Abstract
The immunoregulatory cytokine IL-10 plays an essential role in down-modulating adaptive and innate immune responses leading to chronic inflammatory diseases. In contrast, cysteinyl leukotrienes (cysLTs), important proinflammatory mediators of cell trafficking and innate immune responses, are believed to enhance immune reactions in the pathogenesis of diseases, such as bronchial asthma, atherosclerosis and pulmonary fibrosis. The aim of this study was to determine the IL-10 regulatory role in cysLT-induced activation of human monocytes and monocyte-derived dendritic cells. Here we show that cysLT-induced activation and chemotaxis of human monocytes and monocyte-derived immature dendritic cells (iDC) are inhibited by IL-10 pretreatment. IL-10 down-regulated cysteinyl leukotriene type 1 and 2 receptors mRNA in a time and a concentration dependent fashion. CysLT induced activation of monocytes and iDCs measured by intracellular calcium flux and immediate-early gene expression (FBJ murine osteosarcoma viral oncogen homolog B and early growth response-2) was potently decreased by IL-10 and by the cysLT antagonist, MK571. Chemotaxis of monocytes and iDCs to increasing concentrations of leukotriene D4 (LTD4) was also inhibited by IL-10. LTD4 enhanced iDC migration in response to CCL5. IL-10 selectively inhibited LTD4-induced chemotaxis without affecting migration to CCL5. These data indicate that cysLT-induced activation of human monocytes and dendritic cells may be specifically inhibited by IL-10, suggesting a direct link between the 5-lipoxygenase proinflammatory pathway and IL-10 regulatory mechanisms. Antileukotriene therapies may reproduce some regulatory mechanisms played by IL-10 in inflammatory processes.
Keywords: Human, dendritic cells, cytokines, lipid mediators, inflammation
Introduction
Interleukin-10 (IL-10) is a major regulatory cytokine of inflammatory reactions. It was originally described as cytokine synthesis inhibitor produced by T helper 2 cells (TH2)(1) and its main biological functions seem to be to limit inflammatory responses, block pro-inflammatory cytokine secretion and regulate the differentiation and proliferation of several immune cells such as T cells, B cells, natural killer cells, dendritic cells and mast cells. There is emerging evidence for a major role of IL-10 in controlling allergic inflammatory processes. Respiratory exposure to allergen may induce T cell tolerance and protection against the development of airway hyperreactivity and asthma. It has been shown that IL-10 production by DCs is critical for the induction of tolerance (2), and that TH2 responses, characteristic for allergic manifestations, can be regulated by naturally occurring IL-10 secreting CD4+ regulatory T cells (3). In addition, successful allergen-specific desensitization therapy is believed to work through the action of IL-10 produced by regulatory T cells (4). Significantly less IL-10 is found in the lungs of patients with asthma (5) and an inverse association between IL-10 levels and the severity of allergic and asthmatic disease has been described (6). Collectively, these data indicate that IL-10 plays an important role in regulation of inflammatory responses leading to asthma and allergy.
Cysteinyl leukotrienes (cysLTs) are lipid mediators derived from arachidonic acid through the 5-lipoxygenase pathway that are directly involved in pathogenesis of a variety of chronic inflammatory disorders, including bronchial asthma and allergic diseases. Many studies have elucidated the role of cysLTs in the effector phase of immediate hypersensitivity, showing its potent bronchoconstricting and vasodilatory activity (7). Data support the concept of cysLTs acting also as important mediators of the early stage of immune responses. Robbiani et al. (8) showed that cysLTs are necessary for DC migration to lymph nodes. It has been shown in a murine model of pulmonary inflammation that cysLTs are required for initiation and amplification of antigen-specific TH2-dependent inflammatory responses (9), that cysLTs may modify the cytokine profile of DCs (10) and that cysLTs may potentiate the alloantigen-presenting capacity of DCs (11), underlining the importance of cysLT signaling in specific immune responses. In asthmatic patients, peripheral blood DCs migrate into the bronchial mucosa after allergen exposure and inhibition of cysLT signaling significantly attenuates airway responses and alters circulating DCs (12, 13).
The interactions between pro-inflammatory cysLTs and regulatory IL-10 in human DCs may represent a way to modulate the early phase of specific immune responsiveness. IL-10 plays an important role in down-regulation or modification of DC driven immune responses but the mechanisms by which IL-10 induces changes in these cells are not well understood. In the present study we analyzed the role of IL-10 in the modulation of cysLT-induced activation of human monocytes and monocyte-derived dendritic cells. Our data showed that IL-10 by down-regulating expression for cysLT receptors inhibits cysLT-induced signaling, gene expression and migration of monocytes and DCs. A similar inhibitory effect on cysLT-induced activation of monocytes and DCs was observed when cysLT receptor antagonists were used, suggesting that these drugs could be beneficial in regulating the initial immune response in humans.
Materials and Methods
Materials
Human recombinant GM-CSF, IL-4, IL-10, CCL2, CCL3, CCL5 (R&D Systems, Minneapolis, MN), LTD4, MK571 (Cayman Chemical, Ann Arbor, MI), mouse IgG FITC and PE conjugated isotype controls, anti-CD14 (FITC; M5E2), anti-CD1a (FITC; HI149), anti-CD83 (FITC; HB15e), anti-CD86 (PE; FUN-1), anti-HLA-DR (PE; TU36) (BD Pharmingen, San Diego, CA), anti-CD1c (FITC; AD5-8E7)(Miltenyi Biotec, Auburn, CA), were obtained from the manufacturers.
