Abstract
The ultimobranchial body (UBB) is an outpocketing of the fourth pharyngeal pouch that fuses with the thyroid diverticulum, giving rise to calcitonin-producing C-cells. In this study, we demonstrate that the UBB is composed of two types of cells: one expressing T/ebp/Nkx2.1 and the other expressing p63. The former cell type, accounting for a majority of the UBB, requires T/ebp/Nkx2.1 for their survival. In contrast, the p63-positive cells, even in the absence of T/ebp/Nkx2.1 expression, can proliferate and give rise to a vesicular structure that is lined by a monolayer of p63-negative cells, surrounded by a cluster and/or single layer of p63-positive cells, displaying the basal/stem cell phenotype. T/ebp/Nkx2.1 haploinsufficiency causes abnormal fusion of the UBB with the thyroid diverticulum, which stays as a cluster of C-cells around the vesicular structure, similar to the one observed in mice null for T/ebp/Nkx2.1 expression. These results demonstrate that T/ebp/Nkx2.1 plays a role in the survival of UBB cells, their dissemination into the thyroid diverticulum, and the formation of UBB-derived vesicular structure.
Keywords: T/ebp-null mouse, Nkx2.1, Titf1, ultimobranchial body, ultimobranchial body cyst, thyroid, calcitonin, C-cells, development
INTRODUCTION
The thyroid gland has a dual embryonic origin (Biddinger and Ray, 1993; Manley and Capecchi, 1998; Kaufman and Bard, 1999; Di Lauro and De Felice, 2001). The thyroid primordium is derived from a ventral outpocketing of the floor of the pharynx that migrates caudally at midline and then bilaterally to form the isthmus and the thyroid lobes. To each of the lobes, a caudal lateral outpocketing from the fourth pharyngeal pouches, called the ultimobranchial body (UBB), becomes embedded, and its cellular components disseminate within it, ultimately giving rise to the calcitonin-producing parafollicular or C-cells. These series of events take place before embryonic day (E) 14.5-15 in mouse, at which time the thyroid starts producing thyroid hormones (Biddinger and Ray, 1993; Manley and Capecchi, 1998; Kaufman and Bard, 1999; Di Lauro and De Felice, 2001).
The thyroid gland is mainly composed of spherical and/or spheroidal structures called follicles, each of which is further composed of a single layer of epithelial cells, so-called thyroid follicular cells or thyrocytes (Di Lauro and De Felice, 2001). The C-cells consist of only 0.1% of the epithelial mass of the thyroid gland in humans (Biddinger and Ray, 1993). The thyroid hormone precursor colloid is stored in the follicular lumen, a space present inside the follicle. It has been long known that there are at least two kinds of follicles in many mammalian thyroids (Wetzel and Wollman, 1969; Wollman and Neve, 1971a,b). The most common are those responsible for thyroid hormone production. The second kind of follicle is less common and is characterized by a nonhomogeneous or foamy colloid and the presence of occasional ciliated epithelial cells. This second class of follicles are usually found as the so-called mixed follicles, containing typical thyroid epithelium and atypical cells (Wetzel and Wollman, 1969; Wollman and Neve, 1971b). The atypical cells are believed to be derived from the UBB (Wollman and Hilfer, 1978). The solid cell nest (SCN), known as the embryonic remnant of UBB, has been described in human thyroid (Harach, 1988; Cameselle-Teijeiro et al., 1994; Reis-Filho et al., 2003). The SCN is usually admixed with the mixed follicles in up to 81% cases (Harach, 1988; Reis-Filho et al., 2003). The SCN is usually located in the middle third of the thyroid lateral lobes and is composed of both C-cells and main cells that are oval to polygonal in shape (Harach, 1988; Cameselle-Teijeiro et al., 1994; Reis-Filho et al., 2003).
p63 is a member of the p53 tumor-suppressor gene family (Levrero et al., 2000; Irwin and Kaelin, 2001; McKeon, 2004) and is expressed in the basal/stem cells of several types of epithelia, including skin, esophagus, and urethra, as well as secretory epithelial tissues, including lacrimal glands, mammary glands, and prostate glands (Mills et al., 1999; Yang et al., 1999; Di Como et al., 2002; Reis-Filho and Schmitt, 2002; Kurita et al., 2004; McKeon, 2004). Based on these studies, it was proposed that p63 plays a role in commitment, maintenance, and differentiation of the epithelia. In the adult thyroid, p63 is a highly sensitive marker for the main cells of the SCN, whereas C-cells and other thyroid structures are consistently negative for p63 (Reis-Filho et al., 2003; Preto et al., 2004). This finding suggests that the main cells of SCN display a basal/stem cell phenotype and may represent a pool of stem cells of the adult thyroid (Preto et al., 2004).
A homeodomain transcription factor, T/ebp (also called Ttf1, Titf1, or Nkx2.1) (Guazzi et al., 1990; Mizuno et al., 1991), is one of the transcription factors essential for regulating the expression of genes involved in thyroid hormone synthesis as well as thyroid organogenesis (Kimura et al., 1996; De Felice et al., 1998; Mansouri et al., 1998; Damante et al., 2001; Di Lauro and De Felice, 2001; De Felice and Di Lauro, 2004). Mice carrying a null mutation for T/ebp gene die at birth due to profoundly hypoplastic lungs, a defective hypothalamus, and absence of the thyroid and pituitary glands (Kimura et al., 1996). The thyroid diverticulum is present at E10 in these mouse embryos but is lost by E12-E13 through apoptosis (Kimura et al., 1999). At this stage of development, the UBB has not yet met with the thyroid diverticulum. Expression of the T/ebp transcript in the UBB was reported previously (Mansouri et al., 1998; Meunier et al., 2003). Whether T/ebp plays any role in the development of UBB is not known.
In the present study, the development of UBB, from its outpocketing from the fourth pharyngeal pouch to dissemination into the thyroid gland, was studied using T/ebp-null and heterozygous mice and their wild-type littermates. These data suggest a prominent role for T/ebp in the survival of UBB cells, dissemination into the thyroid diverticulum, and the formation of UBB-derived vesicular structure.
