Abstract
Background/Aims
The aim of this study was to determine which of the estrogen receptor (ER) subtypes plays a predominant role in ameliorating hepatic damage following trauma-hemorrhage.
Methods
Adult male rats were subjected to hemorrhagic shock (40 mmHg for 90 min) and resuscitation. ER-α agonist (PPT) or ER-β agonist (DPN) was administered during resuscitation; rats were sacrificed 24 hrs thereafter.
Results
PPT or DPN decreased elevated plasma α-glutathione S-transferase levels; however, PPT was more effective. PPT or DPN increased hepatic heat shock protein 32 (Hsp32) mRNA/protein expressions above levels observed after trauma-hemorrhage. PPT reduced hepatic NF-κB and AP-1 activity and iNOS expression. Although DPN reduced hepatic NF-κB activity, AP-1 activity remained higher than in shams; hepatic iNOS induction remained elevated. PPT/DPN reduced nitrate/nitrite production and iNOS mRNA in Kupffer cells following trauma-hemorrhage; however, these levels in DPN-treated animals remained higher than sham.
Conclusions
Although both PPT and DPN decreased hepatic injury following trauma-hemorrhage, ER-α agonist PPT appears to be more effective in downregulating NF-κB and AP-1 activity, and iNOS induction. Thus, ER-α appears to play a predominant role in mediating the salutatory effects of E2 in ameliorating hepatic damage following trauma-hemorrhage.
Keywords: Hemorrhagic shock, nitric oxide, NOS-2, liver, heat shock proteins, heme oxygenase
INTRODUCTION
Trauma-hemorrhage impairs hepatic perfusion despite fluid resuscitation (1–4). Furthermore, the hepatic dysfunction reflects the severity of injury and is associated with poor outcome after traumatic injury or hemorrhagic shock (5). Several studies support the concept that maintenance of hepatic circulation following hemorrhagic shock is essential for preventing hepatocellular dysfunction (6;7). Furthermore, nitric oxide (NO) plays a role in maintaining hepatic circulation after hemorrhagic shock (3;8;9). Vodovotz et al., however, suggested that inducible NO synthase (iNOS) can be either protective or damaging to the liver, they delineated two distinct actions of NO: the stimulation of cyclic guanosine monophosphate and the inhibition of caspases by S-nitrosation. In contrast, the iNOS promotes hepatocyte death following hemorrhagic shock (10).
The heat shock response is induced during clinically relevant situations such as ischemia/reperfusion and hemorrhagic shock. Heat shock proteins (Hsps) are implicated in protecting cells and maintaining organ function during harmful conditions (11). Experimental studies have demonstrated that Hsp32 contributes to maintenance of hepatic perfusion in vivo under stressful conditions (12;13). Similarly Hsp70 has been shown to protect cells and organs from harmful insults (14). In a previous study we found that preinduction of Hsp70 protects cardiovascular and hepatocellular functions following trauma-hemorrhage (15).
Studies have shown gender dimorphism in hepatic response following hemorrhagic shock. The mechanisms responsible for the gender dimorphic response include differences in pro-inflammatory cytokine, reactive oxygen species, and vasoregulatory action (16;17). Our previous studies have shown that administration of female sex steroid hormone, 17β-estradiol (E2) (18;19), or androgen receptor antagonist, flutamide (20) protect hepatic function following trauma-hemorrhage. Furthermore, recent studies have suggested that the salutary effects of E2 on organ function after trauma-hemorrhage are mediated in part via upregulation of Hsps (18;18). There are two estrogen receptors (ERs), ER-α and ER-β, which are differentially expressed in different tissues (21). A recent study reported that ER-α may be involved in the reduction of liver ischemia and reperfusion injury in mice (22). Although E2 administration ameliorates hepatic injury following trauma-hemorrhage, it remains unknown which subtype of ER is predominantly responsible for the salutary effects of E2. Since studies have indicated that that plasma α-GST is a more sensitive and specific marker of hepatocellular damage than aminotransferase activity and it correlates better with histolopathological changes (23–25), we measured plasma α-GST as an index of hepatocellular injury. In this regard, patient studies have also advocated α-GST to be a superior maker of hepatocellular damage than the aminotransferase or bilirubin concentrations (26). We therefore examined the effects of ER-α agonist, propyl pyrazole triol (PPT), and ER-β agonist, diarylpropiolnitrile (DPN), on hepatic injury following trauma-hemorrhage. This was carried out by measuring hepatic nuclear factor-kappaB (NF-κB) and activating protein 1 (AP-1) DNA binding activity, and mRNA/protein expressions of Hsp32, Hsp70 and iNOS following trauma-hemorrhage. Moreover, NO has been reported to play an important role in producing hepatic injury (27). Therefore, we also examined the effect of PPT and DPN on NO production by isolated Kupffer cells following trauma-hemorrhage.