Cell culture
Human elutriated monocytes from healthy donors were obtained by an institutional review board-approved protocol from the NIH Blood Bank (Bethesda, MD), resuspended in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and 2 mM L-glutamine (Invitrogen) and allowed to rest overnight before experiments at 37°C in a humidified 5% CO2 incubator. More than 95% of cells were CD14+ as determined by flow cytometry with FITC labeled anti-human CD14 using FACScan flow cytometer (BD Biosciences).
Monocyte-derived immature DCs were generated from elutriated monocytes. Cells were cultured under the same conditions as monocytes in the presence of recombinant human IL-4 (20 ng/ml) and GM-CSF (40 ng/ml) for 5–6 days. Fresh medium with cytokines was added at day 3. After 5–6 days of culture cells expressed high levels of CD1a, CD1c, medium level of CD86 and HLA-DR and low levels of CD83 and CD14 and were used as iDCs.
CysLT1 knockdown
For CysLT1 knockdown experiments Silencer Select pre-designed siRNA (5′GGAAAAGGCUGUCUACAUUtt) and Silencer Select Negative Control siRNA were used (Ambion, Austin, TX). Elutriated monocytes (5×106) were nucleofected with 4 μg of negative control or CysLT1 specific siRNA using a Human Monocyte Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer’s protocol. After 24 hours, media was replaced and cells were used for functional studies.
Real-time PCR
Total RNA was extracted from cells using QIA Shredder columns and RNeasy mini kit and was treated with DNase (Qiagen, Valencia, CA) and quantitated using a NanoDrop spectrophotometer (BioLabNet, Great Falls, VA). mRNA expression for selected genes was measured using real-time PCR performed on an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) using the following commercially available probe and primers sets (Applied Biosystems): CysLT1- Hs00272624_s1, CysLT2- Hs00252658_s1, GPR17- Hs00171137_m1, FosB- Hs00171851_m1, Egr2-Hs00166165_m1. CysLT1 alternatively spliced transcript specific mRNA expression was analyzed with previously described primers and probes (14). Reverse transcription and PCR were performed using an RT kit and TaqMan Universal PCR master mix (Applied Biosystems) according to manufacturer’s directions. Relative gene expression was normalized to GAPDH transcripts and calculated as a fold change compared with control.
Calcium mobilization assay
Calcium mobilization experiments were conducted using a FLIPR Calcium 3 assay kit (Molecular Devices, Sunnyvale, CA) according to the manufacturer’s instructions. Cells (2 × 105 cells/well) were plated into Poly-L–Lysine coated 96-well plates and incubated in RPMI 1640 supplemented with 10 mM HEPES and FLIPR 3 assay reagent. After incubation for 1 hour at 37°C, fluorescence was measured every 4 sec. using the FlexStation (Molecular Devices).
Chemotaxis assay
Chemotaxis experiments were performed using a modified Boyden chamber (Neuro Probe, Gaithesburg, MD). Cells were washed and resuspended in RPMI supplemented with 10 mmol/L HEPES, 2 mmol/L L-glutamine and 1% bovine serum albumin (Sigma). Chemoattractants were resuspended in the same medium and added to the lower wells. Controls wells received medium without chemoattractant. Cells (7.5 × 104) were added to the upper wells and separated by a polycarbonate filter with 5 μm pores (Nero Probe). The number of cells migrated to the lower surface of the filter was counted after 2 hours incubation. In some experiments, cells were pretreated with IL-10 (10 ng/ml) overnight or MK571 (100 nmol/L) for 10 min.
Determination of LTC4 production
The concentration of LTC4 produced spontaneously and after treatment during cell cultures was assayed by means of a competitive enzyme immunosorbent assay (Cayman Chem., Ann Arbor, MI) according to manufacturer’s protocol.
Statistical analysis
Data were analyzed by one-way ANOVA or paired and unpaired Student’s t tests, as appropriate. Differences were considered significant when p < 0.05.
Results
IL-10 downregulates cysLT receptors mRNA in human monocytes and iDCs
The biological action of cysLTs is mediated via three known G-protein coupled receptors, cysLT type I receptor (CysLT1)(15), cysLT type II receptor (CysLT2)(16) and newly characterized GPR17 (17). We have recently shown that CysLT1 mRNA is predominantly expressed in human monocytes and that cysLTs acting through CysLT1 can significantly influence the activation and migration of human monocytes (14). To analyze cysLT induced activities, first steady state mRNA levels for 3 cysLT receptors in elutriated monocytes and monocyte-derived immature DCs (described here as iDCs) were analyzed. Differentiation of monocytes into iDCs was induced by culturing monocytes in the presence of GM-CSF and IL-4 for 5–6 days. Cultured cells expressed high levels of CD1a, CD1c, intermediate levels of CD86 and HLA-DR and low levels of CD83 and CD14 as determined by flow cytometry, characteristic for iDCs. mRNA expression of cysLT receptors in monocytes and iDCs obtained from 4 separate healthy donors and expressed as relative number of specific transcripts per 1000 control GAPDH transcripts was compared. In human iDCs, CysLT1 mRNA was the most highly expressed (12.95 ± 1.66), followed by 10 times lower levels of CysLT2 (1.43 ± 0.12) and GPR17 mRNA was below the detection limits. In comparison to iDC, human monocytes expressed 2 times lower CysLT1 mRNA (6.17 ± 0.5) and similarly low levels of CysLT2 mRNA (0.4 ± 0.1), with GPR17 mRNA being non detectable. Our data suggest that CysLT1 mRNA is also predominantly expressed in cultured iDCs as in elutriated human monocytes.