RESULTS
T/ebp Is Not Required for the Formation and Migration of the UBB
Previous studies have shown that T/ebp is expressed in the UBB as well as in the thyroid diverticulum during thyroid gland organogenesis (Mansouri et al., 1998; Meunier et al., 2003). To study whether T/ebp plays any role in the formation and migration of the UBB, embryos from wild-type, T/ebp-heterozygous, and T/ebp-null mutant mice were collected at various embryonic stages and subjected to histological analysis. At E12.5, ventrocaudal growth of the endoderm from the fourth pharyngeal pouch was observed in all three genotypes, which formed the UBB as an oval structure with a lumen surrounded by stratified cells (Fig. 1A-C). T/ebp expression was observed in the UBB as well as the cells in the laryngotracheal groove in both wild-type and T/ebp-heterozygous mice, whereas no expression was found in T/ebp-null mutants at either location, as expected (Fig. 1A-C). The size of wild-type UBB was slightly larger than the other two genotypes (average number of cells: wild-type, 102.2 ± 4.8; heterozygous, 87.8 ± 3.6; null, 82.6 ± 1.7). Of interest, in the T/ebp-null mutant, the UBB appeared to migrate in the correct direction, similar to that of wild-type or T/ebp-heterozygous mice (Fig. 1C), even though by this stage due to apoptotic degeneration, the thyroid diverticulum, the destination of the UBB, had already disintegrated (Kimura et al., 1996, 1999). These results suggest that T/ebp expression is not required for UBB formation and migration, and that migration of the UBB is determined independent of T/ebp expression in the UBB cells or presence of the thyroid diverticulum.
Fig. 1.
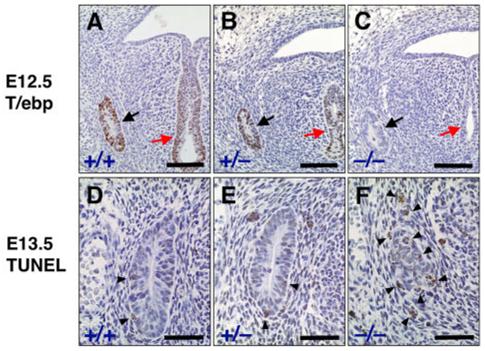
Ultimobranchial body (UBB) of wild-type, T/ebp-heterozygous, and T/ebp-null mutant mice. A-C: Immunostaining for T/ebp on transverse sections of embryonic day (E) 12.5 embryos. T/ebp-positive cells are shown in brown. Dorsal is up. Black arrow indicates UBB. Red arrow points to the laryngotracheal groove. Most cells in the UBB of wild-type (A) and T/ebp-heterozygous mutant mice (B) express T/ebp. Note that the UBB forms and migrates properly in both T/ebp-heterozygous and null mice (B,C) just like wild-type control, as seen in B,C. D-F: Terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) staining of UBBs using transverse sections of E13.5 embryos. Cells stained in brown represent apoptotic cells (shown by arrowheads). Dorsal is up. Note that many cells are positive for apoptosis in the UBB of T/ebp-null mutants (F), whereas a few apoptotic cells are observed in both wild-type (D) and T/ebp-heterozygous mice (E). Scale bar = 100 μm in A-C, 50 μm in D-F.
T/ebp Is Required for the Survival of UBB Cells During Migration
Although there is no significant difference in morphology of the UBB among wild-type, T/ebp-heterozygous, and T/ebp-null mutants at the beginning of its migration, the difference in size between the former two and the latter becomes obvious as their downward migration proceeds. Thus, by E13.5, the UBB of T/ebp-null mutants was considerably smaller compared with that of E12.5, whereas the UBBs of wild-type and T/ebp-heterozygous mice increased in size due to proliferation of cells as they approached the thyroid diverticulum (average number of cells at E13.5: wild-type, 120.4 ± 4.2; heterozygous, 94.8 ± 1.9; null, 39.4 ± 2.3; Fig. 1D-F). To determine whether apoptosis was increased in T/ebp-null mutants, a terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) assay was performed. Several apoptotic cells were detected in the UBB of T/ebp-null mutants, although a few cells in the UBB of wild-type and T/ebp-heterozygous mice were also positive for apoptosis (average number of apoptotic cells: wild-type, 2.6 ± 0.6; heterozygous, 2.4 ± 0.6; null, 9.2 ± 0.8); Fig. 1D-F). These results suggest that T/ebp expression appears to be essential for survival of the UBB cells during its migration. Furthermore, a small subset of cells in the wild-type UBB may also undergo physiological apoptosis during UBB organogenesis.
P63 Is Expressed in a Small Subset of UBB Cells That Survive and Proliferate in the Absence of T/ebp
Because p63 is a highly sensitive marker for the main cells of the SCN in the adult thyroid gland that is considered as an embryonic remnant of the UBB (Reis-Filho et al., 2003; Preto et al., 2004), p63 expression was examined in the UBB during development using immunostaining of E13.5 embryo sections from wild-type, T/ebp-heterozygous, and T/ebp-null mutant mice (Fig. 2A-C). Unexpectedly, in mice of all three genotypes, most cells in the UBBs lacked p63 expression, and only a few p63-positive cells were detected (Fig. 2A-C). Double staining for p63 and T/ebp using wild-type embryo sections showed that p63-positive cells did not express T/ebp. Conversely, T/ebp-positive cells were always negative for p63 (Fig. 2D-F). Thus, p63-positive cells are only a minor constituent in the UBB, and p63- and T/ebp-positive cells are mutually exclusive, suggesting dual origins of the UBB. Of interest, in T/ebp-null mutants, the number of p63-positive cells did not seem to be significantly different between E12.5 and E13.5 (Fig. 3A vs. 2C; average number of p63-positive cells: 6.2 ± 1.3 vs. 6.4 ± 1.1, respectively), despite finding that the total number of UBB cells were markedly reduced approximately by half by E13.5 (from 82.6 ± 1.7 to 39.4 ± 2.3 cells) due to apoptotic degeneration (Figs. 2C, 3A). These results suggest that only p63-negative and T/ebp-positive cells may be susceptible to apoptotic degeneration in the absence of T/ebp.
Fig. 2.
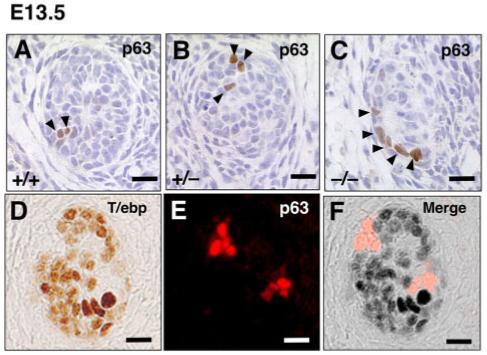
Expression of p63 and T/ebp in ultimobranchial body (UBB) cells. A-C: Transverse sections of UBBs from E13.5 wild-type, T/ebp-heterozygous and T/ebp-null mutant embryos. p63-positive cells are shown in brown. Dorsal is up. Only a few p63-positive cells can be detected per section in all genotypes, and a vast majority of UBB cells are negative for p63. D-F: Double staining for T/ebp (D, brown) and p63 (E, red) using wild-type UBB. Dorsal is up. F: The merged image demonstrates the complementary expression of T/ebp and p63 in the UBB. Scale bar = 20 μm.