MATERIALS AND METHODS
Animals
Male (275–325 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used. All experiments were performed in adherence to National Institutes of Guide for the Care and Use of Laboratory Animals and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. After fasted overnight, rats were anesthetized by isoflurane (Attane, Minrad Inc., Bethlehem, PA) inhalation prior to induction of soft tissue trauma via 5-cm midline laparotomy (28). The abdomen was closed in layers, and catheters were placed in both femoral arteries and right femoral vein (polyethylene [PE-50] tubing; Becton Dickinson & Co., Sparks, MD). The wounds were bathed with 1% lidocaine (Elkins-Sinn Inc., Cherry Hill, NJ) throughout the surgical procedure to reduce postoperative pain. The rats were then placed into a Plexiglas chamber (21×9×5 cm) in a prone position and allowed to awaken after which they were bled rapidly within 10 min to a mean arterial pressure (MAP) of 35–40 mmHg. This level of hypotension was maintained until the animals could no longer maintain a MAP of 35 mmHg unless some fluid in the form of Ringer’s lactate solution was administered. This time was defined as maximal bleed-out (MBO). Following the MBO, MAP was maintained between 35 and 40 mmHg until 40% of the MBO volume was returned in the form of Ringer’s lactate solution (~90 min from the onset of bleeding). The rats were then resuscitated with four times the volume of MBO with Ringer’s lactate over 60 min, catheters were removed, vessels ligated and skin incisions closed with sutures. Sham-operated animals underwent the same surgical procedure, but the animals were neither hemorrhaged nor resuscitated. The animals were returned to their cages, allowed food and water ad libitum, and were sacrificed 24 hrs thereafter.
In the treatment group, ER-α agonist, propyl pyrazole triol (PPT, 5 µg/kg subcutaneously; Sigma, St Louis, MO), and ER-β agonist, diarylpropiolnitrile (DPN, 5 µg/kg subcutaneously, Sigma) was administered at the end of resuscitation. In the vehicle-treated group (control group), rats received the same volume of vehicle (250 µl; 10% DMSO, 90% corn oil; Sigma) at the middle of resuscitation (29).
Measurement of hepatic injury
Twenty-four hrs after resuscitation, blood was obtained and plasma was separated by centrifugation and stored at −80°C until assayed. Hepatic injury was determined by measuring plasma levels of α-glutathione S-transferase (αGST) using a commercially available kit (Biotrin, Dublin, Ireland).
Western blot analysis
Western blots were performed to measure liver Hsp32, Hsp70 and iNOS protein levels (30). Samples were analyzed using electrophoresis on precasted gels (Nupage, Bis-Tris; Invitrogen, Carlsbad, CA). Proteins from the gels were transferred to nitrocellulose membranes. The membranes were incubated with anti-Hsp32, HspSP70 and iNOS antibody (Santa Cruz Biotechnology, Inc., CA) overnight at 4° C followed by horseradish peroxidase-conjugated secondary anti-rabbit antibody for 1 hr at room temperature. Membranes were reblotted with β-actin using mouse monoclonal antibody (Abcam Inc., Cambridge, MA) to confirm equal protein loading in each lane. Signal densities were evaluated by ChemiImager 5500 software (Alpha Inotech, San Leandro, CA). Protein levels were quantified by densitometric evaluation and were corrected to the corresponding β-actin densities.
Electrophoretic mobility shift assays (EMSA)
NF-κB and AP-1 DNA binding activities were determined in liver nuclear extractsf (30). Oligonucleotide probes corresponding either to NF-κB or AP-1 consensus sequences (Santa Cruz Biotechnology, Inc.) were labeled with γ-32P-ATP (≥6000 Ci/mmol, Amersham, Piscataway, NJ). Nuclear extracts, ≥20,000 cpm of radiolabeled double-stranded target oligonucleotide, poly(dI-dC), and incubation buffer (Promega, Madison, WI) were mixed and incubated. After 30 min incubation on RT, each of the samples was loaded onto 6% DNA retardation gel (Novex, Carlsbad, CA) and run at 10–15 mA for 90 min. Following electrophoresis, band intensities were quantified using autoradiography. Signal densities were evaluated by ChemiImager 5500 software (Alpha Inotech).