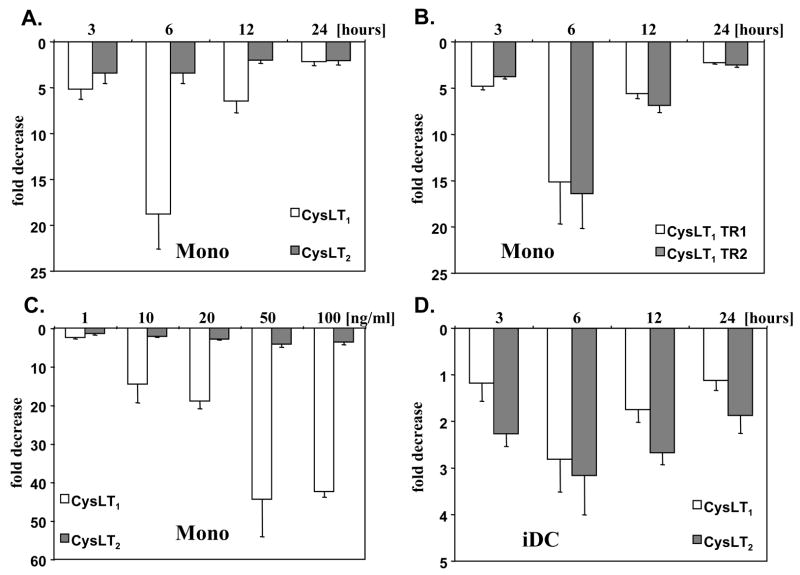
To examine the effects of IL-10 on cysLT receptors mRNA expression in human monocytes, we cultured cells in the presence of IL-10 for up to 24 hours and mRNA for CysLT1 and CysLT2 was measured (Fig. 1 A). IL-10 effectively decreased both, CysLT1 and CysLT2 mRNA expression, with the maximum effect observed after 6 hours. This effect was mediated in a concentration dependent manner (Fig. 1C). The human CysLT1 gene is alternatively spliced in the 5′ untranslated region and 2 transcripts have been detected in human monocytes, both encoding the same protein (18). To further analyze the down regulating effect of IL-10, we measured the expression of CysLT1 splice variants in cells exposed to IL-10 (Fig. 1B). Expression of both CysLT1 transcripts, the transcript 1 (consisting of 3 exons) and the transcript 2 (lacking exon 2) was similarly decreased in a time-dependent manner by IL-10, confirming our data at the level of specific CysLT1 transcripts. We next used cultured iDC to determine whether the similar changes can be induced by IL-10 in these cells. IL-4 is known to up-regulate cysLT receptors expression (18, 19). Therefore, before stimulation with IL-10, iDC were transferred to medium without IL-4. As in monocytes, IL-10 decreased expression of CysLT1 and CysLT2 in iDCs in a time-dependent fashion (Fig. 1D). Both CysLT1 alternative transcripts were also down-regulated in iDCs by IL-10 in a similar way (data not shown). When iDCs were exposed to IL-10 in the presence of IL-4, the down-regulating effect was diminished, suggesting dominant IL-4 up-regulating activity on cysLT receptor expression over the IL-10 down-regulation (data not shown). Altogether these data show that IL-10 effectively down-regulates the expression of cysLT receptors mRNA in human monocytes and iDCs.
Figure 1. IL-10 downregulates cysLT receptors mRNA in human monocytes and iDCs.
Human elutriated monocytes (A) or immature dendritic cells (iDCs) (D) were incubated with or without IL-10 (20 ng/ml) for up to 24 hours or with different concentrations of IL-10 for 6 hours (C) and CysLT1 (white bars) and CysLT2 (grey bars) mRNA expression was measured by TaqMan. Monocytes (B) were stimulated with IL-10 (20 ng/ml) and expression of two CysLT1 alternatively spliced transcripts (CysLT1 TR1, CysLT1 TR2) was measured. Results are normalized to an internal control (GAPDH) and presented as fold decrease from control baseline values. The means ± SD from 3 different donors, done in triplicate, are shown.
In order to analyze the potential role of endogenously produced cysLTs by the studied cells, LTC4 concentrations were measured in media from stimulated and non-stimulated monocytes and iDCs. Very low levels (< 1 nM) of LTC4 were detected in the cell culture media, suggesting a minor role of endogenously produced cysLT in our in vitro experiments (data not shown).
IL-10 inhibits cysLT-induced activation of monocytes and iDCs
To determine whether IL-10 induced changes in mRNA levels correlate with monocyte and iDC responsiveness to cysLTs, we performed calcium flux experiments. Elutriated monocytes were incubated with or without IL-10 (20 ng/ml) overnight, stimulated with different concentrations of LTD4 and intracellular calcium responses were measured. LTD4 induced a concentration dependent intracellular calcium flux in monocytes that was potently inhibited by IL-10 pretreatment (Fig. 2A). Similarly, iDCs treated overnight with IL-10 (20 ng/ml) showed decreased calcium responsiveness to a range of LTD4 concentrations (Fig. 2B). For comparison, intracellular calcium flux was fully inhibited in monocytes and iDCs by the selective CysLT1 inhibitor, MK571 (1 μM), suggesting that in both cell types LTD4 induced calcium signaling through a CysLT1 dependent pathway (data not shown). To further define the role of IL-10 in cysLT-induced activation of monocytes and iDCs, we tested the response to LTD4 at the gene expression level. We have previously showed that LTD4 induces in human monocytes several immediate-early genes, such as FBJ murine osteosarcoma viral oncogene homolog B (FosB) and early growth response 2 (Egr2).(14) IL-10 significantly inhibited LTD4 induced FosB and Egr2 mRNA expression in monocytes (Fig. 3A). The inhibitory effect of IL-10 was similar to the effect obtained by the CysLT1 inhibitor MK571. The effect of cysLTs on gene expression in human iDC is not known. We tested whether the similar immediate-early genes as in monocytes can be induced in cultured iDCs. Stimulation of iDCs with LTD4 (100 nM) for 1 hour caused a significant increase in mRNA expression of FosB and Egr2 (Fig. 3B). This effect was effectively inhibited by MK571 (1 μM) as well as IL-10, suggesting that a similar pathway of gene expression activation is present in monocytes nd iDCs and that IL-10 significantly inhibits cysLT induced activation of these cells.
