances in combination therapy, prognosis for high stage neuroblastoma patients is poor (23) and so there remains a need for more effective, less cytotoxic treatments. Therefore, developing an effective treatment strategy is important. Isothiocyanates possess anti-tumor properties in adult cancer models and negligible toxicity in normal cells, but little is known about the effect of these agents on pediatric cancers. We investigated the effects of iberin on the proliferation, apoptosis, and cell cycle alterations of human neuroblastoma cells.
Materials and methods
Reagents
Iberin was isolated from Lesquerella fendleri seedmeal as described previously (24). DMSO, 4′, 6-diamino-2-phenylindole (DAPI), phenylmethylsulfonylfluoride (PMSF), propidium iodide (PI) and 3–4,5-dimethylthiazol-2-yl–2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma. CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit was purchased from Promega. Fluorochrome labeled inhibitor of caspases (FLICA) was from Immunochemistry Technologies. Cdk2, Cdk4, and Cdk6 antibodies were from Biomeda. Antibodies for caspase-3, caspase-9 and poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signaling. Anti-β-actin antibody was obtained from Abcam. Reagents for electrophoresis and western blotting were obtained from Fisher and Amersham Bioscience, respectively.
Cell Culture
The human neuroblastoma SK-N-AS, SK-N-SH and SK-N-BE(2) cell lines were obtained from American Type Culture Collection (Rockville, MD) and were cultured in DMEM supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 units gg/ml) and maintained at 37°C in a 95% air/5% CO2 humidified incubator. SK-N-BE(2) has a MYCN amplification and nonfunctional mutant p53 whereas SK-N-SH expresses wild-type p53 with a low MYCN copy number. Neuroblastoma cells were treated with iberin at indicated concentrations or the equivalent volume of DMSO (final concentration <0.1%).
Cell proliferation assay
Cells were plated at a density of 1×105 cells/well in microtiter plates and treated with different concentrations of iberin for indicated time periods as mentioned. Then 20 μl of 5 mg/ml MTT in PBS, was added to each well and allowed to incubate for a further 4 h. After 4 h of incubation, 100 μl of DMSO was added to each well to dissolve the formazan crystals. Absorbance values at 550 nm were measured with a microplate reader. The results were presented as percentage of cells treated with vehicle DMSO.
Cytotoxicity assay
To assess cell cytotoxicity, LDH leakage was determined in the extracellular cell-culture medium. Cells were plated in microtiter plates and were treated with different concentrations of iberin for specified time intervals. The cell-free supernatant was obtained by centrifugation (400×g) for 10 min and was used to determine the activity of LDH leaked through cell membranes. LDH activity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit according to the manufacturer’s recommended protocol.
DNA Cell Cycle Analysis
Cells treated with or without iberin were harvested, washed once with PBS, and fixed in 3.7% paraformaldehyde in PBS for 10 min at room temperature. Cells were pelleted, washed once with PBS, and resuspended in a PI solution (50 μg/ml PI, Sigma; 0.1 mg/ml RNase A in PBS, pH 7.4) for 30 min in the dark. Flow cytometry analyses were performed (25) on a flow cytometry system (Beckman Coulter, San Jose, CA). Forward light scatter characteristics were used to exclude the cell debris from the analysis. The sub-G1 population was calculated as an estimate of the apoptotic cell population.
Apoptosis assay
In the initial phase of apoptosis, the caspases become activated and the FLICA binds to these activated caspases. Human neuroblastoma cells were treated with different concentrations of iberin for 24 h. After 24 h the cells were stained with FLICA following the manufacturer’s instructions.
Nuclear Staining with DAPI
Cells were treated with or without iberin, washed twice with PBS and then fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature. Fixed cells were washed with PBS and stained with DAPI solution for 10 min at room temperature. The cells were washed twice with PBS and analyzed via a fluorescence microscope.
SDS-PAGE and Western blot analysis
Cells were treated with different concentrations of iberin for 24 hr. The controls were treated with DMSO. For western blot analysis, Cells ere extracted in a buffer solution containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1mM sodium fluoride, 1mM PMSF, 1 μg/ml aprotinin on ice for 20 min. Samples were subjected to SDS-PAGE and separated proteins were transferred onto membrane followed by blocking of membrane with 5% nonfat milk powder (w/v) in tris- buffered saline (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature or overnight at 4°C. Membranes were probed for the protein levels of CDK2, CDK4, and CDK6 using specific primary antibodies followed by peroxidase-conjugated appropriate secondary antibody, and visualized by an enhanced chemiluminescence detection system. Similarly, for apoptotic molecules, caspase-9, caspase-3, PARP were probed using their specific primary antibodies followed by appropriate secondary antibody and enhanced chemiluminescence visualization. Membranes were stripped and reprobed with β-actin antibody as a protein loading control.
