Abstract
Children of mothers that abused alcohol during pregnancy are often reported to suffer from growth retardation and central nervous system (CNS) abnormalities. The use of prenatal alcohol exposed (PAE) animal models has revealed reductions in body and brain weights as well as regional specific brain deficits in neonatal pups. Recently, we and others reported reductions in the size of the posteromedial barrel subfield (PMBSF) in first somatosensory cortex (SI) associated with the representation of the large mystacial vibrissae in neonatal rats and mice that were exposed to alcohol at various times during gestation. While these reductions in barrel field size were reported in neonates, it was unclear whether similar reductions persisted later in life or whether some catch-up might take place in older animals.
In the present study, we examined the effect of PAE on measures of barrel field size in juvenile (6 weeks of age) and adult (7 months of age) rats; body and brain weights were also measured. Pregnant rats (Sprague-Dawley) were intragastrically gavaged during gestational days 1 to 20 with alcohol (6 g/kg) to simulate a binge-like pattern of alcohol consumption (Alc); 6 g/kg alcohol produced blood alcohol levels ranging between 207.4 and 478.6 mg/dl. Chow-fed (CF), pair-fed (PF) and cross-foster (XF) groups served as normal, nutritional/stress, and maternal controls, respectively for juvenile rats; an XF group was not included for adult rats. The major findings in the present study are: (i) PAE significantly reduced the size of the total barrel field in Alc juvenile rats (13%) and adult rats (9%) compared to CF controls, (ii) PAE significantly reduced the total averaged sizes of individual PMBSF barrels in juvenile (14%) and adult (13%) rats, (iii) PAE did not significantly alter the septal area between barrels or the barrel pattern, (iv) PAE significantly reduced body weight of juvenile rats but only in comparison to PF controls (18%), (v) PAE significantly reduced whole brain (8%) and forebrain (7%) weights of juvenile rats but not adult rats, (vi) no differences were observed in forebrain/PMBSF body ratios nor was forebrain weight correlated with PMBSF area, and (vii) PAE resulted in a greater reduction in anterior barrels compared to posterior barrels. These results suggest that the effects of PAE previously reported in neonate PMBSF areas persist into adulthood.
Keywords: fetal alcohol exposure; FASD; somatosensory cortex; PMBSF, vibrissae; birth defects
1. Introduction
Gestational alcohol exposure damages the developing brain and often leads to life-long behavioral and cognitive deficits. In the most extreme cases, prenatal alcohol exposure (PAE) results in children with growth retardation, craniofacial abnormalities, and central nervous system dysfunction and has led to the clinical diagnosis of fetal alcohol syndrome (FAS) (Jones & Smith, 1973; Jones & Smith, 1975). However, children of mothers that consumed alcohol during pregnancy may not meet all of the criteria for a diagnosis of FAS, but nonetheless suffer from permanent damage to brain structures that may result in a variety of dysfunctions including deficits in central nervous system processing. The term, fetal alcohol spectrum disorder (FASD), has been reserved to describe the range of deficits resulting from early alcohol exposure (Goodlett, Horn & Zhou, 2005; Riley & McGee, 2005).
PAE children often have sensorimotor processing deficits as demonstrated by their poor performance on higher-order cognitive motor tasks (Adnams, et al., 2001), difficulties in maintaining postural balance (Roebuck, et al., 1998), slower premotor and motor reaction times (Simmons, et al., 2002), and deficits in fine motor control (Connor, et al., 2006), and timing (Wass, et al., 2002). Many reported sensorimotor disturbances may be due, in part, to altered visual and auditory capacity (Church, 1987; Church & Abel, 1998; Stromland, 1985; Stromland, 2004), reduction in overall brain size and shape (Roebuck, Mattson & Riley, 1998; Sowell, et al., 2001), corpus callosum (Riley, et al., 1995), basal ganglia (Mattson, et al., 1994), and parietal cortex (Archibald, et al., 2001).
Many of the sensorimotor deficits seen in PAE children are mirrored in PAE animal models, the use of these models may be helpful in uncovering potential deficits that can be further explored in PAE children. Similar to children, animals exposed to prenatal alcohol may also exhibit impaired balance (Kelly, Hulsether & West, 1987), disrupted righting reflexes (Lopez-Tejero, et al., 1986), delayed motor (Molina, et al., 1987; Norton, et al., 1988), and altered corticospinal development (Miller, 1987). Furthermore, PAE has been shown to result in regional specific deficits in cerebellum (Maier, et al., 1999; Maier, Miller & West, 1999; Maier & West, 2001), locus coeruleus (Maier & West, 2003), and somatosensory cortex (Margret, et al., 2005a; Margret, et al., 2005b; Miller & Potempa, 1990; Mooney & Miller, 1999; Powrozek & Zhou, 2005).
We, (Margret, et al., 2006a; Margret, et al., 2006b; Margret, et al., 2005b), and others (Powrozek & Zhou, 2005), have used the rodent barrel field cortex to study the effects of gestational alcohol exposure on the organization of somatosensory cortex. Clusters of cells, called barrels, located within layer IV of the primary somatosensory cortex (SI) are associated with the representation of the body surface. These barrels are readily amenable to quantitative methods that allow comparisons of aerial measurements between alcohol and non-alcohol treatment groups. One subfield of barrels, the posterior medial barrel subfield (PMBSF), is associated with the representation of the large mystacial whiskers on the contralateral face. Recently, Powrozek and Zhou (2005) examined serotonin labeling in mice at postnatal day 7 (P7), and reported that PAE reduced the overall size of serotonin-labeled barrels in the PMBSF as well as the sizes of some of the individual barrels within the PMBSF. Furthermore, PAE not only reduced the number of cells within selected barrels, but in some cases, entire barrels were often reported missing. Similarly, in P9 rats, PAE reduced the size of the overall PMBSF, along with the sizes of individual barrels and septal areas lying between barrels, but did not alter barrel pattern or result in missing barrels (Margret, et al., 2005b).
In the present study, we asked whether area reductions in total PMBSF barrel area and sizes of individual barrels observed in neonatal rats would also be present in six-week-old juvenile rats and seven-month-old adult rats. The results of the present study indicated that PAE exerts a long-term effect on the size of barrel cortex while leaving the barrel pattern unperturbed.
2. Methods
2.1 Animals
A total of 109 Sprague-Dawley pups were used in this study (84 animals were used for the 6-week-old rat experiments, and 25 animals were used for the 7-month-old rat experiments). Pups were produced by placing adult female rats (n=32, 250–300 g) with adult male rats (300–350 g). All females were handled and habituated to the dry gavage procedure 4 days prior to breeding.
2.2 Breeding and treatment groups
Breeding occurred by placing 2–3 adult female rats in a cage overnight with an adult male breeder. Vaginal smears were examined the next morning under a microscope. Sperm positive slides were marked as gestational day one (G1). At that time, pregnant females were weighed, and separated into one of three treatment groups: alcohol (Alc, n=11), pairfed (PF, n=10), and chowfed (CF, n=11). PF mothers were matched in weight to Alc mothers. Pregnant females were separated into individual cages during pregnancy and pup rearing.
2.3 Treatment procedures
The Alc group was gavaged with 25% (w/v) alcohol solution at a 6 g/kg dose from G1–G20. This period is equivalent to the first and second trimesters in humans (Dobbing & Sands, 1973; Dobbing & Sands, 1979). The PF group was gavaged with 25% maltose dextran solution being isocaloric and isovolumetric to the alcohol given the Alc group. PF mothers received only the amount of food consumed a day earlier by a previously matched Alc female. The PF group served as a control for nutrition and stress. The CF group served as a normal control with no diet restriction or gavage treatment (excepting for the initial gavage acclimation given to all females). A cross-foster group (XF) was used in the 6-week-old rat experiment to test for maternal rearing effects. These pups received equal prenatal treatment as the Alc group, however, following delivery, pups were fostered to a CF mother whose own recently delivered pups had been removed. All groups were provided with ad libitum water.