Figure 2. IL-10 inhibits calcium mobilization responses to LTD4.
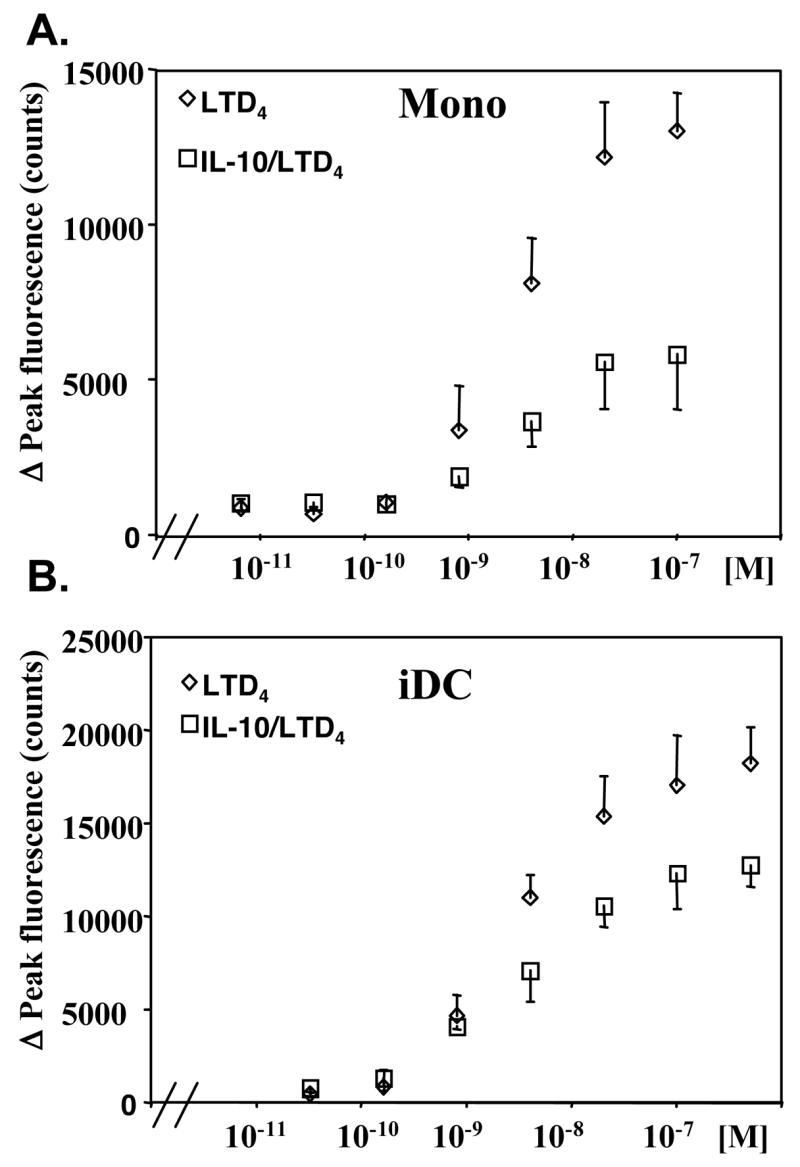
Monocytes (A) and iDCs (B) were incubated with (open square) or without (open diamond) IL-10 (20 ng/ml) overnight and calcium release was measured in response to different concentrations of LTD4 as described in the Methods section. Data are presented as means ± SD from 3 separate experiments performed in triplicate.
Figure 3. IL-10 inhibits LTD4 induced gene expression.
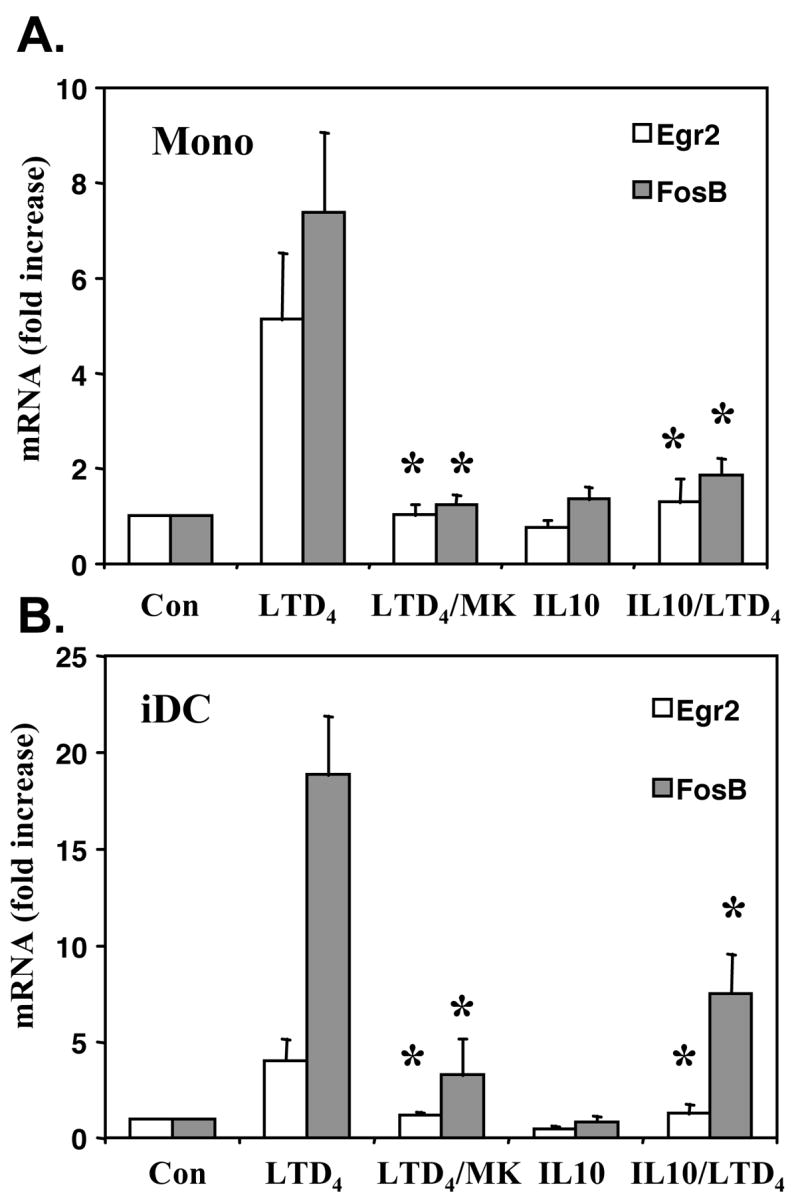
Monocytes (A) and iDCs (B) were incubated overnight with or without IL-10 (20ng/ml) and stimulated with LTD4 (100 nM) for one hour. In some experiments cells were exposed to MK571 (MK) (1 μM) for 5 minutes before stimulation with LTD4. Expression of mRNA for Egr2 and FosB was measured by TaqMan. Results are normalized to an internal control (GAPDH) and presented as fold increase from control vehicle (ethanol) treated samples. The means ± SD from 3 different donors, done in triplicate, are shown. p < 0.001, in comparison to LTD4 stimulated cells, by Student’s t-test.
CysLT1 knockdown inhibits cysLT-induced activation of monocytes