Statistical analysis
Statistical significance of the experimental results was determined by the Student’s t-test. For all analyses p < 0.05 was accepted as a significant probability level.
Results
Iberin inhibits growth of human neuroblastoma cells
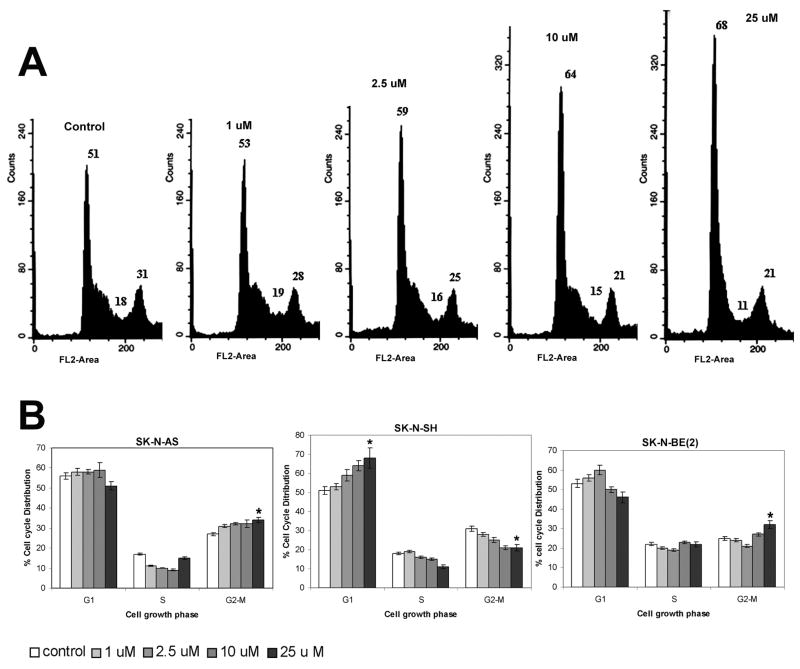
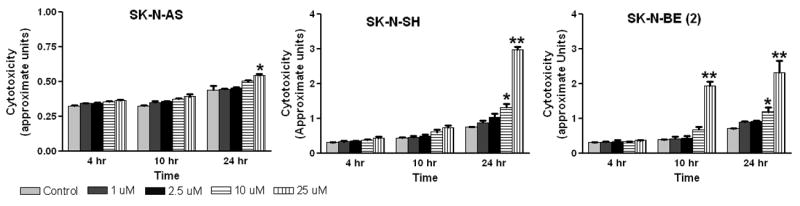
Our aim was to investigate whether iberin treatment imparts an anti-proliferative effect against neuroblastoma cells, as this is the first study assessing the effect of iberin in human neuroblastoma SK-N-AS, SK-N-SH and SK-N-BE(2) cells. To assess the biological activity of iberin in terms of cell growth, neuroblastoma cells were treated with 1, 2.5, 10 and 25 μM doses of iberin for 24 and 48 hours. Iberin showed a strong time dependent inhibition of growth at 25 μM conc in SK-N-AS and SK-N-SH at 24 and 48h respectively whereas there was significant reduction at 10 μM conc in SK-N-BE(2) cells. As shown in Fig 1, iberin treatment resulted in a dose-dependent inhibition of cell growth, as compared to vehicle-treated controls. Iberin treatment also resulted in time dependent inhibition of cell growth and this effect was more pronounced at 48 h post-treatment (Fig 1). These data suggest that iberin is a potent isothiocyanate in inhibiting the growth of neuroblastoma cells.
Figure 1.
Growth inhibitory effects of iberin on SK-N-AS, SK-N-SH and SK-N-BE (2) cells. To assess the effect of iberin on cell growth, human neuroblastoma SK-N-AS, SK-N-SH and SK-N-BE (2) cells were treated with either DMSO vehicle control or 1, 2.5, 10 and 25 μM doses of iberin. After 24 and 48 h of treatment, viable cells were scored using metabolic-dye based MTT assay. Mean ± SE of three independent experiments; each assayed in duplicate. Significant difference from vehicle control, *p < 0.05; **p < 0.01.