2.4 Daily procedures
Food hoppers were removed daily between 0830 and 0930 hours. Daily dam weight and food consumption were recorded. Three hours following food hopper removal, Alc and PF dams were gavaged with their respective solutions. Stainless-steel gavage needles, lubricated with canola oil, were used on virgin females during the habituation period prior to mating. Stainless steel tubes were replaced by plastic oral feeding tubes (Instech Solomon) at the time of gestation. The gavage procedure occurred at the end of a 3-hour fast in order to maximize solution absorption. Each day, Alc and CF dams were given ad libitum access to 50 g of chow (Harlan).
2.5 Parturition and rearing
Gavage treatment continued until G20, at which time dams were transferred to clean cages; two nesting pads were added to the cage. Dams were weighed daily, but otherwise were left undisturbed until parturition, having free access to chow and water. On G22, cages were checked twice daily at 0800 and 1700 hours until delivery. On the day of birth, designated postnatal day 0 (P0), litters were culled to 8 pups. The weight of the dams as well as their food consumption, were noted until P10. Pups were weaned on P28, separated into sex specific and litter specific cages where they remained with ad libitum food and water until testing during the 6th postnatal week.
All animals were maintained in UT animal facility rooms at temperatures of 78–80° F, 35–40% humidity, and a 12-hour light/dark cycle. The experiments conformed to the Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985) and were approved by the Animal Care and Use Committee, University of Tennessee Health Science Center. The Animal Care Facility is AAALAC approved.
2.6 Blood Alcohol Concentration
Blood samples were collected from Alc and PF groups on G13 and G20 at 60, 120, 180, and 240 minutes post-gavage. PF blood samples were taken to match for stress of handling Alc animals during the blood sampling procedure, although the PF samples were not analyzed. Blood was taken by placing the tail in warm water for 5 seconds, wiping off excess water with a paper towel, and cutting the tip of tail with a sharp razor blade. Blood was collected in 20 μl heparinised tubes, centrifuged to separate plasma, and stored at 4° C. Blood alcohol concentration (BAC) was measured using an ANALOX G5 alcohol analyzer.
2.7 Tissue processing
Juvenile rats in their 6th post-natal week were weighed and given a lethal injection of Nembutal (50 mg/kg, i.p.). Rats were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde in 0.15M sodium phosphate buffer saline (NaPBS, pH 7.4, 21° C). The brain was removed from the skull and weighed (whole brain), followed by removal of olfactory bulbs and cerebellum then weighed again (forebrain). The forebrain was hemisected, the white matter of each hemisphere was removed and the remaining gray matter of each hemisphere was flattened between two Plexiglas plates. The hemispheres of juveniles were flattened between two Plexiglas plates that were compressed together with rubber washers fitted around rivet posts. In contrast, hemispheres of adult rats were flattened between two Plexiglas plates by adjusting hexagonal nuts fitted to fine-threaded machine bolts holding the plates together. In the latter case, flattening was more uniform across sections, but it appeared that the adult tissue was less compressed than the juvenile tissue. Therefore, we were unable to make direct comparisons of PMBSF measures between juveniles and adults. The flattened hemispheres were then placed in paraformaldehyde and refrigerated overnight. The following day the flattened tissue was sectioned in the sagittal plane at 120 μm on a Vibratome and placed in test tubes containing 0.01M potassium phosphate buffer solution (KPBS, pH 7.4, 21° C). Sections were washed in KPBS (3 × 10 min) at room temperature. The tissue was stained with cytochrome oxidase using a method modified from Wong-Riley and Welt (Wong-Riley & Welt, 1980). Following final KPBS rinse, test tubes with tissue sections were filled with a solution of 30 ml 0.01M KPBS, 22.5 mg diaminobenzidine (DAB, Sigma), 12 mg cytochrome oxidase (Sigma), and 1.5 mg sucrose (Fisher Chemicals). Test tubes were placed in a 37° C water bath for 2 hrs or until barrels were clearly discernible from interbarrel septal regions. Sections were then rinsed 3 ×10 min in 0.01M KPBS, mounted on gelatin coated slides, and cover-slipped using permount/xylene.
2.8 Quantitative morphometric and data analyses
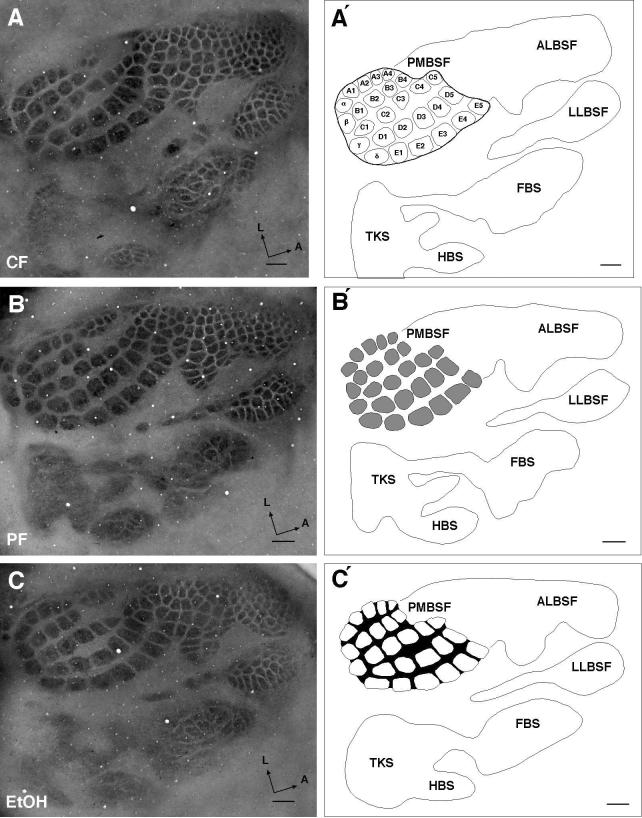
Tissue sections were viewed with a light microscope using the 2 × objective and images were captured with a digital camera. A blue gelatin filter (Kodak, Wratten gelatin filter #47A) was used to visualize the barrel field. Digitized sections containing the PMBSF were aligned, stacked, and reconstructed using Photoshop 7.0. The PMBSF consists of 27 well-demarcated barrels consisting of five rows (Row A–E) and four posteriorly located straddler barrels (α,β,γ,δ). In addition to the PMBSF, other barrel subfields were present and include the anterolateral barrel subfield (ALBSF) representing the upper lip and sinus hairs of the snout, lower lip barrel subfield (LLBSF), forepaw barrel subfield (FBS), hindpaw barrel subfield (HBS), and a nebulously-stained trunk subfield (TKS). The present study focused exclusively on measurements of the total averaged area of the PMBSF, the total averaged area of individual barrels, and the total averaged area of the septal areas lying between barrels. These aerial measurements are illustrated in the line drawings in Fig. 1. The total PMBSF area was drawn by placing a boundary line around the reconstructed PMBSF and this is shown in Fig. 1A′. The total area of the individual barrels is shown in Fig. 1B′, and the septal area is shown by the blackened region in Fig. 1C′. Digital reconstructions of total barrel area, individual barrels, and septal regions were measured using Image J (NIH, Wayne Rashband).
Figure 1.