To confirm that cysLT-induced activation of the studied cells is mediated mainly through CysLT1 we nucleofected elutriated monocytes with CysLT1 siRNA or negative control siRNA to knockdown the receptor. Treatment with CysLT1 specific siRNA resulted in a significant decrease in CysLT1 mRNA (Fig. 4A). To determine whether CysLT1 knockdown correlates with monocyte responsiveness to cysLTs, calcium flux and gene expression experiments were performed. CysLT1 knockdown effectively decreased LTD4 induced calcium flux (Fig. 4B) and LTD4 induced FosB expression (Fig. 4C) confirming that LTD4 signals through CysLT1 in monocytes to induce intracellular calcium and gene expression. The effects obtained by CysLT1 knockdown were similar to IL-10 mediated changes in LTD4-induced activation of monocytes.
Figure 4. CysLT1 knockdown inhibits LTD4-induced activation of monocytes.
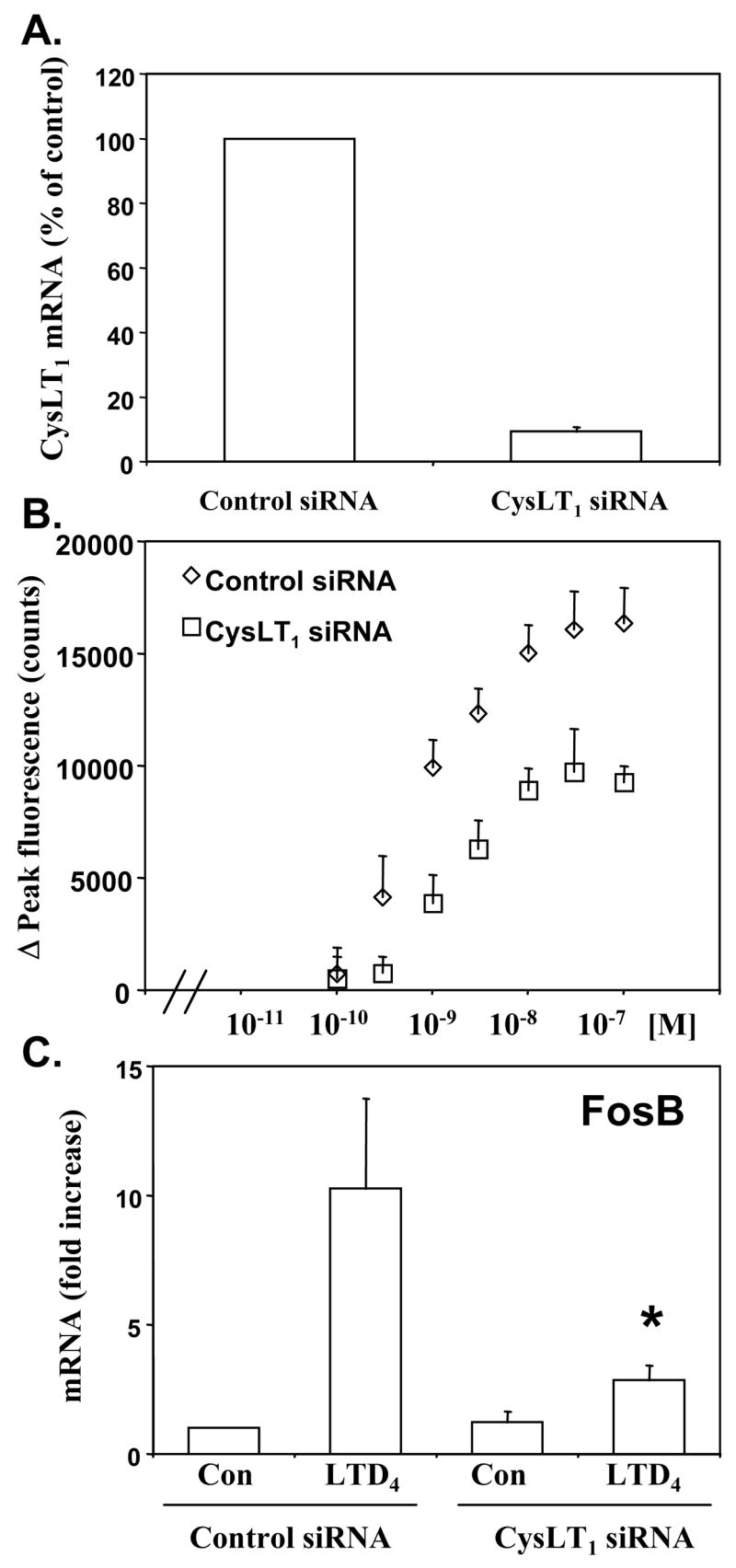
Monocytes were nucleofected with CysLT1 siRNA or negative control oligonucleotides (control siRNA) as described in the Methods section, cultured for 24 hours and CysLT1 mRNA was measured by TaqMan (A), calcium flux was measured in response to LTD4 (B) or LTD4-induced (100 nM) FosB mRNA expression was measured by TaqMan (C). TaqMan results are normalized to an internal control (GAPDH) and presented as fold increase from control (control siRNA) treated samples. The means ± SD from 3 donors, performed in triplicate are shown. * p < 0.001 in comparison to LTD4 stimulated control cells, by Student’s t-test.