Fig. 3.
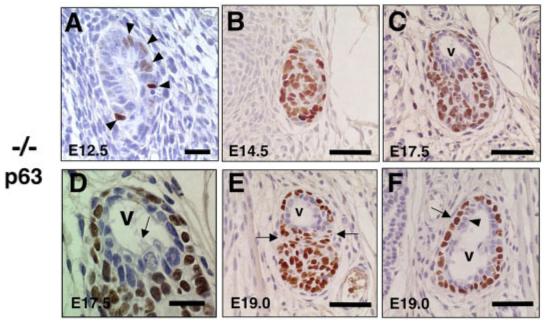
Altered ultimobranchial body (UBB) development and characteristic pattern of p63 expression in T/ebp-null mutants. A-F: Transverse sections of UBBs obtained from various embryonic days of T/ebp-null mutants were subjected to p63 immunostaining. Dorsal is up. Cells stained in brown indicate p63 expression. A: No significant difference was observed in the number of p63-positive cells between E12.5 and E13.5 UBBs (Fig. 2C). B: The p63-positive cells make a solid, oval mass by E14.5 that have taken over most of the UBBs. C,D: By E17.5, a vesicular structure is generated at the dorsal part of the UBB (v in C), which is lined by a monolayer of cells without p63 expression, accompanied by occasional ciliated cells (D, arrow). E: By E19.0, the UBB becomes constricted between the vesicular structure and the mass of p63-positive cells (shown by arrows). F: Cranial to the section shown in E, the vesicular structure displays a well-defined double layer of cells composed of p63-negative inner layer (arrowhead) and p63-positive surrounding layer (arrow). Scale bar = 20 μm in A,D, 50 μm in B,C,E,F.
Of further interest is that the T/ebp-null mutant UBB were diminished in size by E13.5 and began increasing its size at E14.5 (average number of cells: 39.4 ± 2.3 vs. 72.6 ± 3.0, respectively), forming an oval cellular structure, which consists mainly of p63-positive cells (Fig. 2C vs. 3B). By E17.5, the p63-positive population in the UBB of T/ebp-null mutant embryos further increased in cell numbers (from 63.6 ± 3.7 cells at E14.5 to 81.2 ± 2.9 cells at E17.5), and a vesicular structure was generated within a cluster of p63-positive cells that were lined by monolayers of p63-negative cells (Fig. 3C). These structures are never seen in wild-type mouse thyroids (see Fig. 4). Ciliated cells were occasionally found in the lining epithelium of the vesicle (Fig. 3D). This finding demonstrates a striking similarity in appearance with the UB cyst or a second kind of thyroid follicle that has been known for decades (Wetzel and Wollman, 1969; Wollman and Neve, 1971a,b). At E19.0, the vesicle became extended at the peripheral part of the cluster of p63-positive cells (Fig. 3E), which formed a clear bilayer structure consisting of an inner lining of p63-negative cells surrounded by an outer layer of p63-positive cells (Fig. 3F), giving rise to a basal/stem cell appearance (Mills et al., 1999; Yang et al., 1999; Di Como et al., 2002; Reis-Filho and Schmitt, 2002; Kurita et al., 2004; McKeon, 2004). Immunostaining for calcitonin and thyroglobulin (TG) in these sections did not reveal any positives, indicating that the UBB of T/ebp-null mutant embryos do not generate any calcitonin-producing cells and that the vesicle exhibits no colloid retention or TG accumulation in the lumen (data not shown). These findings demonstrate that some UBB cells, including p63-positive cells and vesicular epithelium, survive and proliferate in the absence of T/ebp, and neither calcitonin-producing cells nor UBB cell-derived thyroid follicles are developed if functional T/ebp is not present (see below).
Fig. 4.
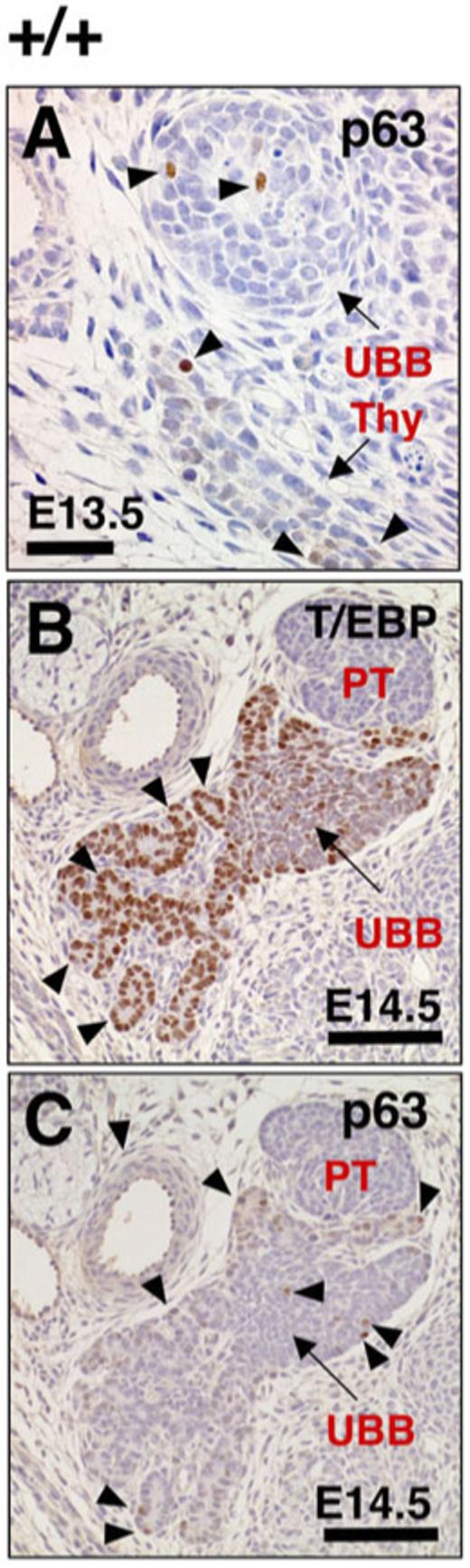
Images of p63 and T/ebp expression in the ultimobranchial body (UBB) and thyroid diverticulum of wild-type embryos. A: The p63 immunostaining can be detected in thyroid diverticulum as well as UBB (arrowheads, shown in brown) in transverse sections of embryonic day (E) 13.5 embryo. Dorsal is up. Thy, thyroid diverticulum. B,C: Immunostaining for T/ebp and p63 (both shown in brown) using serially prepared E14.5 embryo transverse sections. Dorsal is up. The UBB has already been fused but has not yet been completely incorporated into the thyroid diverticulum at this stage. The thyroid diverticulum begins to form many primitive follicles (B, arrowheads) with high levels of T/ebp expression, making a sharp contrast to solid appearance and faint T/ebp expression of partly fused UBB (B, C, arrows). C: P63-positive cells are sparsely distributed in both UBB and primitive thyroid follicles (arrowheads). PT, parathyroid. Scale bar = 50 μm in A,100 μm in B,C.