Barrel field pattern and measures in 6-week-old (juvenile) chow-fed (CF), pair-fed (PF) and alcohol-treated (Alc) rats. A Digital image of the barrel field in layer IV in a CF rat. A′ Barrel field reconstruction delineating the major subfields of the rodent barrel cortex (posterior medial barrel subfield, PMBSF; anterolateral barrel subfield, ALBSF; lower lip barrel subfield, LLBSF; forepaw barrel subfield, FBS; hindpaw barrel subfield, HBS; trunk subfield, TKS). Line surrounding the 27 barrels of PMBSF indicates the measured area defining the total PMBSF barrel area. Nomenclature within PMBSF barrels indicates the orientation of the anterior-posterior running barrel rows (A through E) and the locations of the lateral-medial running arcs (barrels 1 through 5). The most posterior barrels comprising the straddler barrels lie between barrel rows; these are designated as α, β, γ, δ barrels). B Digital image of barrel cortex in PF rat. B′ The shaded region within the PMBSF shows the area of each barrel and is used to define the total individual barrel area. C Digital image of barrel field in Alc rat. C′ The blackened inter-barrel region within the PMBSF denotes the septal area; measurement of the septal area was made by subtracting total individual barrel area from total PMBSF barrel area. Note that the general barrel field pattern is not noticeably different between the treatment groups. Scale bar indicates 500 μm.
When two hemispheres from the same animal were available, both hemispheres were measured and averaged. All treatment groups were compared using ANOVA with a P-value set at (P<0.05). All comparisons that resulted in a significant main effect were further analyzed using Tukey-Kramer's post-hoc test, also set at a significance level of (P<0.05). Statview (5.1) or DataDesk (6.1) were used to perform all statistical analyses. To examine possible differential region effects following PAE, Alc data was normalized, and expressed as a percentage change from CF. The brain/body ratio was determined by dividing the whole brain weight by the body weight of each animal respectively. This ratio examines possible brain/body differential effects following PAE. Forebrain weight and total PMBSF area were compared using a Pearson-Product-Moment Correlation and linear regression analyses (t-ratio).
3. Results
3.1 Maternal Blood Alcohol Concentrations
Blood samples were taken on G13 and G20. On G13, Alc dams had an average peak blood alcohol concentration of 285.8 ± 12.8 (range; 210.4–327.6 mg/dl) whereas peak BAC levels measured on G20 were 329.1 ± 21.0 (range; 207.4–478.6 mg/dl).
3.2 Effects of PAE measured in juvenile rats at 6-weeks of age
3.2.1 Body weight
A significant main effect in body weight occurred between treatment groups [F(3,80) = 4.348, P = 0.0069]. The body weight of Alc rats was significantly reduced compared to PF alone. No significant differences in body weight were observed between other treatment group comparisons. These data are shown in Table 1. When the Alc data was normalized, by taking the mean body weight and expressing this as a percent change from the CF group, the Alc body weight was reduced 13.6 ± 3.3% compared to the CF group.
Table 1.
The effect of PAE on juvenile body and brain weights (g)
| Treatment | Body weight (g) | Whole brain weight (g) | Forebrain weight (g) |
|---|---|---|---|
| Alc (n =17) | 156.12b (6) | 1.48a,b (0.03) | 1.07a,b (0.02) |
| XF (n =15) | 179.67 (11) | 1.47c,d (0.03) | 1.06c,d (0.02) |
| CF (n =25) | 180.75 (5) | 1.61 (0.01) | 1.15 (0.01) |
| PF (n =27) | 191.86 (7) | 1.63 (0.02) | 1.18 (0.01) |
Data are presented as mean ± S.E.M.
All significant differences (P<0.05)
Alc vs. CF
Alc vs. PF
XF vs. CF
XF vs. PF
3.2.2 Effects of PAE on PMBSF pattern
The PMBSF pattern is illustrated in Fig. 1 for CF, PF, and Alc juvenile rats. Note that the five-row barrel arrangement and the presence of the posteriorly located straddler barrels are seen in each treatment group. The PMBSF barrel pattern, consisting of 27 barrels, likely reflects a common plan of organization that is independent of treatment. A similar PMBSF barrel pattern was also seen in adult rats (data not shown). Thus, PAE does not appear to alter the overall PMBSF pattern in juvenile and adult rats.
3.2.3 Whole brain and forebrain weights
A significant main effect was observed for whole brain [F(3,80) = 19.804, P < 0.0001] and forebrain [F(3,80) = 17.094, P < 0.0001] weights for treatment groups. The mean Alc whole brain and forebrain weights were significantly less than controls and these results are presented in Table 1. No significant differences in brain weight were found between Alc and XF rats or between CF and PF rats. Alc whole brain and forebrain weights were reduced by 8.3 ± 1.6% and 6.9 ± 1.5%, respectively compared to CF rats.
3.2.4 Total area of the PMBSF
A significant main effect was observed for total PMBSF area [F(3,80) = 6.357, P = 0.0006] for treatment groups. Total averaged PMBSF area was significantly reduced in Alc rats compared to CF. These data are illustrated in Table 2. No significant differences were observed between Alc and XF rats or between CF and PF. Normalization of the data showed that the total Alc PMBSF area was 13.1 ± 1.9% smaller than in CF rats.
Table 2.
PAE effect on PMBSF barrels and septal areas (mm2) and forebrain/PMBSF ratio in juvenile rats
| Treatment | Total PMBSF barrel area (mm2) |
Total individual PMBSF barrel area (mm2) |
Total PMBSF septal area (mm2) |
Forebrain/PMBSF ratio |
|---|---|---|---|---|
| Alc (n =17) | 4.69a,b (0.10) | 3.27a,b (0.07) | 1.43 (0.04) | 0.23 (0.01) |
| XF (n =15) | 4.74c,d (0.15) | 3.29c,d (0.09) | 1.45 (0.06) | 0.23 (0.01) |
| CF (n =25) | 5.40 (0.10) | 3.81 (0.07) | 1.59 (0.04) | 0.21 (0.01) |
| PF (n =27) | 5.22 (0.16) | 3.74 (0.11) | 1.48 (0.05) | 0.23 (0.01) |
Data are presented as mean ± S.E.M.
All significant differences (P<0.05)
Alc vs. CF
Alc vs. PF
XF vs. CF
XF vs. PF
3.2.5 Total individual PMBSF barrel area
Analysis of total individual barrel area revealed a significant main effect [F(3,80) = 7.589, P < 0.0002] for treatment groups. The averaged area of the total individual barrels was significantly reduced in Alc rats compared to non-alcohol controls. These data are shown in Table 2. No significant differences were seen between Alc and XF rats or between CF and PF rats. Normalization of the data indicated that the total individual barrel area of Alc rats was 14.3 ± 1.9% smaller than CF rats.
3.2.6 Total septal area of PMBSF
A significant main effect for treatment groups was not observed for the septal region [F(3,80) = 2.546, P = 0.0618]. The averaged septal area is shown in Table 2 for each treatment group. While no significant differences were observed between alcohol and non-alcohol treatment groups, normalization of the data showed that the septal region in Alc rats was nonetheless 10.4 ± 2.4% smaller than in CF rats.
3.2.7 Forebrain weight and PMBSF area
PAE significantly reduced both forebrain weight and PMBSF area. A forebrain/PMBSF ratio was constructed to determine whether alcohol played a greater role on either brain weight or PMBSF area, or whether PAE affected weight and area equally. A significant main effect was not observed between treatment groups since the ratios were nearly identical between groups, suggesting that PAE equally affected both brain weight and PMBSF area. Ratio data are shown in Table 2 for each treatment group.