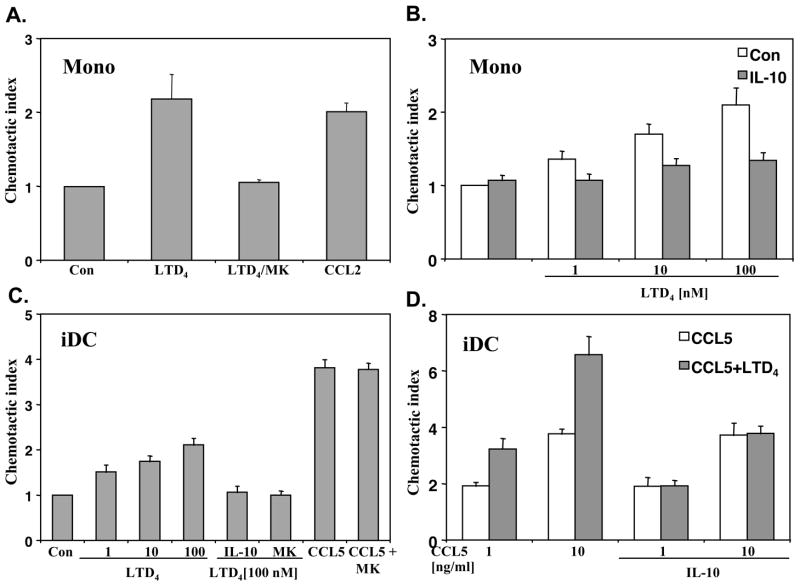
IL-10 inhibits LTD4 induced chemotaxis of monocytes and iDCs
To address the question of cysLT effects on monocyte and iDC migration we studied in vitro migration to a range of LTD4 concentrations. Monocytes and iDCs showed a significant concentration-dependent chemotactic activity that was fully inhibited by pretreatment of cells with MK571 (Fig. 5 A–C), consistent with previous observations (14, 20). IL-10 also effectively inhibited LTD4 induced migration of monocytes and iDCs (Fig. 5 B–C), confirming its down-regulating activity on the cysLT pathway. We next tested whether human iDC migration might be enhanced by cysLTs. Human iDCs respond chemotactically to CCL3, CCL5 and CCL20 (21). When cultured iDCs were pretreated with LTD4 for 30–60 min. and chemotaxis to CCL5 or CCL3 was measured, an increased chemotaxis was not observed (data not shown). However, chemotaxis to CCL5 was potently enhanced by LTD4 co-stimulation (Fig. 5D). A similar additive effect of LTD4 was observed for another chemokine, CCL3 (data not shown). Interestingly, IL-10 preincubation inhibited specifically only LTD4 enhanced migration, without affecting CCL5 chemotaxis (Fig. 5D). These data suggest that cysLTs and IL-10 might play a regulatory role in iDC migration.
Figure 5. IL-10 inhibits LTD4 induced chemotaxis.
Chemotactic activity of monocytes (A) to LTD4 (100 nM) or CCL2 (5 nM) as a positive control was measured as described in the Methods section. Where indicated cells were preincubated with MK571 (MK)(1 μM) for 10 min. before stimulation with LTD4. Monocytes (B) were incubated with (gray bars) or without (white bars) IL-10 (20 ng/ml) overnight and chemotaxis was measured in response to different concentrations of LTD4. Chemotactic activity of iDCs (C) was measured in response to LTD4 or CCL5 (10 ng/ml). Where indicated cells were treated with MK571 (MK) (1 μM) or IL-10 (20 ng/ml) overnight. Chemotactic activity of iDCs (D) in response to CCL5 (white bars) and CCL5 + LTD4 (100 nM) (gray bars) was measured with or without preincubation with IL-10 (20 ng/ml) overnight. Data from 3 different donors are presented as the migration index in comparison with vehicle controls (means ± SD).