Cytotoxicity
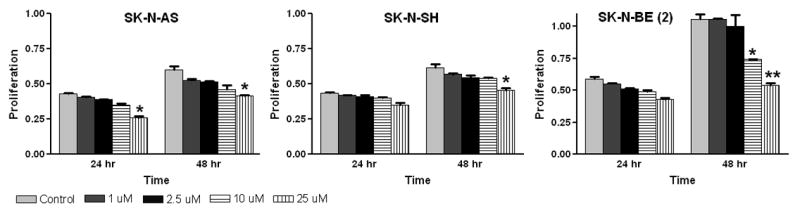
The method that we used to evaluate cytotoxicity was the LDH release assay. An increase in the number of plasma membrane–damaged cells results in an increase in LDH activity in the culture supernatant. To evaluate the cytotoxic effects of iberin, neuroblastoma cells were exposed to varying concentrations of iberin for 24 h and LDH activity was measured in medium. The results show the concentration-dependent LDH release from iberin exposed tumor cells. As shown in Fig 2, treatment of SK-N-SH and SK-N-BE(2) cells with doses of 10 μM of iberin for 24 hours resulted in a significantly increased the LDH release compared to that of DMSO-treated vehicle controls; however, significant cytotoxic effects have been found in SK-N-AS cells only at 25 μM dose of iberin treatment for 24 hr. These results indicate that iberin exposure causes significant damage to the plasma membrane of the neuroblastoma cells.
Figure 2.
Effect of iberin on cytotoxicity in human neuroblastoma SK-N-AS, SK-N-SH and SK-N-BE (2) cells. Cells were cultured in complete medium, and treated with either DMSO vehicle control or 1 to 25 μM doses of iberin. After the indicated treatment times, LDH release into the medium was measured using Cytotoxicity Assay Kit as described in Section 2. Data represent Mean ± SE of three independent experiments; each assayed in duplicate. Significant difference from control, *p < 0.05; **p < 0.01.
Induction of apoptosis
Apoptosis is a controlled form of cell death. To assess whether the cytotoxic effects of iberin, might be mediated by apoptosis, we treated neuroblastoma cells with iberin under similar condition as in other studies, and then analyzed the cells by fluorescence microscopy following DAPI and FLICA staining. Within 24 h of treatment of 10 μM iberin, SK-N-AS cells clearly exhibited significant morphological changes and chromosomal condensation, which is indicative of apoptotic cell death (Fig 3A). Such results imply that the cytotoxic action of iberin was due to its ability to induce apoptosis. It was shown earlier by others (26) that the proportion of cells reactive to FLICA was strongly correlated with the fraction of apoptotic cells identified by the presence of nuclear fragmentation. The detection of activated caspases by application of FLICA was performed with fluorescence microscopy. As shown in Figure 3B, the 10 μM concentration of iberin effectively induced 30–35% apoptotic cell population following 24 h of treatment in neuroblastoma cells compared with controls; iberin treatment at a 25 μM dose resulted in >40% apoptotic cells following 24 h treatment. In the present study a strong correlation was also seen between the percentage of cells labeled with FLICA and those cells labeled with DAPI exhibiting nuclear fragmentation (Fig 3A). These findings demonstrate that iberin activates induction of apoptosis in human neuroblastoma cells.
Figure 3.
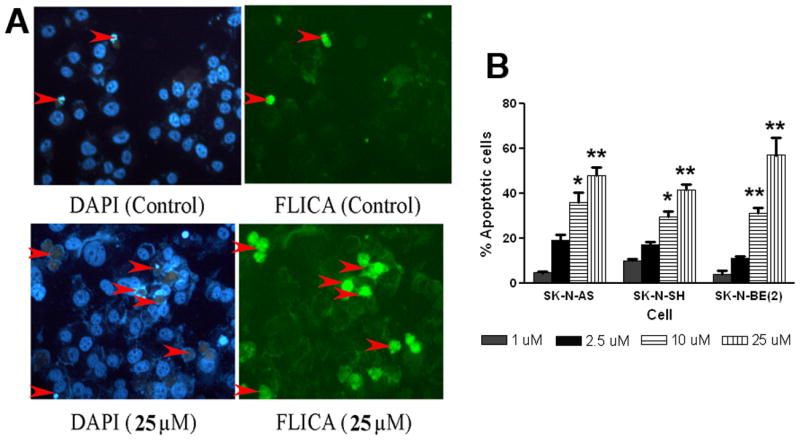
Effects of iberin treatment on apoptosis induction in human neuroblastoma cells using FLICA/DAPI staining. A. The human neuroblastoma SK-N-AS cells were exposed to 25 μM of iberin for 24h. An equal volume of vehicle (DMSO) was added to the controls. To detect activation of caspases and chromatin condensation and fragmentation, cells were stained with FLICA and DAPI respectively as described in section 2 and subsequently apoptotic cells were quantified by fluorescent microscopy. B. Percentage of apoptotic cells are shown and each column represents the mean + SE of the data obtained from three independent experiments. Significant difference from the controls, *p<0.05; **p<0.01.