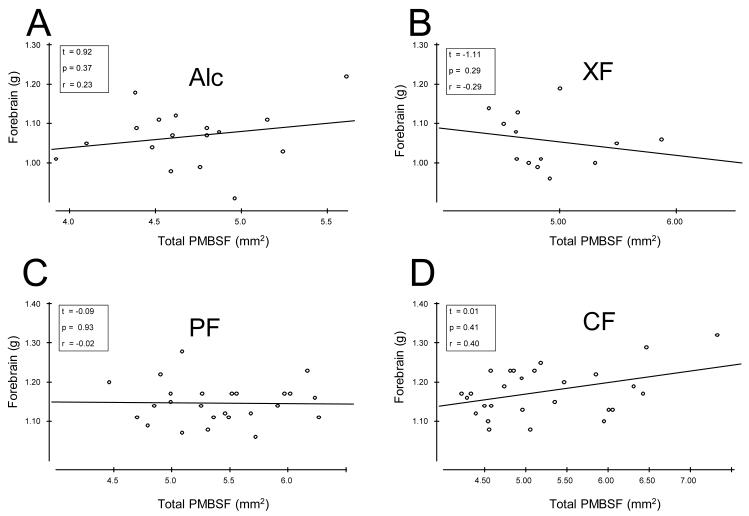
We next addressed whether a relationship existed between forebrain weight and PMBSF area. Forebrain weight was compared with total PMBSF area for each treatment group using a Pearson Product-Moment Correlation (r) and no significant correlations were found for any of the treatment groups. These data are presented in scatter-plot format and a simple regression line (least-squares line) through the data points are shown in Fig. 2.
Figure 2.
Pearson Product-Moment Correlation and linear regression analyses [t-ratio] of forebrain weight and total PMBSF area for each treatment group in 6-week-old rats. A Forebrain weight and total PMBSF area correlation and regression analyses for Alc treated rats. Inset provides t-ratio, probability (p), and correlation coefficient (r) values. B: Measures for XF treated rats, C: PF treated rats, and D: CF treated rats.
3.2.8 Regional vulnerability
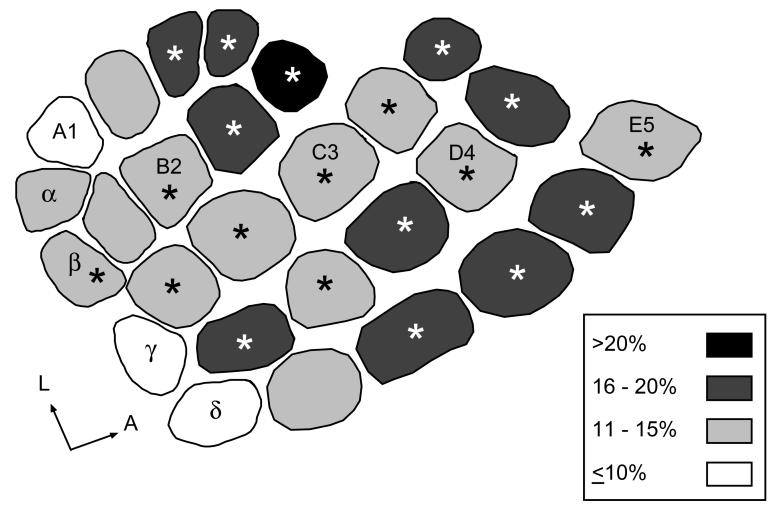
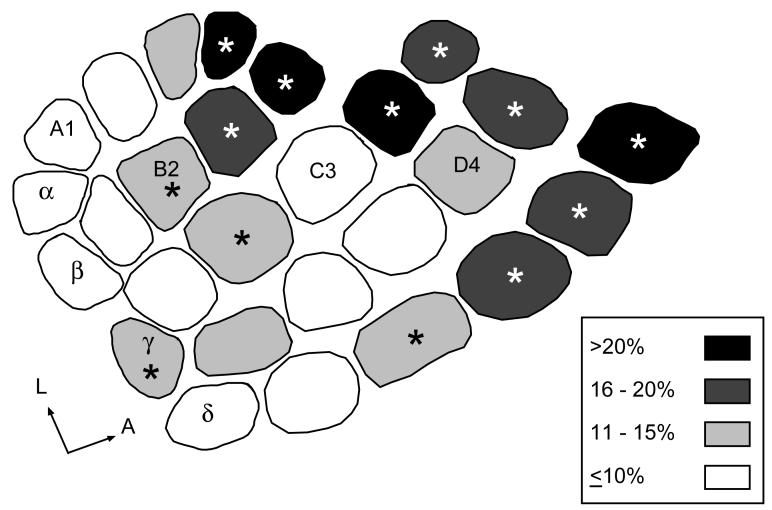
To determine whether individual barrels within the PMBSF were uniformly reduced following PAE, or whether regional differences in vulnerability exist, as previously reported in P10 pups (Margret, et al., 2005b) we measured and compared the averaged barrel area for each of the 27 barrels for alcohol and non-alcohol treated rats. Individual barrels for Alc and CF rats were compared on a barrel-by-barrel basis and 74% (20/27) of the barrels were significantly smaller in Alc rats compared to CF rats. These data are shown in Fig. 3; asterisks show significant differences between individual Alc and CF barrels. No significant differences in barrel sizes were observed for Alc and XF comparisons, or between PF and CF comparisons. Mean areas of Alc barrels were then normalized and expressed as a percent difference from the mean area of the corresponding CF barrel, and categorized as follows: (i) severely reduced (>20%), (ii) greatly reduced (16–20%), (iii) moderately reduced (11–15%), (iv) slightly reduced (≤10%). Inspection of Fig. 3 showed that anterior barrels in Alc rats showed a greater reduction in size compared to CF, while posterior barrels in Alc rats exhibited a modest reduction in size compared to CF rats.
Figure 3.
Comparison of averaged individual barrel area in PMBSF between Alc and CF rats at 6-weeks of age. Shading denotes averaged percent barrel reduction for Alc barrels normalized to CF barrels. Percent reduction was categorized into 4 groups: i) unshaded barrels were ≤10% reduced in Alc rats, ii) light-gray barrels were 11% to 15% reduced in Alc rats, iii) dark-gray barrels were 16% to 20% reduced in Alc rats, iv) blacked-barrels were greater than 20% reduced in Alc rats. Anterior barrels showed a greater reduction in size compared to posterior barrels. Asterisks denote barrels that were significantly reduced in Alc rats compared to CF rats (P<0.05).
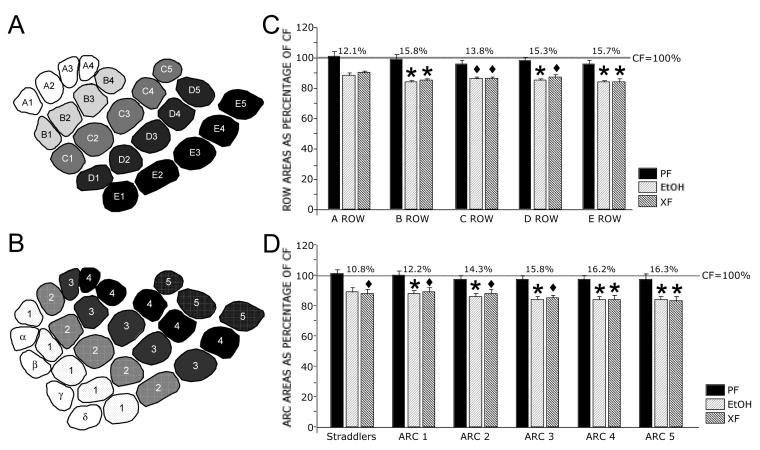
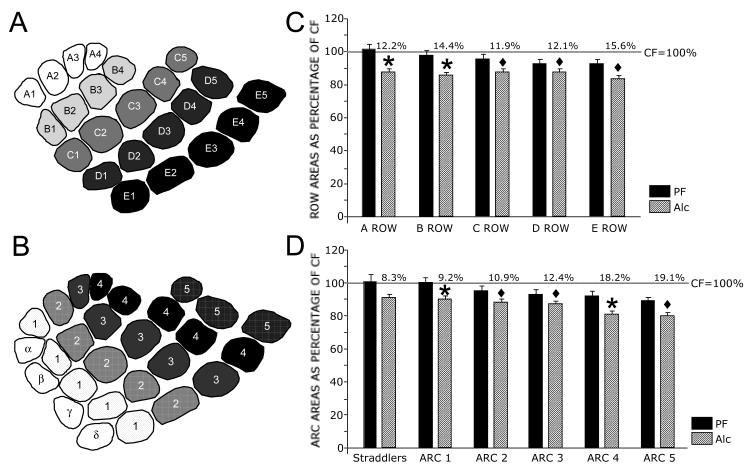
We next compared barrel rows (A–E) and arcs (1 to 5 and straddlers) between treatment groups. Normalization of the Alc row data as a percent change from CF showed little difference in percent reductions between lateral (A1–A4) to medial (E1–E5) rows (12.1%, 15.8%, 13.8%, 15.3%, and 15.7%, respectively), while normalization of arc data showed a lesser reduction in posterior arcs compared to anterior arcs (10.8%, 12.2%, 14.3%, 15.8%, 16.2%, and 16.3%, respectively). These results are shown in Fig. 4. All row and arc comparisons resulted in significant main effects between treatment groups. Post-hoc analysis (Tukey-Kramer's) revealed that all arcs (except the straddler arc) and all rows (except Row A) were significantly reduced in Alc compared to CF (Fig. 4).