NO production by Kupffer cells
For Kupffer cell isolation (31), liver was perfused with 0.03% collagenase (Sigma, St. Louis, MO), and cell suspension obtained was filtered through a sieve and centrifuged at 50 × g for 3 min to separate parenchymal cells and non-parenchymal cells. Non-parenchymal cells were centrifuged over 16% Histodenz (Sigma) for 45 min (2000 × g; 4° C).The cells appearing at the interface were collected, washed with HBSS (450 × g, 10 min, 4° C) and resuspended in William's E medium (GIBCO-BRL) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO-BRL). After assessing the cell viability by Trypan blue exclusion (average viability >95%), the concentration was adjusted to 1×106 cells/mL in William's E medium and 1.0 mL of cell suspension was added per well to a 24-well culture dish. Plates were washed with warm HBSS to remove non-adherent cells and 1.0 mL of William's E medium was added. The adherent Kupffer cells were incubated with or without LPS (1 µg/mL) for 24 hrs. LPS was used to stimulate macrophages. Cell-free supernatants were collected, cells removed and placed in Trizol reagent (Invitrogen) for RNA isolation. NO production in Kupffer cell culture supernatants was evaluated by measuring total nitrate/nitrite using a colorimetric assay kit (Cayman Chemical, Ann Arbor, MI).
mRNA expression in liver and Kupffer cells
Hsp32, Hsp70, and iNOS in liver, as well as iNOS in Kupffer cells were determined by real-time PCR using ABI Prism 7900HT (Applied Biosystems, Foster City, CA) as previously described (30). RNA was isolated from whole liver using TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. cDNA was generated from the total RNA samples using TaqMan Reverse Transcription Reagents (Applied Biosystems). TaqMan Gene Expression Assays (Applied Biosystems) for those target genes were purchased as probe and primer sets. All samples were tested in triplicate, and average values were used for quantification. 18S rRNA was used as endogenous control. The comparative CT method (ΔΔCT, cycle threshold) was used for quantification of gene expression. Analysis was performed using SDS v2.2 software (Applied Biosystems) according to the manufacturer’s instructions.
Statistical analysis
Data are presented as mean ± SEM. Statistical differences among groups were determined by one way analysis of variance (ANOVA) followed by Fisher’s LSD as a post hoc test. Differences were considered significant if p<0.05.
RESULTS
Plasma αGST levels
Trauma-hemorrhage significantly increased plasma αGST levels (Fig. 1); PPT treatment attenuated the increase in plasma αGST but the levels remained higher than shams. DPN treatment also significantly decreased trauma-hemorrhage-induced increase in plasma αGST compared to vehicle-treated group.
Figure 1.
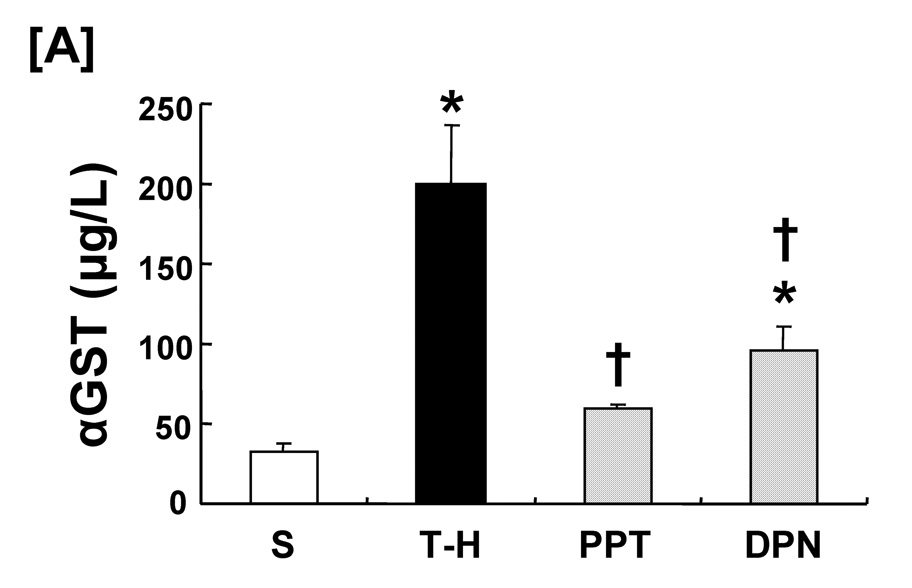
Effect of PPT and DPN on plasma αGST levels at 24 hrs after trauma-hemorrhage (T-H). Data are presented as mean ± SEM (n = 6–7 animals/group). *p<0.05 vs. sham, †p<0.05 vs. T-H. S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H.