Induction of cell-cycle arrest
Inhibition of deregulated cell cycle progression in tumor cells is an effective strategy to control tumor growth (27). To assess whether iberin-induced growth inhibition of cells is mediated via alterations in cell cycle, we evaluated the effect of iberin on cell-cycle distribution. We performed DNA cell-cycle analysis with growing neuroblastoma cells followed by treatment with varying concentrations of iberin for 24 h. As summarized in Figure 4B, treatment of SK-N-SH cells with iberin for 24 h resulted in a significantly higher number of cells in the G1 phase at the following concentrations used, 1μM (53%), 2.5 μM (59%,), 10 μM (64%) and 25 μM (68%, P < 0.05), compared with the vehicle treated control (46%). Similar observations were obtained on the analysis of the effects of iberin treatment on cell cycle progression of SK-N-BE(2) cells. Although, the 10 and 25 μM doses of iberin did not induce G1 arrest, there was a significant accumulation of cells in the G2-M phase at 25 μM dose (P < 0.05) in SK-N-BE(2). Remarkably, unlike in SK-N-SH cells, the fraction of SK-N-AS cells in the G1 phase was not affected by iberin at any of the concentrations evaluated, but 25 μm concentration showed an increase in G2-M cell population at 24 h treatment. These results suggest that inhibition of deregulated cell cycle progression could be one of the molecular events associated with selective anticancer efficacy of iberin in neuroblastoma cells.
Figure 4.
Effect of iberin on cell cycle progression in SK-N-AS, SK-N-SH and SK-N-BE (2) cells. Cells were cultured in complete medium, and treated with either DMSO vehicle control or 1 to 25 μM doses of iberin. After 24 hours of these treatments, cells were collected, washed with PBS, and then cellular DNA was stained with propidium iodide as detailed in section 2. The distribution of cells in G1, S and G2-M phase was analyzed by flow cytometry. A, PI fluorescence pattern for cell cycle distribution of SK-N-SH cells in different treatments of iberin. B, the percentage of cell cycle distribution data for each treatment group of SK-N-AS, SK-N-SH and SK-N-BE (2) cells. Three independent experiments were performed and mean ± S.E. are presented. Significant difference from vehicle control, *p < 0.05; **p < 0.01.
Down regulation of protein levels of G1 regulatory CDKs
Based on the above findings showing that iberin causes cell cycle arrests in neuroblastoma cells and the cell cycle is controlled by expression and activation of several cyclins and Cdks, we asked whether their expression levels changed after cell exposure to iberin. Total cell lysates were prepared following iberin treatment of neuroblastoma cells at 1, 2.5, 10 and 25 μM doses for 24 h and cell lysates were assayed for Cdk2, Cdk4, and Cdk6 using immunoblot analysis. As shown in Figure 5A, compared with control, iberin treatment resulted in almost complete inhibition in Cdk2 in SK-N-SH cells, however, the inhibitory effect of iberin in SK-N-AS and SK-N-BE(2) was of lower magnitude. In terms of its effect on Cdk4, iberin caused a decrease in Cdk4 levels in tested neuroblastoma cell lines; it showed profound effect on the reduction of Cdk4 level in SK-N-SH cells at 2.5 μM, in SK-N-BE(2) cells at 10 μM and in SK-N-AS at 25 μM in comparison to control cells following 24 hours of treatment (Fig 5A). Iberin also decreased the expression of Cdk6 levels in SK-N-AS cells; however it showed effect at 25 μM in SK-N-SH and SK-N-BE(2) cells. These results suggest that the suppressive effects of the iberin on the growth of neuroblastoma cells are partly caused by downregulating the levels and activities of specific Cdks. Taken together, these results suggest that alterations in the levels of cell cycle regulators by iberin play a major role in its effect on human neuroblastoma cells in terms of cell cycle arrest and cell growth inhibition together with possible apoptosis induction.
Figure 5.