Figure 4.
Averaged PMBSF row and arc areas in Alc, XF, and PF rats compared to CF rats at 6-weeks of age. Schematic diagrams showing barrel rows (A) and arcs and straddler barrels (B). C Graph showing averaged row area for Alc, XF, and PF rats normalized as a percentage change of CF rats. Significant area reductions occurred between Alc and CF rats for rows B–E. No significant row differences occurred between CF and PF or between Alc and XF. Percent reduction in row area between Alc and CF is indicated above the bars for each of the rows. D Graph showing averaged arc and straddler areas for Alc, XF, and PF rats normalized as a percentage change of CF rats. The straddler arc showed no significant difference between Alc and CF, while arcs 1–5 were significantly reduced. No significant differences occurred between CF and PF or between Alc and XF arc areas. Percent reduction in arc area between Alc and CF is indicated above the bars of each arc/straddler. Note the gradient moving from posterior to anterior arcs, which is not seen between rows. Gray lines in (C) and (D) indicate normalized CF values at 100%. Diamonds (◆) indicate significant differences between Alc and CF or between XF and CF. Asterisks (*) indicate significant differences between Alc and both CF and PF groups or between XF and both CF and PF groups.
3.3 Effects of PAE measured in adult rats at 7-months of age
3.3.1 Body and brain weights
No significant main effects were observed in body or brain weights between treatment groups. Mean and SEM for each group are presented in Table 3. Normalization of body and brain weights indicated that Alc rats weighed 4.1 ± 10% less than CF rats, whole brain weights were 4.7 ± 2.8% lower than CF rats, and forebrain weights were 3.3 ± 3.2% less than CF rats.
Table 3.
PAE effect on adult body and brain weights (g)
| Treatment | Body weight (g) | Whole brain weight (g) | Forebrain weight (g) |
|---|---|---|---|
| Alc (n=9) | 383.83 (40) | 1.80 (0.05) | 1.27 (0.04) |
| CF (n=8) | 400.31 (40) | 1.89 (0.04) | 1.31 (0.03) |
| PF (n=8) | 405.94 (37) | 1.93 (0.05) | 1.35 (0.03) |
Data are presented as mean ± S.E.M.
No significant differences between any treatment group comparisons (P <0.05).
3.3.2 Total PMBSF area
A significant main effect was observed for total PMBSF area [F(2,22) = 5.017, P = 0.016] for treatment groups. Total PMBSF area was significantly smaller in Alc rats compared to PF and CF rats. These data are shown in Table 4. No significant differences were observed between CF and PF rats. Normalization of the total PMBSF area showed a 8.9 ± 2.0% reduction in Alc rats compared to CF rats.
Table 4.
PAE effect on adult PMBSF and septal areas (mm2)
| Treatment | Total PMBSF barrel area (mm2) |
Total individual PMBSF barrel area (mm2) |
Total PMBSF Septal area (mm2) |
|---|---|---|---|
| Alc (n=9) | 4.30a,b (0.09) | 2.98a,b (0.06) | 1.33 (0.06) |
| CF (n=8) | 4.73 (0.12) | 3.41 (0.12) | 1.32 (0.04) |
| PF (n=8) | 4.76 (0.13) | 3.28 (0.10) | 1.48 (0.06) |
Data are presented as mean ± S.E.M.
No significant differences occurred between any treatment groups (P<0.05).
Alc vs. CF
Alc vs. PF
3.3.3 Total individual PMBSF barrel area
A significant group main effect was found for the total individual PMBSF barrel area [F(2,22) = 5.938, P = 0.0087]. The total averaged individual barrel area of Alc rats was significantly smaller compared to PF and CF rats; these data are illustrated in Table 4. No significant differences were found between non-alcohol groups. PAE reduced mean total individual barrel area by 12.7 ± 1.7% in Alc rats compared to CF.
3.3.4 Total septal area of PMBSF
No significant group main effect was observed for the total septal area [F(2,22) = 2.741, P = 0.0865]. Results are shown in Table 4. The septal region in Alc rats is 0.7 ± 4.5% larger than in CF rats.
3.3.5 Regional vulnerability
Similar to results from juvenile rats, total individual PMBSF barrel areas were significantly smaller in adult Alc rats compared to CF and PF rats. When the areas of individual barrels in Alc rats were compared to the areas of identical barrels in juvenile rats, 74% (20/27) of the barrels were smaller in Alc rats compared to 48% (13/27) in adult rats. Asterisks in Fig. 5 indicate significantly smaller barrels. No significant differences resulted between individual PF and CF PMBSF barrels. We next normalized barrels in Alc rats as a percent change from CF rats and categorized the results as shown in Fig. 5. Again, anterior barrels showed greater reduction in size compared to posterior barrels. Examination of rows and arcs revealed little change in percent reduction across rows (A row = 12.2%, B row = 14.4%, C row = 11.9%, D row = 12.1%, E row = 15.6%). In contrast, clear reductions were observed between posterior and anterior (straddlers = 8.3%, Arc 1 = 9.2%, Arc 2 = 10.9%, Arc 3 = 12.4%, Arc 4 = 18.2%, Arc 5 = 19.1%). Tukey-Kramer's post-hoc analysis revealed that all Alc rows and arcs (excepting the straddler arc) were significantly smaller than PF or CF, respectively as shown in Fig. 6.
Figure 5.
Comparison of averaged individual barrel area in PMBSF between Alc and CF rats at 7-months of age. Shading denotes averaged percent barrel reduction for Alc barrels normalized to CF barrels. Percent reduction was categorized as in Fig. 3. Anterior barrels showed a greater reduction in size compared to posterior barrels. Asterisks denote barrels that were significantly reduced in Alc rats compared to CF rats (P<0.05).
Figure 6.
Averaged PMBSF row and arc areas in Alc and PF rats compared to CF rats at 7-months of age. Schematic diagrams showing barrel rows (A) and arcs and straddler barrels (B). C Graph showing average percent row area for Alc and PF rats normalized as a percentage change of CF rats. Significant reductions in row area were observed between Alc and non-alcohol rats. No significant row differences occurred between CF and PF groups. Percent reduction in row area between Alc and CF is indicated above the bars of each row. D Graph showing averaged percent arc and straddler areas for Alc and PF rats normalized as a percentage change of CF rats. The straddler arc showed no significant difference between Alc and CF, while arcs 1–5 were significantly reduced from CF or both CF and PF groups. No significant differences occurred between CF and PF arc areas. Percent reduction in arc area between Alc and CF is indicated above the bars of each arc/straddler. Gray lines in C and D indicate normalized CF values at 100%. Diamond (◆) indicates significant difference between Alc and CF. Asterisk (*) indicates significant difference between Alc and both non-alcohol groups.