Hepatic Hsps and iNOS mRNA/protein expression
Following trauma-hemorrhage, liver Hsp32 mRNA and protein expressions were increased compared to shams. PPT and DPN administration further increased hepatic Hsp32 expressions following trauma-hemorrhage. There was no significant difference in hepatic Hsp32 mRNA and protein expressions between PPT- and DPN-treated trauma-hemorrhage animals (Fig. 2). No significant difference was observed in hepatic Hsp70 mRNA and protein expressions in sham and vehicle-treated trauma-hemorrhage groups. PPT and DPN administration following trauma-hemorrhage significantly increased hepatic Hsp70 mRNA expression compared to sham and vehicle-treated groups. However, Hsp70 protein expression was increased only in the DPN-treated trauma-hemorrhage animals (Fig. 3). Trauma-hemorrhage induced a significant elevation in hepatic iNOS mRNA/protein levels; PPT treatment significantly attenuated the increase in hepatic iNOS mRNA/protein. However, the hepatic iNOS expression in DPN-treated group remained at the same level as in vehicle-treated animals (Fig. 4).
Figure 2.
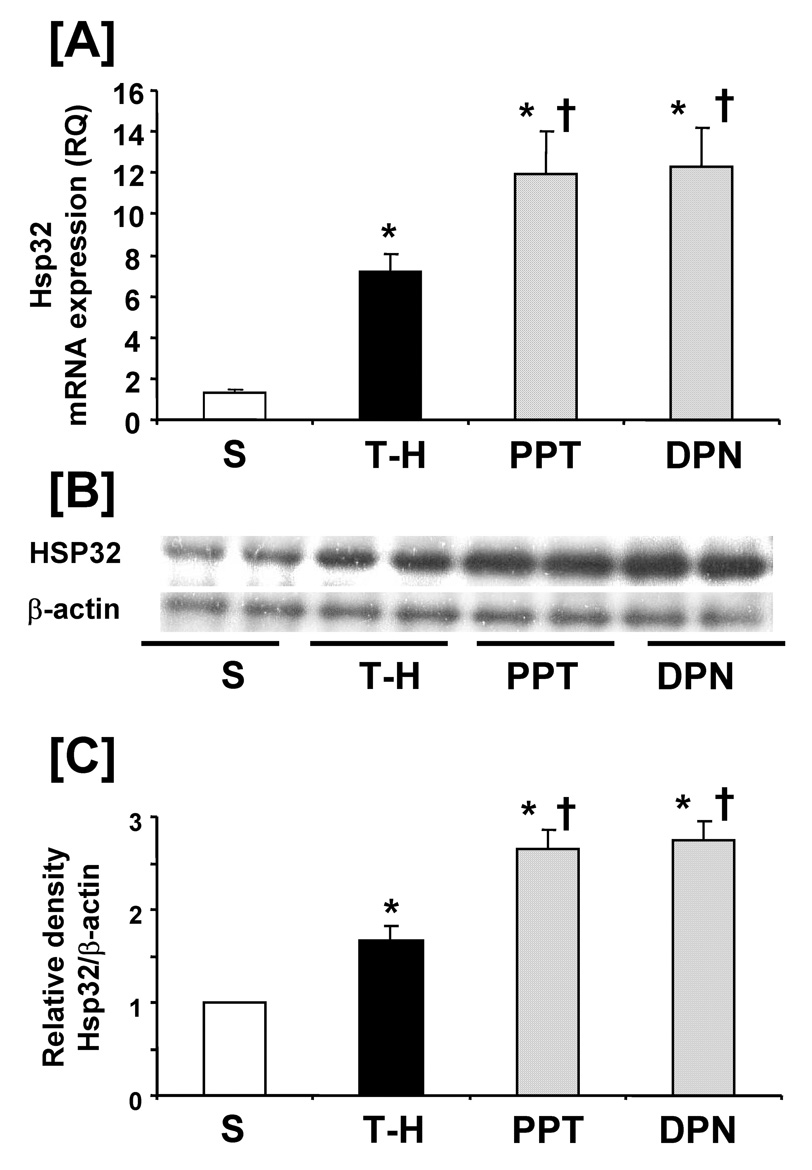
Effects of PPT and DPN on hepatic Hsp32 mRNA and protein expression at 24 hrs after trauma-hemorrhage (T-H). [A] hepatic Hsp32 mRNA expression, [B] hepatic Hsp32 protein expression, [C] folds induction of hepatic Hsp32 protein expression compared to sham. Data are presented as mean ± SEM (n = 3–5 animals/group). *p<0.05 vs. sham, †p<0.05 vs. T-H, S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H, RQ: relative quantity.