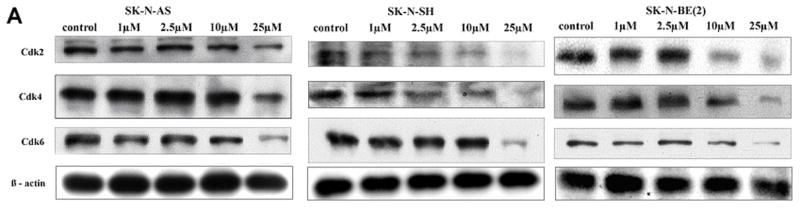
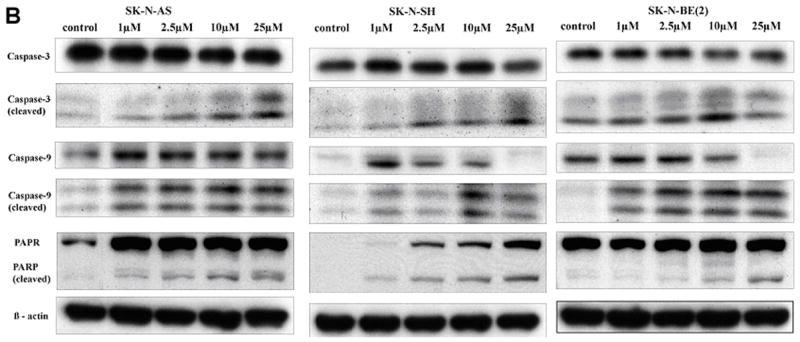
A. Effect of iberin on cell cycle regulators in human SK-N-AS, SK-N-SH and SK-N-BE (2) neuroblastoma cells. Cells were treated with DMSO or 1 to 25 μM doses of iberin for 24 h. At the end of treatment time, total cell lysates were prepared and subjected to SDS-PAGE followed by Western immunoblotting. Membranes were probed with anti-Cdk2, Cdk4, and Cdk6 antibodies followed by peroxidase-conjugated appropriate secondary antibodies, and visualized by enhanced chemiluminescence detection system. The same blots were stripped and re-probed with antibody against β-actin. B. Apoptotic effect of iberin on SK-N-AS, SK-N-SH and SK-N-BE(2) neuroblastoma cells. Tumor cells were cultured in complete medium, and treated with either DMSO vehicle control or 1 to 25 μM doses of iberin for 24 h. At the end of treatments, cell lysates were prepared and SDS-PAGE and Western blot analysis were performed for caspase-9, caspase-3, and PARP using specific antibodies as described in section 2. Membranes were also stripped and reprobed with anti- β-actin antibody for protein loading correction.
Role of caspase activation in iberin-caused apoptosis
Caspase-3 activation and PARP cleavage are characteristic indicators of apoptosis. Based on the above results showing induction of apoptosis by iberin, we determined the effect of iberin on the activation of caspase-9 and caspase-3 following 24 hours of treatment. Cleavage of caspases is directly related to their activation status. Treatment of neuroblastoma cells with iberin (0, 2.5, 10, and 25 μM) for 24 hours caused an increase in cleaved caspase-9 and caspase-3, which were very prominent at 25 μM dose of iberin (Fig 5B). We also assessed PARP cleavage, a nuclear protein that is specifically cleaved by activated caspases. Consistent with the cleavage of caspases, iberin also caused a strong increase in PARP cleavage (Fig 5B) when compared with the cells, which were not treated with iberin; equal protein loading was confirmed by probing the same membrane with β-actin antibody. Taken together, these results show that iberin induced apoptosis in a dose-dependent manner, and that apoptosis is mediated by caspase activation.
Discussion
Conventional chemotherapy of advanced malignant tumors has done little to improve the treatment outcomes in human patients. Prevention and therapeutic intervention by dietary phytochemicals is a newer approach in cancer management (28). Different epidemiological studies have indicated that diet and cancers are closely associated and people who consume higher amount of fruits and vegetables have a lower risk of various types of cancers (29, 30). Previous studies have revealed that isothiocyanates are potent inducers of the expression of enzymes implicated in detoxification of a variety of chemical carcinogens, and are highly effective in chemically induced cancer in animals (31, 32). Isothiocyanates are known to inhibit the growth of cancer cells and to induce apoptosis (14, 33, 34) but the mechanisms are still only partially understood. The isothiocyanate iberin, a sulforaphane sulfoxide analog, has been reported to exhibit some biological effects (12, 20) but their anticancer mechanism is still elusive. In this study, we demonstrated that the possible roles of iberin on the human neuroblastoma cells were 1) to decrease the percentage of viable cells in a dose- and time-dependent manner, 2) to arrest the cell cycle via downregulation of CDKs, and 3) to induce apoptosis via activation of caspase-3 and caspase –9 followed by cleavage of PARP.