4. Discussion
We, and others, have reported that PAE altered the size of the PMBSF in neonatal rats (Margret, et al., 2006b; Margret, et al., 2005b) and mice (Powrozek & Zhou, 2005), but did not disrupt the barrel pattern with the exception that an occasional barrel was reported missing in mice. One goal of the present study was to determine whether the effects of similar gestational alcohol exposure from G1–G20 were restricted to neonatal rats or whether PAE exerted long-term reductions in barrel field size in juvenile rats and adult rats. The mitochondrial stain, cytochrome oxidase, was used to visualize the PMBSF; barrels in this subfield are large with wide intervening septal spaces and are easily distinguishable from neighboring barrels. The important findings in the present study are: a) PAE significantly reduced the total averaged PMBSF area in juvenile and adult rats compared to non-alcohol treated rats, b) PAE significantly reduced the sizes of individual PMBSF barrels in juvenile and adult rats, c) PAE did not effect the area of the inter-barrel septal area of PMBSF in either juvenile or adult rats, d) intra-barrel subfield differences were observed within the PMBSF whereby anterior located barrels had a greater reduction in size compared to CF controls than did posterior barrels. e) PAE significantly reduced body weight in juvenile rats but only in comparison with PF controls, f) PAE significantly reduced brain weights of juvenile rats but not the brain weights of adult rats. These findings support the notion that the effects of PAE on the cortical barrel field persist into adulthood, while the effects of PAE are less apparent on body and brain weights in older animals.
4.1 PAE has long-term effects on the somatosensory cortex
Previously we used intragastric gavage to administer a high dose of alcohol (6 g/kg) to pregnant rat dams during the first twenty days of gestation and reported reductions in body and brain weight as well as a reduction in overall PMBSF size, sizes of individual barrels in PMBSF, and septal regions between barrels in neonatal pups examined at P10 (Margret, et al., 2005b). Since body, brain, and cortical representation were all reduced it could not be ruled out that PAE exerted a global reduction rather than a specific effect on somatosensory cortex. However, a recent study in mice, showed that alcohol administered over a more limited period of gestation (G8 to G18; typical gestation length is 19 days) reduced the size of the PMBSF but did not significantly reduce body or brain weights although the averaged body and brain weights of Alc mice were less than the nutritional controls (Powrozek & Zhou, 2005). These results suggested that PAE had a direct effect on barrel field cortex that was not confounded by concomitant reductions in body and brain weight. In the present study body and brain weights and PMBSF area were compromised in juvenile rats, while only the overall area of the PMBSF and the areas of individual barrels were significantly reduced in adult rats. Interestingly, the septal regions between barrels were not significantly reduced in either juvenile or adult rats, in contrast to previous findings in neonates (Margret, et al., 2005b), and this may reflect differences in afferent input between septae (Gil, Needleman & Huntley, 2002) and barrels (Molnar, Higashi & Lopez-Bendito, 2003; Senft & Woolsey, 1991) as well as possible differences in cortical circuitry (Kim & Ebner, 1999). Dendrites and spines of layer V pyramidal neurons in SI cortex are particularly affected by PAE (al-Rabiai & Miller, 1989; Galofre, et al., 1987). However, it remains to be determined whether dendrites and spines of barrels and/or septae are similarly affected by PAE, that could possibly account for PAE-related changes in the PMBSF reported here.
Effects of PAE are also seen in the somatosensory cortex of rats examined at approximately 12 weeks of age. These include reduction in overall cortical volume, reductions of neurons and glial cells (Miller & Potempa, 1990), ultrastructural changes in corticospinal somata (al-Rabiai & Miller, 1989), and altered glucose utilization following whisker stimulation (Miller & Dow-Edwards, 1993). Reductions in cortical packing density and thickness of layer V were found following PAE, while thickness of layers II/III increased in 6-week-old juvenile rats (Fakoya & Caxton-Martins, 2006). These findings, along with the present results, support the conclusion that PAE has long-term effects on somatosensory cortex, and specifically on the cortical barrel field.
The present results are relevant for understanding deficits in fine motor control and higher-order cognitive functions (Adnams, et al., 2001) reported in sensorimotor systems in humans following gestational alcohol exposure. PAE children often have difficulties with fine motor control (Connor, et al., 2006) and timing (Wass, et al., 2002), exhibit slower reaction times (Simmons, et al., 2002), and perform poorly on higher-order cognitive motor tasks (Adnams, et al., 2001). It is well established that the motor cortex controls voluntary movements but more recently has been shown to play a role in motor learning and possibly even in cognitive functioning (Sanes & Donoghue, 2000). It is also well known that the somatosensory cortex modulates the motor cortex output through direct corticocortical connections. A somatosensory cortex that is smaller in overall size with fewer neurons and glia likely communicates a compromised sensory message to the motor cortex that may help explain, in part, the deficits in sensorimotor behavior observed in children with FASD.
4.2 Regional and intraregional vulnerability
It is well established that PAE results in regional vulnerability whereby some brain regions are affected while others are spared. Previous studies have indicated that PAE administered specifically during neurogenesis of locus coeruleus (LC), cerebellar purkinje cells or inferior olive cells only reduced the number of cells in LC (Maier & West, 2003). PAE significantly reduced CA1 hippocampal pyramidal cells without a concomitant reduction in CA2 through CA4 pyramidal cells or in dentate gyrus granule cells (Barnes & Walker, 1981; Gibson, et al., 2000). Cell number and volume of ventrobasal thalamus (Mooney & Miller, 1999), and ventral lateral thalamus (Livy, et al., 2001) do not appear vulnerable in pups exposed to alcohol during neurogenesis even though thalamocortical afferents were reported delayed in reaching barrel cortex (Margret, et al., 2005a). In contrast, cortical thickness (Fakoya & Caxton-Martins, 2006), neuronal number (Miller, 1987), glucose metabolism (Miller & Dow-Edwards, 1988), and neuronal migration (Miller, 1993) are disrupted in rats exposed to gestational alcohol.
Here we report that while the area of individual PMBSF barrels is reduced in PAE rats, normalization of individual Alc barrel areas in comparison to CF controls indicated intraregional differences in the amount of reduction between posterior and anterior barrels. Anterior PMBSF barrels have a greater reduction in area compared to CF rats than do the posterior barrels. Several potential mechanisms may be responsible for this regional gradient in barrel area first identified in neonates (Margret, et al., 2005b) and now in juvenile and adult rats. Previous examinations of the mystacial whiskers and follicle-sinus complexes (FSC) indicated that not only are posterior whiskers longer and thicker than anterior whiskers (Brecht, Preilowski & Merzenich, 1997), but posterior FSCs are innervated by a greater number of axons than the more anteriorly located follicles (Welker & Van der Loos, 1986). Vibrissal Merkel cell development also progresses in a posterior to anterior gradient during embryonic and early postnatal development (Nurse & Farraway, 1988). Within the PMBSF, large posterior vibrissae are represented by larger and more cell dense cortical barrels than their anterior counterparts (Lee & Woolsey, 1975; Welker & Van der Loos, 1986). During whisking, not only do posterior whiskers palpate objects more frequently than anterior whiskers (Carvell & Simons, 1990), but posterior whiskers have distinctly lower fundamental resonance tuning frequencies (60-100Hz) than do anterior (∼750Hz) whiskers which may reflect differential tactile encoding properties at anterior and posterior locations across the whisker pad (Neimark, et al., 2003), and across anterior and posterior regions of PMBSF (Andermann, et al., 2004). 2-deoxyglucose uptake levels also appear greater in posterior barrels following whisker stimulation than uptake levels in anterior barrels (McCasland, et al., 1991; Shin, et al., 2005) which may reflect differences in levels of inhibitory interaction in posteriorly and anteriorly located barrels (McCasland, et al., 1991). While possible differential usage of anterior and posterior whiskers may play some part in establishing the gradient, other factors must also contribute since a similar gradient was observed in P10 pups (Margret, et al., 2005b), and whisking behavior is not reported until approximately P15 (Landers & Philip Zeigler, 2006).