Strong p63-Positive Cells With Basal/Stem Cell Appearance Arise in the Primitive Follicles of T/ebp-Heterozygous Thyroids
Although p63 is exclusively expressed in the SCN within the adult human thyroid gland (Reis-Filho et al., 2003), expression has also been observed in some cells of the isthmus and the thyroglossal duct cyst (Burstein et al., 2004). Immunostaining of mouse embryos demonstrated that p63-expressing cells exist in both the thyroid diverticulum and UBB. Thus, at E13.5, a stage just before the UBB reaches the thyroid diverticulum, a few cells expressing p63 were detected in the thyroid diverticulum of wild-type (Fig. 4A) and T/ebp-heterozygous mutant (data not shown) embryos. At E14.5, the time when the UBB has already fused with the thyroid diverticulum, these two different tissues remain as separate entities, clearly distinguishable from each other by the intensity of T/EBP expression and by the solid appearance of UBB embedded in the thyroid diverticulum that is composed of a large number of primitive follicles (Fig. 4B). Of particular interest was that only a few cells in the embedded UBB express T/ebp at this stage of wild-type mouse embryo development. It should be noted that no apoptotic cells were found at this stage in wild-type UBB as judged by both the appearance of cells and the TUNEL assay (data not shown), suggesting that the down-regulation of T/ebp expression is not associated with apoptotic degeneration after the UBB has fused with the thyroid diverticulum. Similar to E13.5, p63 expression was mostly negative, and very weak signals were only sparsely detected in both the thyroid diverticulum and the fused UBB cells (Figs. 4C, 5A).
Fig. 5.
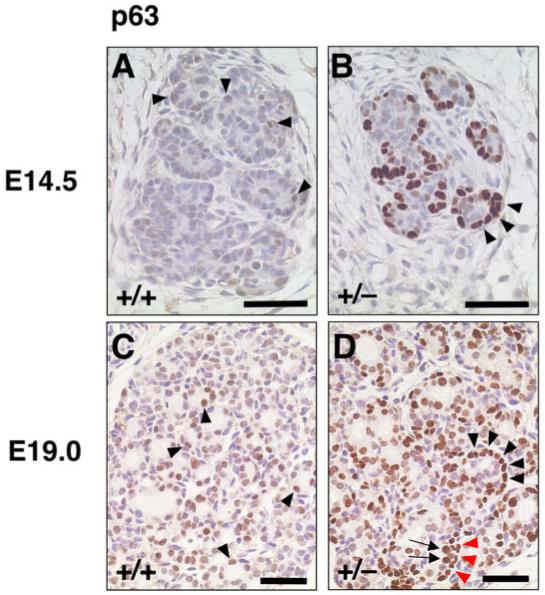
Immunostaining for p63 in primitive thyroid follicles of wild-type and T/ebp-heterozygous mutant embryos. A-D: The p63 expression is shown in brown. Dorsal is up. A,B: At embryonic day (E)14.5, the p63 expression level is mostly low or undetectable in primitive follicles of wild-type embryo thyroids (A, arrowheads), whereas almost all primitive follicles in T/ebp-heterozygous mutant thyroids are clearly rimmed with strongly p63-positive cells (B, arrowheads). C,D: At E19.0, many but not all follicular cells in primitive follicles of wild-type thyroid exhibit low p63 expression (C, arrowheads), whereas strong p63 expression is evident not only in follicular cells of primitive follicles (D, arrows) but also cells surrounding the primitive follicle (D, arrowheads) in T/ebp-heterozygous mutants, clearly showing a bilayered appearance in some part (D, arrows and red arrowheads). Scale bar = 50 μm.
Surprisingly, in marked contrast to wild-type embryos, E14.5 T/ebp-heterozygous embryos demonstrated strong positive p63 staining in several cells in the thyroid diverticulum that were found at the periphery of the primitive thyroid follicles (Fig. 5A vs. B). This staining shows a striking similarity to the pattern of p63 expression found in the basal/stem cells of epithelia such as prostate and breast tissues (Barbareschi et al., 2001; Reis-Filho and Schmitt, 2002; Kurita et al., 2004). These results suggest that the haploinsufficiency of T/ebp expression might lead to the formation of a particular cell type at the periphery of the primitive follicles. By E19.0, p63 begins to be expressed in some cells in the primitive follicles of wild-type mouse thyroids (Fig. 5C). In T/ebp-heterozygous mice, the rim of p63-positive cells surrounding the primitive follicles becomes slightly obscure, although in some cases they are still visible (Fig. 5D). This condition can be seen in as late as 1-month-old thyroids (data not shown). In most newborns, however, the expression of p63 is distinct in thyrocytes and is scattered throughout the thyroid lobes in both wild-type and T/ebp-heterozygous mice (data not shown). In this regard, it is interesting to note that no p63 expression was reported in the thyrocytes of human thyroid gland (Di Como et al., 2002; Reis-Filho et al., 2003).