Discussion
Several studies suggested IL-10 as a major regulatory cytokine involved in modulation of inflammatory reactions leading to allergic inflammation and asthma (3, 22). Consistent with this hypothesis are observations that less IL-10 is produced by alveolar macrophages and peripheral blood mononuclear cells of patients with asthma and that decreased IL-10 levels correlated with the severity of asthmatic disease (5, 6). In IL-10 knockout mice an exaggerated allergen-induced airway inflammation was observed compared to wild type mice (23). Another line of evidence for the IL-10 involvement in presentation of asthma was revealed by genetic association study, showing that a haplotype related to low IL-10 production was found significantly more often in patients with severe asthma (24). However, the mechanism of an IL-10 regulatory role in asthma and allergic diseases has not been fully defined. Early studies suggested that IL-10 can inhibit allergic inflammation by reducing inflammatory cytokine and chemokine production as well as antigen presentation and dendritic cell maturation (25–27). Recent observations emphasized the role of IL-10 in promoting the induction of IL-10 secreting regulatory T cells and in regulation of tolerance to an antigen (3).
We propose here a new mechanism by which IL-10 can regulate allergic inflammation. The biosynthesis of cysLTs in the airways is a key component of asthma pathogenesis and allergic reactions. Our data suggest that IL-10 through downregulation of cysLT receptors inhibits cysLT-induced signaling, gene expression and migration of human monocytes and iDCs. CysLTs induce in human monocytes expression of several immediate-early genes, acting through CysLT1 (14). A similar acitivity of cysLTs can be demonstrated in human iDC, as LTD4 stimulation potently increased expression of two immediate-early genes, FosB and Egr2 in these cells. Similar to cysLTs signaling in monocytes, cysLT-induced calcium flux and gene expression in iDCs were fully inhibited by the CysLT1 specific antagonist, MK571, suggesting a dominant role of CysLT1 signaling in iDCs, too. The role of cysLT-induced immediate-early genes in monocytes and iDCs has not been studied. Interestingly, both families of transcription factors (Egr and Fos) have been shown to be involved in the regulation of cell differentiation and inflammatory processes (28, 29). CysLTs can activate the NF-κB pathway in lung derived mononuclear cells (30) and direct interactions between NF-κB proteins and Egr and Fos proteins in the regulation of inflammatory gene expression have been demonstrated (31, 32). It has also been shown that monocyte-derived DCs require for differentiation multidrug resistance protein 1 (ABCC1) transporter activity, which actively transports LTC4 out of the cell (33). Altogether, these data suggest that cysLTs may play an important role in induction and modulation of proinflammatory phenotypes in monocytes or iDCs. The observation that IL-10 can effectively inhibit cysLT-induced signaling and gene expression suggests a new mechanism for IL-10 modulatory action in cysLT related inflammatory processes.
Airway DCs are a very dynamic population that can rapidly increase in number following local inflammatory stimuli. Inhalation of allergen by atopic asthmatics induces release of mediators such as histamine and cysLTs causing immediate bronchoconstriction, which is followed then by a late reaction, characterized by inflammatory cell infiltration. Allergen challenge induces a rapid decrease in the number of circulating DCs (34) which are recruited to the bronchial mucosa (12), where they can acquire antigen and carry it to regional lymph nodes. Monocytes have been mainly viewed as a pool of precursor cells for tissue macrophages and DCs that does not participate directly in the immune response, but it has been shown recently that monocytes can process antigen in the bone marrow and effectively present it later in lymph nodes (35). It has been demonstrated that activity of cysLTs was crucial for CCL19 induced migration of DCs to lymph nodes (8). Pretreatment of DCs with LTD4 significantly increased chemotaxis to CCL19, suggesting the chemotaxis promoting activity of cysLTs. We did not observe such LTD4 promoting activity in iDCs in response to CCL3 or CCL5. We showed that cysLTs can induce direct chemotaxis of monocytes and iDCs, consistent with other observations (19, 20) and that chemotaxis to these chemokines can be further enhanced by coincubation with LTD4. Monocyte-derived iDCs do not express CCR6 (chemotactic receptor for CCL20) and CCR7 (chemotactic receptor for CCL19), similar to human peripheral blood CD11c+ iDCs (21), so chemotaxis to CCL19 and CCL20 could not be assessed in our model. It is possible that cysLTs may have a different activity in promoting chemotaxis depending on the level of DCs maturation. CysLTs may enhance chemotaxis of iDCs in response to CCL3 and CCL5 and have promoting activity in mature DCs in response to CCL19.
When cysLTs are released after allergen exposure in vivo, they can increase migration of circulating iDCs to inflamed tissue and potentially only cells expressing cysLT receptors should be specifically affected. IL-10, in contrast, by down-regulating cysLT receptors could have an inhibitory effect on migration and activation of a subpopulation of circulating DCs. It is interesting to note that the same inhibitory activity on iDC migration was observed also after CysLT1 antagonist treatment. It has been shown in asthmatic patients that treatment with the cysLT antagonist pranlukast attenuated not only allergen induced methacholine airway hyperresponsiveness, but it also affected the circulating DC population (13). The increased airway cysLT levels in asthmatic patients may be involved in enhanced mobilization of iDCs from the circulation through two mechanisms. First, our data suggest that cysLTs acting with other chemokines can directly attract iDCs to inflamed airways. The second mechanism can be related to cysLT induced chemokine production in the lung. It has been shown that cysLTs can increase production of chemokines chemotactic for iDC, such as CCL3 and CCL5 and this effect may be prevented by cysLT antagonist treatment (13, 30). Described here, the inhibitory action of IL-10 on cysLT induced activation of monocytes and iDCs may be significant for understanding of regulatory processes in allergic inflammation. IL-10 production by DCs or IL-10 secreting T regulatory cells is critical for the induction of tolerance to antigen introduced by the respiratory route (3). In a recent study, Koya et al. (36) demonstrated that IL-10 treated DCs are potent suppressors of the development of airway hyperresponsivenes and TH2 cytokine production.
Our data suggest a new mechanism of IL-10 regulatory activity through inhibition of cysLT-induced activation of monocytes and iDCs. To our knowledge, this is the first evidence that IL-10 can effectively influence a cysLT mediated proinflammatory pathway in human cells. The observation that some of the IL-10 inhibitory activities may be reproduced by cysLT antagonists, might have important implications for pharmacological approaches to interfere with chronic inflammatory reactions.