4.3 PAE effect on body weight
In the present study, a main treatment effect was found for body weight in juvenile rats with a significant effect only between Alc and PF rats, while normalization of the data showed that Alc body weight was 13.6% reduced from CF and 18.7% reduced from PF rats. In contrast, no significant differences in body weight were found for any treatment group comparisons in adult rats, suggesting a catch-up in older animals. Other investigators that used a similar dose and administration regiment reported a reduction in body weight at 4 weeks of age (McMechan, et al., 2004), while liquid-diet administration of alcohol during gestation did not result in differences in body weight at 3 weeks (Christie, et al., 2005), 12 weeks (Miller & Potempa, 1990), or 16 weeks (Miller & Dow-Edwards, 1988; Mooney, Napper & West, 1996), or between 21 and 28 weeks (Savage, et al., 2002) of age. On the otherhand, when alcohol (4 g/kg) was administered to pregnant guinea pigs by gavage throughout gestation, no differences in body weight of alcohol and non-alcohol treated offspring were found at 8 weeks of age (Bailey, Brien & Reynolds, 2001; Bailey, Brien & Reynolds, 2004). These results are interesting in light of reported reductions in body weight, height (Streissguth, et al., 1991), and body-mass index (Day, et al., 2002; Klug, et al., 2003) in adolescents. However, as adolescents reach adulthood differences become less apparent and may be somewhat ameliorated by environmental circumstances (Nordstrom-Klee, et al., 2002).
4.4 PAE effect on brain weight
Our data showed significant differences in whole brain and forebrain weights in juvenile rats, but these differences did not persist in adult animals even though the mean brain weights of Alc rats remained lower than the non-alcohol controls. In a similar study where alcohol (5.8 g/kg) was delivered by gavage, a reduction in brain weight was also reported in 6-week-old juvenile rats (Fakoya & Caxton-Martins, 2006). Liquid-diet delivery of alcohol during gestation only reduced brain weight at 12 (Miller & Potempa, 1990) and 16 (Miller, 1987; Mooney, Napper & West, 1996) weeks of age but not after 16 weeks (Allan, et al., 1998; Savage, et al., 2002). These results suggest that the significant differences in brain weight reported in neonates (Maier, et al., 1997; Maier, Miller & West, 1999; Margret, et al., 2005b) disappear in adult rats. One hallmark feature, of children whose mothers consumed alcohol during pregnancy is microcephaly (Clarren, et al., 1978; Ferrer & Galofre, 1987) that persists through adolescence and into adulthood (Sowell, et al., 2001; Sowell, et al., 2002). In the present study, even though the mean brain weights of PAE adults was not significantly different from controls, the mean brain weights of both juvenile and adult rats were always lower than brain weights in non-alcohol controls suggesting that PAE likely influences overall brain weight. Nonetheless, the finding that brain weights between Alc and non-alcohol controls were significantly different for juvenile but not for adult rats is puzzling since housing conditions and food and water availability were equivalent for all treatment groups. Interestingly, body weighs were also not significantly different between adult treatment groups. Normalizations of both body and brain weights in adult Alc rats as a percent reduction from CF rats showed similar reductions for each weight comparison; noteworthy is the fact that the reductions seen in adults were two to three times less than those observed for similar measures in juvenile rats. If adult Alc rats consumed proportionally more chow than control rats then nutrition may play some role in the body and brain catch-up.
In conclusion, the present results suggest that deficits in the size of the vibrissae barrel field in SI cortex previously reported in neonatal pups exposed to alcohol during gestation persist in juvenile and adult rats. In contrast, body and brain weights of adult Alc rats were not significantly different from non-alcohol controls, although averaged body and brain weights of adult Alc rats were always lower than for controls. Our findings are relevant for understanding deficits in information processing reported for children with FASD who often perform poorly on behavioral tasks requiring sensorimotor integration.
Acknowledgements
This research was supported by NIH grant AA-013437-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA. Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcohol Clin Exp Res. 2001;25(4):557–62. [PubMed] [Google Scholar]
- al-Rabiai S, Miller MW. Effect of prenatal exposure to ethanol on the ultrastructure of layer V of mature rat somatosensory cortex. J Neurocytol. 1989;18(6):711–29. doi: 10.1007/BF01187226. [DOI] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284(1):250–7. [PubMed] [Google Scholar]
- Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron. 2004;42(3):451–63. doi: 10.1016/s0896-6273(04)00198-9. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54. [PubMed] [Google Scholar]
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases GABA(A) receptor subunit protein expression in the adult guinea pig cerebral cortex. J Neurosci. 2001;21(12):4381–9. doi: 10.1523/JNEUROSCI.21-12-04381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters the proportion of GABAergic neurons in layers II/III of the adult guinea pig somatosensory cortex. Neurotoxicol Teratol. 2004;26(1):59–63. doi: 10.1016/j.ntt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Walker DW. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Brain Res. 1981;227(3):333–40. doi: 10.1016/0165-3806(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84(12):81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10(8):2638–48. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21(6):1719–26. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Church MW. Chronic in utero alcohol exposure affects auditory function in rats and in humans. Alcohol. 1987;4(4):231–9. doi: 10.1016/0741-8329(87)90017-6. [DOI] [PubMed] [Google Scholar]
- Church MW, Abel EL. Fetal alcohol syndrome. Hearing, speech, language, and vestibular disorders. Obstet Gynecol Clin North Am. 1998;25(1):85–97. doi: 10.1016/s0889-8545(05)70359-4. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Alvord EC, Jr., Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92(1):64–7. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44(5):744–51. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–91. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48(10):757–67. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fakoya FA, Caxton-Martins EA. Neocortical neurodegeneration in young adult Wistar rats prenatally exposed to ethanol. Neurotoxicol Teratol. 2006;28(2):229–37. doi: 10.1016/j.ntt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Galofre E. Dendritic spine anomalies in fetal alcohol syndrome. Neuropediatrics. 1987;18(3):161–3. doi: 10.1055/s-2008-1052472. [DOI] [PubMed] [Google Scholar]
- Galofre E, Ferrer I, Fabregues I, Lopez-Tejero D. Effects of prenatal ethanol exposure on dendritic spines of layer V pyramidal neurons in the somatosensory cortex of the rat. J Neurol Sci. 1987;81(23):185–95. doi: 10.1016/0022-510x(87)90095-5. [DOI] [PubMed] [Google Scholar]
- Gibson MA, Butters NS, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol Teratol. 2000;22(2):183–92. doi: 10.1016/s0892-0362(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Gil OD, Needleman L, Huntley GW. Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. J Comp Neurol. 2002;453(4):372–88. doi: 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230(6):394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12(1):1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Hulsether SA, West JR. Alterations in sensorimotor development: relationship to postnatal alcohol exposure. Neurotoxicol Teratol. 1987;9(3):243–51. doi: 10.1016/0892-0362(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrels field cortex. J Comp Neurol. 1999;408(4):489–505. [PubMed] [Google Scholar]