Haploinsufficiency of the T/ebp Gene Affects Normal Incorporation and Dissemination of UBB Cells Into the Thyroid Lobes
The UBB fuses with the thyroid diverticulum around E14.5 (Fig. 4B). The UBB cells then disseminate into the thyroid diverticulum and are eventually distributed throughout the thyroid lobes and can be seen as calcitonin-positive cells in E19.0 (Fig. 6A,C). In T/ebp-heterozygous mouse embryos, the UBB also fuses with the thyroid diverticulum at the correct developmental timing and place, similar to wild-type embryos (data not shown). However, the UBB of T/ebp-heterozygous embryos when examined at E19.0, appeared to be incompletely incorporated into the thyroid diverticulum and remained at the dorsal part of the thyroid lobe (Fig. 6B,D). This incomplete fusion occurred in all T/ebp-heterozygous embryos examined (five cases), and vesicular structures consistently were seen that were lined by p63-negative cuboidal to columnar epithelium, surrounded by strongly p63-positive cells (Fig. 7A). These structures were identical to those observed in the UBB of T/ebp-null mutants (Fig. 3C-F) and were not found in wild-type thyroids. Unlike the UBB vesicles in the T/ebp-null mutants, however, the vesicular walls in T/ebp-heterozygous mice were intermittent, and partly interrupted by a cluster of C-cell precursors and small thyroid follicles, which expressed calcitonin and T/ebp, respectively (Fig. 7A-C). The calcitonin-producing C-cells were preferentially localized around UBB vesicles and never disseminated into the thyroid lobe (Fig. 6B,D). This finding is in sharp contrast to the even distribution of C-cells throughout the thyroid lobe seen in wild-type mice (Fig. 6A,C). To examine if the UBB defects seen in T/ebp-heterozygous mice merely represent a delayed incorporation of the UBB cells into the thyroid lobe, or indicate complete defects of the UBB dissemination into the thyroid lobe, histological analysis was performed on 10-month-old adult thyroid glands of T/ebp-heterozygous mice. In all cases examined (five cases), the vesicle was observed at the dorsal part of the thyroid gland (Fig. 6E). In the vicinity of the vesicles, clusters of calcitonin-producing cells were found, and some of the lining cells along the vesicle also expressed calcitonin, whereas no calcitonin-positive cells were obtained in the thyroid lobe. These results show incomplete integration of the UBB cells into the thyroid lobe in T/ebp gene haploinsufficiency.
Fig. 6.
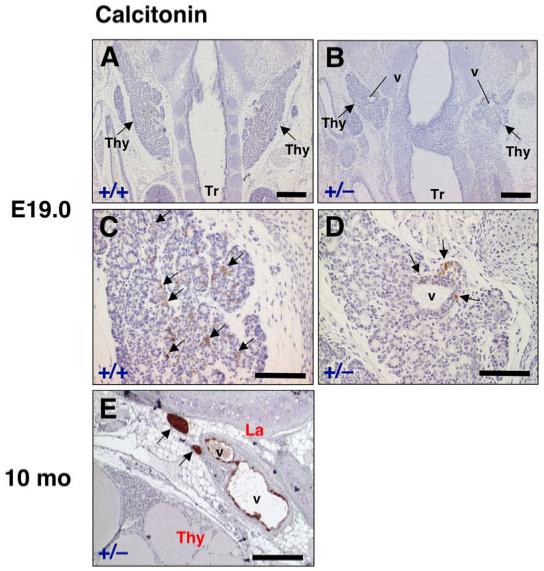
Calcitonin expression in the thyroid of wild-type and T/ebp-heterozygous mutant mice. A-D: Frontal sections of embryonic day (E) 19.0 embryos from wild-type and T/ebp-heterozygous mice were subjected to calcitonin immunostaining. Arrows point to bilateral thyroid lobes (Thy). Tr, trachea. Calcitonin expression is shown in brown. A,B: Cranial is up. T/ebp-heterozygous mutants always form vesicular structures in bilateral thyroid lobes (v in B), whereas the vesicular structure never appears in wild-type (A). C,D: Higher magnification of the same section shown in A and B, respectively. In wild-type, calcitonin-positive cells are diffusely disseminated into the thyroid lobe (C, arrows), whereas in T/ebp-heterozygous mutant, calcitonin expression is found only around the vesicular structure (D, arrows). E: Immunostaining for calcitonin on a thyroid section from 10-month-old adult T/ebp-heterozygous mutant. Dorsal is right. Vesicular structures persist between the thyroid lobe (Thy) and the larynx (La). Calcitonin is expressed in some cells lining the vesicles and strong expression found in clumps of cells localized in the vicinity of the vesicles (arrows). Scale bar = 300 μm in A,B, 100 μm in C,D, 200 μm in E.
Fig. 7.
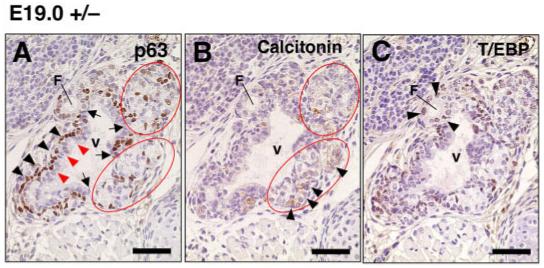
Defective ultimobranchial body (UBB) development and abnormal merging with the thyroid diverticulum. A-C: Immunostaining for p63, calcitonin, and T/ebp using serially prepared sections from embryonic day (E) 19.0 T/ebp-heterozygous mutant embryos. Dorsal is up. The expression is shown in brown for each staining. Vesicular structure (v) consistently appears at the dorsal part of the embryonic thyroid. Similar to the vesicular structures seen in T/ebp-null mutants (Fig. 3C-F), the vesicular wall consists of inner p63-negative (red arrowheads) and outer p63-positive cells (arrowheads). This wall is frequently intermittent (A, arrows) and interrupted by a cluster of C-cell precursors (A, B, circled) and small follicles (F in A-C), which express calcitonin (B, arrowhead) and T/ebp (C, arrowhead), respectively. Scale bar = 50 μm.
DISCUSSION
Role of T/ebp in the UBB Development
In this study, we show that T/ebp is not required for the formation and migration of the UBB but is essential for survival of the UBB cells. This article is the first report on the detailed examination of T/ebp protein expression in individual UBB cells, although the expression of the T/ebp transcript in the UBB was reported previously (Mansouri et al., 1998; Meunier et al., 2003). The results showed that most cells in the UBB of wild-type embryos have strong T/ebp protein expression at levels similar to those seen in cells of the thyroid diverticulum, especially at the beginning of UBB migration. Of interest, p63 is expressed in a small subset of cells that always lack T/ebp expression, indicating that the UBB is organized heterogeneously and may be derived from dual origins. Because T/ebp is expressed in C-cells in addition to thyroid follicular cells (Suzuki et al., 1998) and the UBB is composed mainly of C-cell precursors originated from the neural crest (Pearse and Carvalheira, 1967; Moseley et al., 1968; Pearse and Polak, 1971; Le Lievre and Le Douarin, 1975; Williams et al., 1989), it seems possible that T/ebp-expressing cells that account for the largest part of the UBB may have originated from the neural crest-derived cells. On the other hand, a small number of p63-positive, T/ebp-negative cells in the UBB are likely to have originated from the epithelium of the fourth pharyngeal pouch, in which p63 begins to be expressed around E9.5, a time before the UBB forms (Kusakabe and Kimura, unpublished observation). These p63-positive cells might mingle with the neural crest-derived cells that are infiltrating into the fourth pouch and then migrate together as the UBB.