Previous studies showed that iberin demonstrate their chemopreventive effects in laboratory animals (12, 20) and this takes place by induction of phase II enzymes, which function in carcinogen detoxification. In the present study, the strong growth inhibitory activity of iberin compared to sulforaphane in cultured human cancer cells prompted us to study its mechanism of action (16). The results from the present study indicate that iberin inhibits human neuroblastoma cell proliferation in a concentration- and time-dependent manner. It is noteworthy that the range of effective doses of iberin (1–25 μM) in neuroblastoma cells is comparable with that shown to be active by sulforaphane in other tumor cell lines such as meduloblastoma (35), colon (36) and prostate cancer cells (37). Moreover, it has been demonstrated that iberin was approximately 2 times more effective than sulforaphane in human myeloid leukemia HL60 cells and its drug-resistant sublines (12). The observation is that these doses of iberin may have significant antitumor effects in other tumor cell lines and correspond to clinically achievable pharmacological concentrations of the drug.
In this study, we demonstrated that iberin is cytotoxic to neuroblastoma cells and that cytotoxicity is result of iberin induced apoptosis via changes of cell cycle and caspase cascade activation. There are several reports that isothiocyanates induce cell arrest in the G1 or G2/M phase depending upon molecular targets of different signaling pathways (38–40). To better understand the inhibitory effect of iberin on the proliferation of neuroblastoma cells, we tested whether iberin has the capacity to block cell cycle progression by flow cytometric analysis of PI-stained cells. These studies showed significant changes of cell cycle distribution in SK-N-AS, SK-N-SH and SK-N-BE (2) cells following iberin treatment at 24 h. Iberin induced G2/M accumulation after 24-h treatment in SK-N-AS and SK-N-BE(2) cells and this is similar to the effects of sulforaphane in colon and prostate cancer cells (39, 40). In SK-N-SH, the proportion of cells in the G1 phase was increased and those in the S phase decreased after 24 hr treatment at all tested concentrations. It has been shown that sulforaphane induced a G2/M cell cycle arrest at 15 μM (41) and at higher doses (>25 μM) a G1 cell cycle arrest in HT-29 cells (42). Our data demonstrate that the iberin induced distribution of cells in the cell cycle changes depending on the histotype of neuroblastoma cells. Moreover, these findings suggest, as is common with therapeutic agents, that the response can vary depending upon the properties of the particular tumor cell type that is being targeted.
The results of The DAPI and FLICA staining assays indicated marked apoptosis occurred in all three tested neuroblastoma cell lines following iberin treatment. Neuroblastomas can acquire a sustained high-level drug resistance during chemotherapy. p53 mutations are rare in primary neuroblastomas, but a loss of p53 function could play a role in multidrug resistance (43). Advanced neuroblastoma frequently relapses, and it is possible that p53 mutations develop later. Many chemotherapeutic agents act via p53 and presence of a mutation, a deletion or functional inactivation of p53, that renders the tumor cells often resistant towards chemotherapeutic treatment (44). Therefore, compounds, which are able to induce apoptosis in cancer cells independent of p53 are of special interest. The essential role of p53 for the induction of apoptosis in Jurkat cells by sulforaphane was suggested previously (45). As expected, the p53-dependent apoptosis pathway may involve in the mechanisms of iberin–induced apoptosis since SK-N-AS and SK-N-SH cells have normal p53. However, the SK-N-BE(2) cell line that was used in this study has impaired p53 expression (46). These observations are in accordance with other studies.Sulforaphane was also reported to induce apoptosis in p53 deficient or mutant p53 expressing the human cancer cell lines (47, 48). Our results suggested that p53 is not the only mediation of apoptotic effects of iberin in neuroblastoma cells. Thus, we conclude that iberin could induce neuroblastoma cell apoptosis by both p53-dependent and p53-independent pathways and may have significant therapeutic effect for tumors in neuroblastoma tumors. Interestingly, iberin is a potent inducer of apoptosis in MYCN-amplified SK-N-BE(2) cells. The data presented in this paper provide the first evidence that the isothiocyanate iberin causes apoptosis in human neuroblastoma cell lines and we demonstrated that apoptosis induced by iberin was independent of p53 and MYCN alterations.
Cell cycle control is a highly regulated process that involves a complex cascade of events. Modulation of the expression and function of the cell cycle regulatory proteins including Cdks provides an important mechanism for inhibition of growth. Isothiocyanates have been shown capable of blocking cell cycle progression through the inhibition of multiple CDK activity (49,50). The exact molecular targets of iberin are currently unknown. Cell cycle arrest occurs by loss in the activity of cdks and we tested the hypothesis that iberin will impart antiproliferative effects through cyclin dependent kinase (CDK) machinery. We next investigated by western blotting analysis the effects of iberin on the expression of CDKs in neuroblastoma cells, the major regulators of the cell cycle. The results from the immunoblotting analyses demonstrated that iberin did affect the intracellular protein levels of Cdk2, and Cdk6; however, the levels of Cdk4 protein were down-regulated in a concentration-dependent manner. Our data therefore indicate that iberin has specific mechanisms for inducing cell cycle arrest.