- Klug MG, Burd L, Martsolf JT, Ebertowski M. Body mass index in fetal alcohol syndrome. Neurotoxicol Teratol. 2003;25(6):689–96. doi: 10.1016/j.ntt.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Landers M, Philip Zeigler H. Development of rodent whisking: trigeminal input and central pattern generation. Somatosens Mot Res. 2006;23(12):1–10. doi: 10.1080/08990220600700768. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Woolsey TA. A proportional relationship between peripheral innervation density and cortical neuron number in the somatosensory system of the mouse. Brain Res. 1975;99(2):349–53. doi: 10.1016/0006-8993(75)90035-9. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Maier Se, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the ventrolateral nucleus of the thalamus. Alcohol Clin Exp Res. 2001;25(5):774–80. [PubMed] [Google Scholar]
- Lopez-Tejero D, Ferrer I, M LL, Herrera E. Effects of prenatal ethanol exposure on physical growth, sensory reflex maturation and brain development in the rat. Neuropathol Appl Neurobiol. 1986;12(3):251–60. doi: 10.1111/j.1365-2990.1986.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, Chen WJ, Miller JA, West JR. Fetal alcohol exposure and temporal vulnerability regional differences in alcohol-induced microencephaly as a function of the timing of binge-like alcohol exposure during rat brain development. Alcohol Clin Exp Res. 1997;21(8):1418–28. doi: 10.1111/j.1530-0277.1997.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23(4):726–34. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, West JR. Prenatal binge-like alcohol exposure in the rat results in region-specific deficits in brain growth. Neurotoxicol Teratol. 1999;21(3):285–91. doi: 10.1016/s0892-0362(98)00056-7. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23(1):49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Alcohol and nutritional control treatments during neurogenesis in rat brain reduce total neuron number in locus coeruleus, but not in cerebellum or inferior olive. Alcohol. 2003;30(1):67–74. doi: 10.1016/s0741-8329(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Margret CP, Chappell TD, Li CX, Jan TA, Matta SG, Elberger AJ, Waters RS. Prenatal alcohol exposure (PAE) reduces the size of the forepaw representation in forepaw barrel subfield (FBS) cortex in neonatal rats: relationship between periphery and central representation. Exp Brain Res. 2006a:1–10. doi: 10.1007/s00221-005-0339-9. [DOI] [PubMed] [Google Scholar]
- Margret CP, Elberger AJ, Li CX, Chappell TD, Matta SG, Waters RS. Prenatal alcohol exposure (PAE) delays thalamocortical input to barrel field cortex in early postnatal rats: A DiI labeling study. Alcohol Clin Exp Res. 2005a;29:128A. [Google Scholar]
- Margret CP, Li CX, Chappell TD, Elberger AJ, Matta SG, Waters RS. Prenatal alcohol exposure delays the development of the cortical barrel field in neonatal rats. Exp Brain Res. 2006b doi: 10.1007/s00221-005-0319-0. [DOI] [PubMed] [Google Scholar]
- Margret CP, Li CX, Elberger AJ, Matta SG, Chappell TD, Waters RS. Prenatal alcohol exposure alters the size, but not the pattern, of the whisker representation in neonatal rat barrel cortex. Exp Brain Res. 2005b;165(2):167–78. doi: 10.1007/s00221-005-2287-9. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol. 1994;16(3):283–9. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Carvell GE, Simons DJ, Woolsey TA. Functional asymmetries in the rodent barrel cortex. Somatosens Mot Res. 1991;8(2):111–6. doi: 10.3109/08990229109144735. [DOI] [PubMed] [Google Scholar]
- McMechan AP, O'Leary-Moore SK, Morrison SD, Hannigan JH. Effects of prenatal alcohol exposure on forepaw digit length and digit ratios in rats. Dev Psychobiol. 2004;45(4):251–8. doi: 10.1002/dev.20035. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of prenatal exposure to alcohol on the distribution and time of origin of corticospinal neurons in the rat. J Comp Neurol. 1987;257(3):372–82. doi: 10.1002/cne.902570306. [DOI] [PubMed] [Google Scholar]
- Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17(2):304–14. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Dow-Edwards DL. Structural and metabolic alterations in rat cerebral cortex induced by prenatal exposure to ethanol. Brain Res. 1988;474(2):316–26. doi: 10.1016/0006-8993(88)90445-3. [DOI] [PubMed] [Google Scholar]
- Miller MW, Dow-Edwards DL. Vibrissal stimulation affects glucose utilization in the trigeminal/somatosensory system of normal rats and rats prenatally exposed to ethanol. J Comp Neurol. 1993;335(2):283–4. doi: 10.1002/cne.903350211. [DOI] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293(1):92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Spear LP, Spear NE. Sensorimotor maturation and alcohol responsiveness in rats prenatally exposed to alcohol during gestational day 8. Neurotoxicol Teratol. 1987;9(2):121–8. doi: 10.1016/0892-0362(87)90088-2. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Higashi S, Lopez-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13(6):661–9. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Effects of prenatal exposure to ethanol on systems matching: the number of neurons in the ventrobasal thalamic nucleus of the mature rat. Brain Res Dev Brain Res. 1999;117(1):121–5. doi: 10.1016/s0165-3806(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Napper RM, West JR. Long-term effect of postnatal alcohol exposure on the number of cells in the neocortex of the rat: a stereological study. Alcohol Clin Exp Res. 1996;20(4):615–23. doi: 10.1111/j.1530-0277.1996.tb01663.x. [DOI] [PubMed] [Google Scholar]
- Neimark MA, Andermann ML, Hopfield JJ, Moore CI. Vibrissa resonance as a transduction mechanism for tactile encoding. J Neurosci. 2003;23(16):6499–509. doi: 10.1523/JNEUROSCI.23-16-06499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom-Klee B, Delaney-Black V, Covington C, Ager J, Sokol R. Growth from birth onwards of children prenatally exposed to drugs: a literature review. Neurotoxicol Teratol. 2002;24(4):481–8. doi: 10.1016/s0892-0362(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Norton S, Terranova P, Na JY, Sancho-Tello M. Early motor development and cerebral cortical morphology in rats exposed perinatally to alcohol. Alcohol Clin Exp Res. 1988;12(1):130–6. doi: 10.1111/j.1530-0277.1988.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Farraway L. Development of Merkel cell populations with contrasting sensitivities to neonatal deafferentation in the rat whisker pad. Somatosens Mot Res. 1988;6(2):141–62. doi: 10.3109/08990228809144671. [DOI] [PubMed] [Google Scholar]
- Powrozek TA, Zhou FC. Effects of prenatal alcohol exposure on the development of the vibrissal somatosensory cortical barrel network. Brain Res Dev Brain Res. 2005;155(2):135–46. doi: 10.1016/j.devbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19(5):1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):339–44. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(9):1992–7. [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26(11):1752–8. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1(4):308–35. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Shin JW, Lee DJ, Jung HS, Sohn NW. Metabolic barrel representations with various patterns of neonatal whisker deafferentation in rats. Int J Dev Neurosci. 2005;23(6):537–44. doi: 10.1016/j.ijdevneu.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Simmons RW, Wass T, Thomas JD, Riley EP. Fractionated simple and choice reaction time in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 2002;26(9):1412–9. doi: 10.1097/01.ALC.0000030563.14827.29. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12(3):515–23. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12(8):856–65. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. Jama. 1991;265(15):1961–7. [PubMed] [Google Scholar]
- Stromland K. Ocular abnormalities in the fetal alcohol syndrome. Acta Ophthalmol Suppl. 1985;171:1–50. [PubMed] [Google Scholar]
- Stromland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addict Biol. 2004;9(2):153–7. doi: 10.1080/13556210410001717024. discussion 159-60. [DOI] [PubMed] [Google Scholar]
- Wass TS, Simmons RW, Thomas JD, Riley EP. Timing accuracy and variability in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 2002;26(12):1887–96. doi: 10.1097/01.ALC.0000042221.73478.4F. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6(11):3355–73. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980;77(4):2333–7. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]