It was proposed that T/ebp may regulate some genes involved in the maintenance of calcium homeostasis in C-cells (Suzuki et al., 1998). However, the role of T/ebp in C-cells remains largely unclear. The present studies using T/ebp-null mutants demonstrate that (1) calcitonin-producing C-cells are not generated in the absence of T/ebp and (2) without T/ebp gene expression, the majority of UBB cells degenerate due to apoptosis during its migration, although the UBB usually forms normally. These findings suggest that T/ebp may be essential for the differentiation and survival of C-cell precursors but is dispensable for its initial specification. Of interest, the thyroid diverticulum also presents similar abnormalities in the absence of T/ebp; the rudiment forms but undergoes apoptotic degeneration in the early phase of migration (Kimura et al., 1999). Because apoptotic degeneration takes place in both the UBB and thyroid diverticulum while they are migrating, it is tempting to speculate that T/ebp expression during their migration may be particularly crucial for the survival of these organs. Supporting this hypothesis, apoptosis does not occur in the thyroid diverticulum if the T/ebp gene is disrupted after the completion of migration (Kusakabe and Kimura, unpublished observation). Furthermore, even though T/ebp expression becomes almost undetectable in the UBB cells by E14.5, the time at which the UBB meets the thyroid diverticulum and their dissemination begins, no apoptotic cells are observed. Thus, it might be possible that some kind of cell-toxic stress is present when migrating organs are passing through mesenchymal tissues. In this case, T/ebp could exert some protection against stress while the UBB and the thyroid diverticulum are migrating, and this function becomes no longer necessary when migration has ended. Currently, little is known about genes regulated by T/ebp during early thyroid development. Identification of these genes is necessary for further understanding how the T/ebp gene participates in the development of UBB and thyroid gland.
Role of T/ebp in Fusion of the UBB to the Thyroid Diverticulum
It remains unclear how the thyroid diverticulum and the UBB recognize each other and fuse. The present study using T/ebp-heterozygous mutant mice demonstrated that T/ebp gene haploinsufficiency causes defective UBB that partially fuses with the thyroid lobe, and C-cells fail to be disseminated into the thyroid follicular cells, even though the UBB correctly recognizes and reaches the thyroid diverticulum. This failure indicates that full T/ebp activity may be required for proper mixing of UBB cells into thyroid diverticulum. Similar UBB defects have been reported previously in mutant mice of the Hox group 3 paralogous genes (Manley and Capecchi, 1995, 1998) and Eya1 gene (Xu et al., 2002). Manley and Capecchi carefully studied the roles of Hoxa3, Hoxb3, and Hoxd3 genes in the development of both the thyroid diverticulum and UBB by using mutant mice that lack various combinations of these genes (Manley and Capecchi, 1998). Whereas T/ebp-heterozygous mutants had a 100% penetration of partially fused UBB in bilateral lobes of the thyroid, double and triple compound mutants among three Hox group 3 paralogs exhibited various degrees of UBB defects, including partially fused UBB, absence of UBB and a persistent UBB that remains as a distinct entity from the thyroid lobe and contains calcitonin-producing Ccells as well as colloid-containing follicles (Manley and Capecchi, 1998). These defects could even vary between two lobes of the thyroid in the same animal. Partially fused UBB were seen particularly in Hoxa3 and Hoxb3, and Hoxb3 and Hoxd3 double mutants. T/ebp and Hox group 3 paralogs or the combinations of these genes may cooperatively regulate genes in common, which are responsible for the UBB migration and interaction with the thyroid lobes. Based on the fact that the T/ebp-heterozygous mouse always has the same UBB defect, it seems likely that the T/ebp gene pre-dominates over Hox genes in directing development of the UBB. The relationship between T/ebp gene and Hox group 3 genes remains largely unknown. Only Hoxb3 has been shown to regulate T/ebp gene expression by in vitro transient transfection assays (Guazzi et al., 1994), although no defect was found in both the thyroid gland and the UBB of Hoxb3 single mutant mice (Manley and Capecchi, 1998). Considering that Hox genes cooperatively function to mediate a developmental program by controlling common target genes through common cis elements (Manley and Capecchi, 1997) and that a similar UBB defect is found in both T/ebp-heterozygous and some combination of Hox genes mutants, it is tempting to speculate that T/ebp may be one of the target genes regulated by combinations of Hox group3 paralogs. On the other hand, Eya1-null embryos show thyroid hypoplasia with severe reduction in the number of parafollicular cells as well as the size of the thyroid lobes, and the lack of fusion between the UBB and the thyroid lobe (Xu et al., 2002). T/ebp is expressed in both the thyroid diverticulum and the UBB of these embryos at similar levels to that of wild-type, whereas Hoxa3 expression in the pharyngeal arch mesenchyme and pharyngeal pouch endoderm is not affected (Xu et al., 2002). This finding suggests that the UBB defect seen in Eya1-null mutants may be due to additional mechanisms independent of T/ebp and/or Hox group3 paralogs, or Eya 1 may be downstream of T/ebp/Hox group3 paralogs.
Significance of p63 Expression in the UBB and the Thyroid Diverticulum
Previous studies proposed the possibility that UBB might be a source for both follicular cells and C-cells in man (Harach, 1985; Williams et al., 1989). In fact, it was shown in dogs, in which C-cells derived from the UBB remain as C-cell complexes without complete incorporation into the thyroid lobes, that follicular thyroid cells are found within the C-cell complexes that exhibit positive thyroglobulin staining (Kameda and Ikeda, 1980). More recently, a hypothesis was proposed that p63-positive cells in the SCN, known to be a remnant of UBB, display a basal/stem cell phenotype (Reis-Filho et al., 2003) and possess pluripotent stem-cell ability (Burstein et al., 2004). Whether or not p63-positive cells could actually generate both follicular cells and C-cells has not yet been clarified. In this study, we observed that both a cluster of C-cells and mature follicles appeared to be generated directly from the unusual UBB vesicle composed of p63-positive cells in T/ebp-heterozygous mutant mice. It is possible that the T/ebp-negative, p63-positive cells have the potential to differentiate into cells that can express calcitonin and T/ebp. The presence of a functional T/ebp gene seems to be critical for this event to occur because no C-cells or follicles arose around the UBB vesicles in T/ebp-null mutant embryos even though these vesicles had identical characteristics to those of T/ebp-heterozygous mice and were constituted by p63-positive cells. This capacity of p63-expressing cells may be related to the basal/stem cell phenotypes, however the potential for differentiation appears to be exerted only within cells of the thyroid lineage; thus, these cells may not be pluripotent. Recent studies have revealed critical roles for p63 in mouse development (Mills et al., 1999; Yang et al., 1999), although its role in commitment, proliferation, or maintenance of stem cells has yet to be determined (McKeon, 2004). A possibility cannot be excluded, however, that UBB-derived C-cell precursors and/or thyroid diverticulum-derived cells that are T/ebp-heterozygous and are inconspicuously present around the vesicle, may contribute to the development of C-cells and/or follicles. Further experiments are needed to address these questions.