Abbreviations used in this paper
- CysLTs
cysteinyl leukotrienes
- CysLT1
cysteinyl leukotriene type 1 receptor
- CysLT2
cysteinyl leukotriene type 2 receptor
- FosB
FBJ murine osteosarcoma viral oncogene homolog B
- Egr
early growth response
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 3.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John M, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 6.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 7.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 8.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 10.Machida I, Matsuse H, Kondo Y, Kawano T, Saeki S, Tomari S, Obase Y, Fukushima C, Kohno S. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172:1833–1838. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 11.Okunishi K, Dohi M, Nakagome K, Tanaka R, Yamamoto K. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J Immunol. 2004;173:6393–6402. doi: 10.4049/jimmunol.173.10.6393. [DOI] [PubMed] [Google Scholar]
- 12.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823–826. doi: 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O’Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J Allergy Clin Immunol. 2004;114:73–79. doi: 10.1016/j.jaci.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Woszczek G, Chen LY, Nagineni S, Kern S, Barb J, Munson PJ, Logun C, Danner RL, Shelhamer JH. Leukotriene D4 induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL, Jr, Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 16.Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 17.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woszczek G, Pawliczak R, Qi HY, Nagineni S, Alsaaty S, Logun C, Shelhamer JH. Functional characterization of human cysteinyl leukotriene 1 receptor gene structure. J Immunol. 2005;175:5152–5159. doi: 10.4049/jimmunol.175.8.5152. [DOI] [PubMed] [Google Scholar]
- 19.Thivierge M, Stankova J, Rola-Pleszczynski M. IL-13 and IL-4 up-regulate cysteinyl leukotriene 1 receptor expression in human monocytes and macrophages. J Immunol. 2001;167:2855–2860. doi: 10.4049/jimmunol.167.5.2855. [DOI] [PubMed] [Google Scholar]
- 20.Thivierge M, Stankova J, Rola-Pleszczynski M. Toll-like receptor agonists differentially regulate cysteinyl-leukotriene receptor 1 expression and function in human dendritic cells. J Allergy Clin Immunol. 2006;117:1155–1162. doi: 10.1016/j.jaci.2005.12.1342. [DOI] [PubMed] [Google Scholar]
- 21.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–968. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Tournoy KG, Kips JC, Pauwels RA. Endogenous interleukin-10 suppresses allergen-induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy. 2000;30:775–783. doi: 10.1046/j.1365-2222.2000.00838.x. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Crawley E, Woo P, Barnes PJ. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- 25.Stampfli MR, Cwiartka M, Gajewska BU, Alvarez D, Ritz SA, Inman MD, Xing Z, Jordana M. Interleukin-10 gene transfer to the airway regulates allergic mucosal sensitization in mice. Am J Respir Cell Mol Biol. 1999;21:586–596. doi: 10.1165/ajrcmb.21.5.3755. [DOI] [PubMed] [Google Scholar]
- 26.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–762. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 27.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 28.Kharbanda S, Nakamura T, Stone R, Hass R, Bernstein S, Datta R, Sukhatme VP, Kufe D. Expression of the early growth response 1 and 2 zinc finger genes during induction of monocytic differentiation. J Clin Invest. 1991;88:571–577. doi: 10.1172/JCI115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrovets T, Jendrach M, Rohwedder A, Schule A, Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. 2001;97:3941–3950. doi: 10.1182/blood.v97.12.3941. [DOI] [PubMed] [Google Scholar]
- 30.Kawano T, Matsuse H, Kondo Y, Machida I, Saeki S, Tomari S, Mitsuta K, Obase Y, Fukushima C, Shimoda T, Kohno S. Cysteinyl leukotrienes induce nuclear factor kappa b activation and RANTES production in a murine model of asthma. J Allergy Clin Immunol. 2003;112:369–374. doi: 10.1067/mai.2003.1636. [DOI] [PubMed] [Google Scholar]
- 31.Thompson C, Cloutier A, Bosse Y, Thivierge M, Gouill CL, Larivee P, McDonald PP, Stankova J, Rola-Pleszczynski M. CysLT1 receptor engagement induces activator protein-1- and NF-kappaB-dependent IL-8 expression. Am J Respir Cell Mol Biol. 2006;35:697–704. doi: 10.1165/rcmb.2005-0407OC. [DOI] [PubMed] [Google Scholar]
- 32.Wieland GD, Nehmann N, Muller D, Eibel H, Siebenlist U, Suhnel J, Zipfel PF, Skerka C. Early growth response proteins EGR-4 and EGR-3 interact with immune inflammatory mediators NF-kappaB p50 and p65. J Cell Sci. 2005;118:3203–3212. doi: 10.1242/jcs.02445. [DOI] [PubMed] [Google Scholar]
- 33.van de Ven R, de Jong MC, Reurs AW, Schoonderwoerd AJ, Jansen G, Hooijberg JH, Scheffer GL, de Gruijl TD, Scheper RJ. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. 2006;176:5191–5198. doi: 10.4049/jimmunol.176.9.5191. [DOI] [PubMed] [Google Scholar]
- 34.Upham JW, Denburg JA, O’Byrne PM. Rapid response of circulating myeloid dendritic cells to inhaled allergen in asthmatic subjects. Clin Exp Allergy. 2002;32:818–823. doi: 10.1046/j.1365-2222.2002.01375.x. [DOI] [PubMed] [Google Scholar]
- 35.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, Dakhama A, Gelfand EW. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007;119:1241–1250. doi: 10.1016/j.jaci.2007.01.039. [DOI] [PubMed] [Google Scholar]