Apoptosis is suggested as one of the major mechanisms for the targeted therapy of various cancers including neuroblastoma (51,52), since impaired apoptosis is involved in the pathogenesis of cancer. Thus, apoptosis is an emerging therapeutic target of bioactive agents of diet (53). Apoptosis involves activation of members of the caspase family of cysteine proteases in a hierarchical cascade, with caspases functioning as triggers and executioners of the apoptotic process. This may be regulated by various mitochondrial apoptogenic-mediators. Caspase-3 is a major executioner protease, responsible for initiating the apoptotic program and it is activated via cleavage by other caspases including caspase-9 (54,55). The implication of caspase 3 in the apoptotic mechanism has been described previously in other systems. To explore the possible mechanisms of iberin-induced apoptosis, the expression and activation of caspase-3, caspase-9, and PARP were examined by western blotting. Activation of caspase-9 and caspase-3 has been recognized as hallmarks of mitochondrial cell death in a variety of different cell types (56). Iberin produced the cleavage of procaspase-3 and caused specific cleavage of the caspase-3 substrate PARP, indicating specific evidence of apoptosis. Taken together, these findings indicate that iberin-induced apoptosis of human neuroblastoma cells is mediated via caspase activation and the associated events.
To our knowledge, ours is the first systematic study to demonstrate the direct, selective anti-proliferative/pro-apoptotic effects of iberin against human neuroblastoma cells. In conclusion, our data demonstrate that MYCN-amplified or unamplified neuroblastoma cells with normal or functionally defective p53 can be induced to undergo apoptosis by relatively low concentrations of iberin and that this response is proceeded by cell cycle arrest associated with caspase activation. This raises the possibility that iberin, at physiologically attainable concentrations, may have chemopreventive and even therapeutic potential for human neuroblastoma. Our present findings warrant its further in vivo efficacy studies in preclinical human neuroblastoma cancer models.
Acknowledgments
This work was supported in part by Peoria NEXT grant and NIH Grant R01-NS-051625 (to S.M.).
Abbreviations
- CDK
cyclin-dependent kinase
- DAPI
4′, 6-diamino-2-phenylindole
- FLICA
fluorochrome labeled inhibitor of caspases
- LDH
lactate dehydrogenase
- MTT
3-(4,5 dimethyl-2 thiazolyl)-2,5 diphenyl-2H tetrazolium bromide
- PARP
poly (ADP-ribose) polymerase
- PI
propidium iodide
References
- 1.Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Gemignani F, Chabrier A, Hall J, Hung RJ, Boffetta P, Canzian F. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomization approach. Lancet. 2005;366:1558–1560. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 2.Hu J, La Vecchia C, Negri E, Chatenoud L, Bosetti C, Jia X, Liu R, Huang G, Bi D, Wang C. Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer. 1999;81:20–23. doi: 10.1002/(sici)1097-0215(19990331)81:1<20::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132:2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA. Cancer prevention by natural compounds. Drug Metab Pharmacokinet. 2004;19:245–263. doi: 10.2133/dmpk.19.245. [DOI] [PubMed] [Google Scholar]
- 5.Keck AS, Finley JW. Cruciferous vegetables: cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr Cancer Ther. 2004;3:5–12. doi: 10.1177/1534735403261831. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc Nutr Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- 7.Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug News Perspectives. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 9.Bianchini F, Vainio H. Isothiocyanates in cancer prevention. Drug Metab Rev. 2004;36:655–667. doi: 10.1081/dmr-200033468. [DOI] [PubMed] [Google Scholar]
- 10.Plate AY, Gallaher DD. Effects of indole-3-carbinol and phenethyl isothiocyanate on colon carcinogenesis induced by azoxymethane in rats. Carcinogenesis. 2006;27:287–292. doi: 10.1093/carcin/bgi210. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 13.Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer. 2005;117:356–362. doi: 10.1002/ijc.21191. [DOI] [PubMed] [Google Scholar]
- 14.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 15.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 16.Jakubikova J, Bao Y, Sedlak J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005;25:3375–3386. [PubMed] [Google Scholar]