UBB-derived cystic structures, which sometimes appear as a so-called mixed follicle and that contain typical thyroid epithelium and atypical cells (Wetzel and Wollman, 1969; Wollman and Neve, 1971b), have been known for decades in mice and humans (Wetzel and Wollman, 1969; Harach, 1985). In this study, a typical UBB vesicle was not found in wild-type thyroids as characterized by p63-negative cells surrounded by p63-positive cells. It is possible, however, that the UBB-derived cyst and the UBB vesicle we found in T/ebp-heterozygous and T/ebp-null mutant mice are ontogenetically identical. We do not know why typical UBB vesicles are not found in wild-type thyroids. This may be related to the ability of UBB cells to disseminate into thyroid lobes, depending on T/ebp activity. Once UBB cells are completely scattered throughout the thyroid lobes, they might lose the ability to form vesicular structures.
It is of interest that strongly p63-positive cells appear at the periphery of primitive thyroid follicles of only T/ebp-heterozygous mutant mice, showing stem cell-like partitioning of p63 expression. Because p63-null mutant mice present no gross histological abnormalities in the developing thyroid compared with wild-type mice at least at E18.0 (Kusakabe and Kimura, unpublished observation; p63-null embryos were a kind gift from Dr. A. Mills at the Cold Spring Harbor Laboratory), it is evident that p63 is not essential for the commitment of endodermal epithelium to thyroid primordium, nor for thyroid organogenesis. Considering that thyroid diverticulum is significantly smaller in T/ebp-heterozygous mutant mice compared with wild-type (data not shown), strongly p63-positive cells surrounding the primitive follicles found in T/ebp-heterozygous mutant thyroids may have arisen to compensate for the hypoplasia of thyroid diverticulum through an analogous stem cell-based biological process. Further experiments are required to address these questions.
In conclusion, we have shown a critical role for T/ebp in UBB development and its dissemination to the thyroid diverticulum. In the absence of T/ebp, the majority of cells in the UBB cannot survive during development, except for a small group of cells that express p63 that can proliferate and give rise to a vesicular structure. Furthermore, T/ebp haploinsufficiency causes abnormal fusion of the UBB with the thyroid diverticulum, which keeps C-cells from disseminating into thyroid lobes and stays in the vicinity of the vesicular structure, similar to the one observed in mice null for T/ebp expression.
EXPERIMENTAL PROCEDURES
Mouse Genotyping
Mice heterozygous for a disrupted T/ebp gene were intercrossed to produce wild-type, heterozygous, and homozygous embryos. The mutant allele has an insertion of the neo cassette within exon 2 that contains the homeodomain as previously described (Kimura et al., 1996). At noon on the day when a vaginal plug was found was considered as E0.5. Genotyping was performed by polymerase chain reaction (PCR) analysis of yolk sac or tail DNAs using the following PCR conditions: 1 cycle for 3 min at 94°C, 30 cycles for 30 sec at 94°C, 15 sec at 60°C, 15 sec at 72°C, and 1 cycle for 5 min at 72°C. The sequences of the primers used for the detection of wild-type allele were the following: 5′F, 5′-GGCGAGCGGCATGAATATGA-3′ and 3′R, 5′-TCTTGTAGCGGTGGTTCTGGA-3′. The neo primer (5′-TCGCCTTCTATCGCCTTCTTGA-3′) was designed within the neo cassette to be paired with the 3′R primer for the detection of targeted allele.
Immunohistochemistry
For histological analysis, at least five embryos or thyroid glands were used for each genotype and stage analyzed. Morphological abnormalities and status of immunostaining were estimated by serially prepared sections of whole cervical regions or thyroid glands. Mouse embryos collected at different developmental stages and adult thyroid gland (10-month-old) dissected together with the larynx and trachea were fixed with 4% paraformaldehyde overnight at 4°C, embedded in paraffin, and sectioned at 4 μm. Deparaffinized sections were subjected to immunohistochemistry using mouse monoclonal anti-TTF-1 (T/ebp) antibody (1:1,000 dilution; DAKO, Carpinteria, CA), mouse monoclonal anti-p63 antibody (1:1,000 dilution; BD Biosciences, San Diego, CA), and rabbit polyclonal anti-calcitonin antibody (1:1,000 dilution; ICN, Irvine, CA). Briefly, deparaffinized sections were incubated in 1% H2O2 in methanol for 30 min to inactivate endogenous peroxidase, followed by rinsing three times for 10 min each with PBS. Heat-induced epitope retrieval in citrate buffer (0.01 M, pH 6.0) was performed for all immunostainings before tissues were blocked with 5% horse serum for T/ebp and p63 staining, and with goat serum for calcitonin staining. Tissues were incubated overnight at 4°C with first antibodies in a humidified chamber. After rinsing three times in PBS for 10 min, the tissues were processed by the ABC method using commercially available kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Immunocomplexes were visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB; DAKO). Cell numbers were counted on the largest transverse section from each serially sectioned UBB, and the mean was obtained from five UBBs. Cell numbers are expressed as the mean ± SD.
For double immunostaining for p63 and T/ebp, anti-p63 antibody was applied first as described above, then visualized by using Alexa Fluor 594-conjugated anti-mouse IgG (Invitrogen, Carlsbad, CA). After the image of p63 expression was saved using a fluorescent microscope, the section was heated in boiled citrate buffer (0.01 M, pH 6.0) for 10 min to denature bound antibody molecules. This step was necessary to completely block crossreactivity between sequential stainings (Lan et al., 1995), because both primary anti-p63 and anti-TTF1 antibodies used for double staining were raised in mouse. T/ebp staining was carried out as described above, and the expression was visualized with DAB (DAKO).
Detection of Apoptotic Cells
TUNEL assay was performed to detect apoptotic cells in tissue sections using commercially available kit (Promega, Madison, WI). Tissue sections were processed according to the manufacturer’s instruction, through which biotinylated nucleotide was incorporated at the 3′-OH end of fragmented DNAs. Biotin incorporation was detected with horseradish peroxidase-conjugated streptavidin using DAB as a chromogen.
ACNOWLEDGMENTS
We thank Dr. Frank Gonzalez for his critical review of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Grant sponsor: NIH, National Cancer Institute, Center for Cancer Research.
REFERENCES
- Barbareschi M, Pecciarini L, Cangi MG, Macri E, Rizzo A, Viale G, Doglioni C. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–1060. doi: 10.1097/00000478-200108000-00010. [DOI] [PubMed] [Google Scholar]
- Biddinger PW, Ray M. Distribution of C cells in the normal and diseased thyroid gland. Pathol Annu. 1993;28(Pt 1):205–229. [PubMed] [Google Scholar]
- Burstein DE, Nagi C, Wang BY, Unger P. Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto’s thyroiditis: a stem cell hypothesis of papillary carcinoma oncogenesis. Hum Pathol. 2004;35:465–473. doi: 10.1016/j.humpath.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Cameselle-Teijeiro J, Varela-Duran J, Sambade C, Villanueva JP, Varela-Nunez R, Sobrinho-Simoes M. Solid cell nests of the thyroid: light microscopy and immunohistochemical profile. Hum Pathol. 1994;25:684–693. doi: 10.1016/0046-8177(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- De Felice M, Ovitt C, Biffali E, Rodriguez-Mallon A, Arra C, Anastassiadis K, Macchia PE, Mattei MG, Mariano A, Scholer H, Macchia V, Di Lauro R. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–398. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- Di Lauro R, De Felice M. Thyroid gland: anatomy and development. In: DeGroot L, Jameson J, editors. Endocrinology. Saunders; Philadelphia: 2001. pp. 1268–1278. [Google Scholar]
- Guazzi S, Price M, De Felice M, Damante G, Mattei MG, Di Lauro R. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631–3639. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzi S, Lonigro R, Pintonello L, Boncinelli E, Di Lauro R, Mavilio F. The thyroid transcription factor-1 gene is a candidate target for regulation by Hox proteins. EMBO J. 1994;13:3339–3347. doi: 10.1002/j.1460-2075.1994.tb06636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach HR. Solid cell nests of the thyroid. An anatomical survey and immunohistochemical study for the presence of thyroglobulin. Acta Anat. 1985;122:249–253. [PubMed] [Google Scholar]
- Harach HR. Solid cell nests of the thyroid. J Pathol. 1988;155:191–200. doi: 10.1002/path.1711550303. [DOI] [PubMed] [Google Scholar]
- Irwin MS, Kaelin WG., Jr Role of the newer p53 family proteins in malignancy. Apoptosis. 2001;6:17–29. doi: 10.1023/a:1009663809458. [DOI] [PubMed] [Google Scholar]
- Kameda Y, Ikeda A. Immunohistochemical study of the C-cell complex of dog thyroid glands with reference to the reactions of calcitonin, C-thyroglobulin and 19S thyroglobulin. Cell Tissue Res. 1980;208:405–415. doi: 10.1007/BF00233873. [DOI] [PubMed] [Google Scholar]
- Kaufman M, Bard J. The anatomical basis of mouse development. Academic Press; London: 1999. [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ward JM, Minoo P. Thyroid-specific enhancer-binding protein/thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie. 1999;81:321–327. doi: 10.1016/s0300-9084(99)80077-7. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem. 1995;43:97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113(Pt 10):1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev Biol. 1997;192:274–288. doi: 10.1006/dbio.1997.8765. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195:1–15. doi: 10.1006/dbio.1997.8827. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- McKeon F. p63 and the epithelial stem cell: more than status quo? Genes Dev. 2004;18:465–469. doi: 10.1101/gad.1190504. [DOI] [PubMed] [Google Scholar]
- Meunier D, Aubin J, Jeannotte L. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev Dyn. 2003;227:367–378. doi: 10.1002/dvdy.10325. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Gonzalez FJ, Kimura S. Thyroid-specific enhancer-binding protein (T/EBP): cDNA cloning, functional characterization, and structural identity with thyroid transcription factor TTF-1. Mol Cell Biol. 1991;11:4927–4933. doi: 10.1128/mcb.11.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JM, Matthews EW, Breed RH, Galante L, Tse A, MacIntyre I. The ultimobranchial origin of calcitonin. Lancet. 1968;1:108–110. doi: 10.1016/s0140-6736(68)92720-7. [DOI] [PubMed] [Google Scholar]
- Pearse AG, Carvalheira AF. Cytochemical evidence for an ultimobranchial origin of rodent thyroid C cells. Nature. 1967;214:929–930. doi: 10.1038/214929a0. [DOI] [PubMed] [Google Scholar]
- Pearse AG, Polak JM. Cytochemical evidence for the neural crest origin of mammalian ultimobranchial C cells. Histochemie. 1971;27:96–102. doi: 10.1007/BF00284951. [DOI] [PubMed] [Google Scholar]
- Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro JF, Silva P, Reis-Filho JS, Reyes-Santias RM, Alfonsin-Barreiro N, Forteza J, Sobrinho-Simoes M. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol. 2004;17:819–826. doi: 10.1038/modpathol.3800124. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Schmitt FC. Taking advantage of basic research: p63 is a reliable myoepithelial and stem cell marker. Adv Anat Pathol. 2002;9:280–289. doi: 10.1097/00125480-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simoes M. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol. 2003;16:43–48. doi: 10.1097/01.MP.0000047306.72278.39. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kobayashi Y, Katoh R, Kohn LD, Kawaoi A. Identification of thyroid transcription factor-1 in C cells and parathyroid cells. Endocrinology. 1998;139:3014–3017. doi: 10.1210/endo.139.6.6126. [DOI] [PubMed] [Google Scholar]
- Wetzel BK, Wollman SH. Fine structure of a second kind of thyroid follicle in the C3H mouse. Endocrinology. 1969;84:563–578. doi: 10.1210/endo-84-3-563. [DOI] [PubMed] [Google Scholar]
- Williams ED, Toyn CE, Harach HR. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol. 1989;159:135–141. doi: 10.1002/path.1711590208. [DOI] [PubMed] [Google Scholar]
- Wollman SH, Hilfer SR. Embryologic origin of the various epithelial cell types in the second kind of thyroid follicle in the C3H mouse. Anat Rec. 1978;191:111–121. doi: 10.1002/ar.1091910110. [DOI] [PubMed] [Google Scholar]
- Wollman SH, Neve P. Postnatal development and properties of ultimobranchial follicles in the rat thyroid. Anat Rec. 1971a;171:247–258. doi: 10.1002/ar.1091710205. [DOI] [PubMed] [Google Scholar]
- Wollman SH, Neve P. Ultimobranchial follicles in the thyroid glands of rats and mice. Recent Prog Horm Res. 1971b;27:213–234. doi: 10.1016/b978-0-12-571127-2.50030-4. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129:3033–3044. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]