- 17.Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 18.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 19.Solt DB, Chang K, Helenowski I, Rademaker AW. Phenethyl isothiocyanate inhibits nitrosamine carcinogenesis in a model for study of oral cancer chemoprevention. Cancer Lett. 2003;202:147–152. doi: 10.1016/j.canlet.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Staack R, Kingston S, Wallig MA, Jeffery EH. A comparison of the individual and collective effects of four glucosinolate breakdown products from brussels sprouts on induction of detoxification enzymes. Toxicol Appl Pharmacol. 1998;149:17–23. doi: 10.1006/taap.1997.8340. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Wang S, Howie AF, Beckett GJ, Mithen R, Bao Y. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. J Agric Food Chem. 2005;53:1417–1421. doi: 10.1021/jf048153j. [DOI] [PubMed] [Google Scholar]
- 22.Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 23.de Cremoux P, Jourdan-Da-Silva N, Couturier J, Tran-Perennou C, Schleiermacher G, Fehlbaum P, Doz F, Mosseri V, Delattre O, Klijanienko J, Vielh P, Michon J. Role of chemotherapy resistance genes in outcome of neuroblastoma. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20853. [DOI] [PubMed] [Google Scholar]
- 24.Vaughn SF, Berhow MA. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Industr Crops Prod. 2005;21:193–202. [Google Scholar]
- 25.Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22:392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- 26.Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000;259:308–313. doi: 10.1006/excr.2000.4955. [DOI] [PubMed] [Google Scholar]
- 27.Carnero A. Targeting the cell cycle for cancer therapy. Br J Cancer. 2002;87:129–133. doi: 10.1038/sj.bjc.6600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 29.Zhang SM, Hunter DJ, Rosner BA, Giovannucci EL, Colditz GA, Speizer FE, Willett WC. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin’s lymphoma among women. Cancer Epidemiol Biomarkers Prev. 2000;9:477–485. [PubMed] [Google Scholar]
- 30.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 31.Smith TK, Mithen R, Johnson IT. Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis. 2003;24:491–495. doi: 10.1093/carcin/24.3.491. [DOI] [PubMed] [Google Scholar]
- 32.Sticha KR, Kenney PM, Boysen G, Liang H, Su X, Wang M, Upadhyaya P, Hecht SS. Effects of benzyl isothiocyanate and phenethyl isothiocyanate on DNA adduct formation by a mixture of benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Carcinogenesis. 2002;23:1433–1439. doi: 10.1093/carcin/23.9.1433. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc Nutr Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- 34.Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol Cancer Ther. 2005;4:1250–1259. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- 35.Gingras D, Gendron M, Boivin D, Moghrabi A, Theoret Y, Beliveau R. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004;203:35–43. doi: 10.1016/j.canlet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 37.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- 39.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 41.Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, Gamet-Payrastre L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48:198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- 42.Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 43.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, Triche TJ, Reynolds CP. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 44.Fisher DE. The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis. 2001;6:7–15. doi: 10.1023/a:1009659708549. [DOI] [PubMed] [Google Scholar]
- 45.Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 46.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 47.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 48.Wu X, Kassie F, Mersch-Sundermann V. Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds. Mutat Res. 2005;589:81–102. doi: 10.1016/j.mrrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Suh SJ, Moon SK, Kim CH. Raphanus sativus and its isothiocyanates inhibit vascular smooth muscle cells proliferation and induce G(1) cell cycle arrest. Int Immunopharmacol. 2006;6:854–861. doi: 10.1016/j.intimp.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Lee JY, Moon SK, Hwang CW, Nam KS, Kim YK, Yoon HD, Kim MG, Kim CH. A novel function of benzyl isothiocyanate in vascular smooth muscle cells: the role of ERK1/2, cell cycle regulation, and matrix metalloproteinase-9. J Cell Physiol. 2005;203:493–500. doi: 10.1002/jcp.20257. [DOI] [PubMed] [Google Scholar]
- 51.Nimmanapalli R, Bhalla K. Targets in apoptosis signaling: promise of selective anticancer therapy. Methods in Molecular Biology. 2003;223:465–483. doi: 10.1385/1-59259-329-1:465. [DOI] [PubMed] [Google Scholar]
- 52.Izbicka E, Izbicki T. Therapeutic strategies for the treatment of neuroblastoma. Curr Opin Investig Drugs. 2005;6:1200–1214. [PubMed] [Google Scholar]
- 53.Martin KR. Targeting apoptosis with dietary bioactive agents. Exp Biol Med. 2006;231:117–129. doi: 10.1177/153537020623100201. [DOI] [PubMed] [Google Scholar]
- 54.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29:486–494. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]