Figure 3.
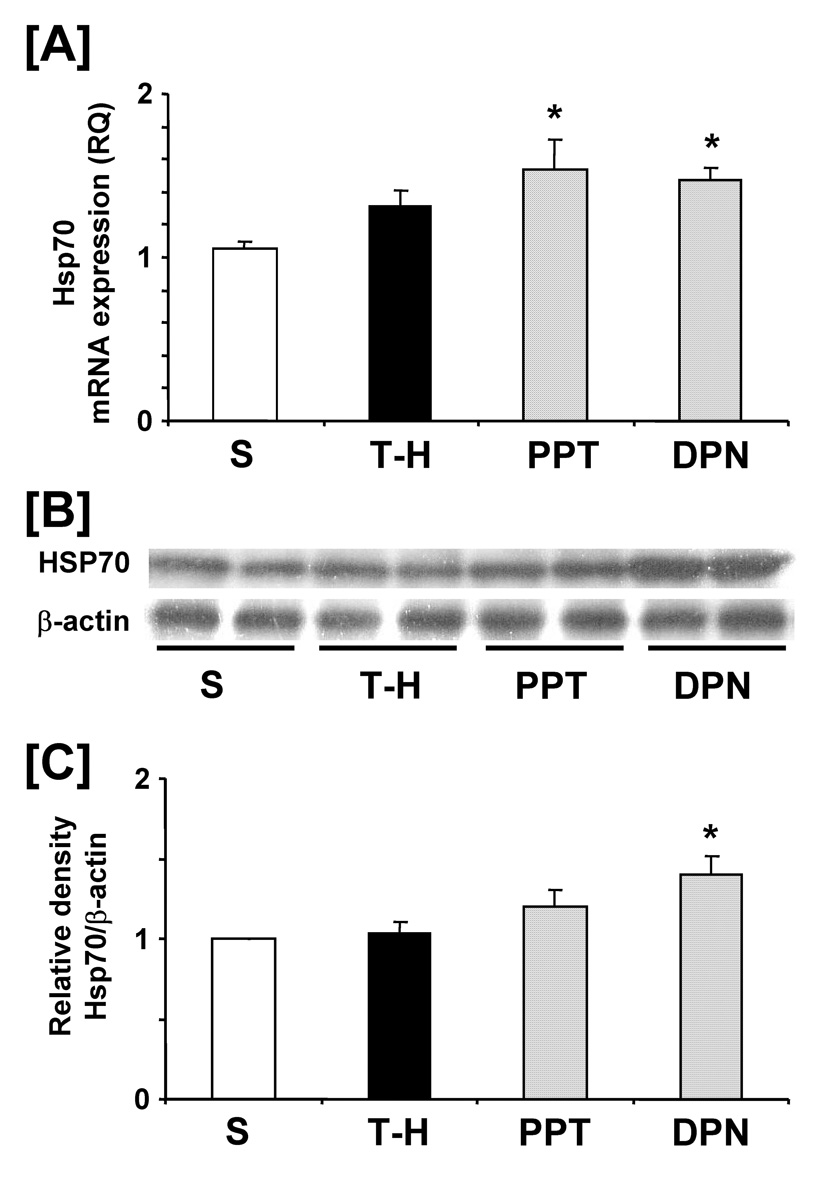
Effects of PPT and DPN on hepatic Hsp70 mRNA and protein expression at 24 hrs after trauma-hemorrhage (T-H). [A] hepatic Hsp70 mRNA expression, [B] hepatic Hsp70 protein expression, [C] folds induction of hepatic Hsp70 protein expression compared to sham. Data are presented as mean ± SEM (n = 3–5 animals/group). *p<0.05 vs. sham, S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H, RQ: relative quantity.
Figure 4.
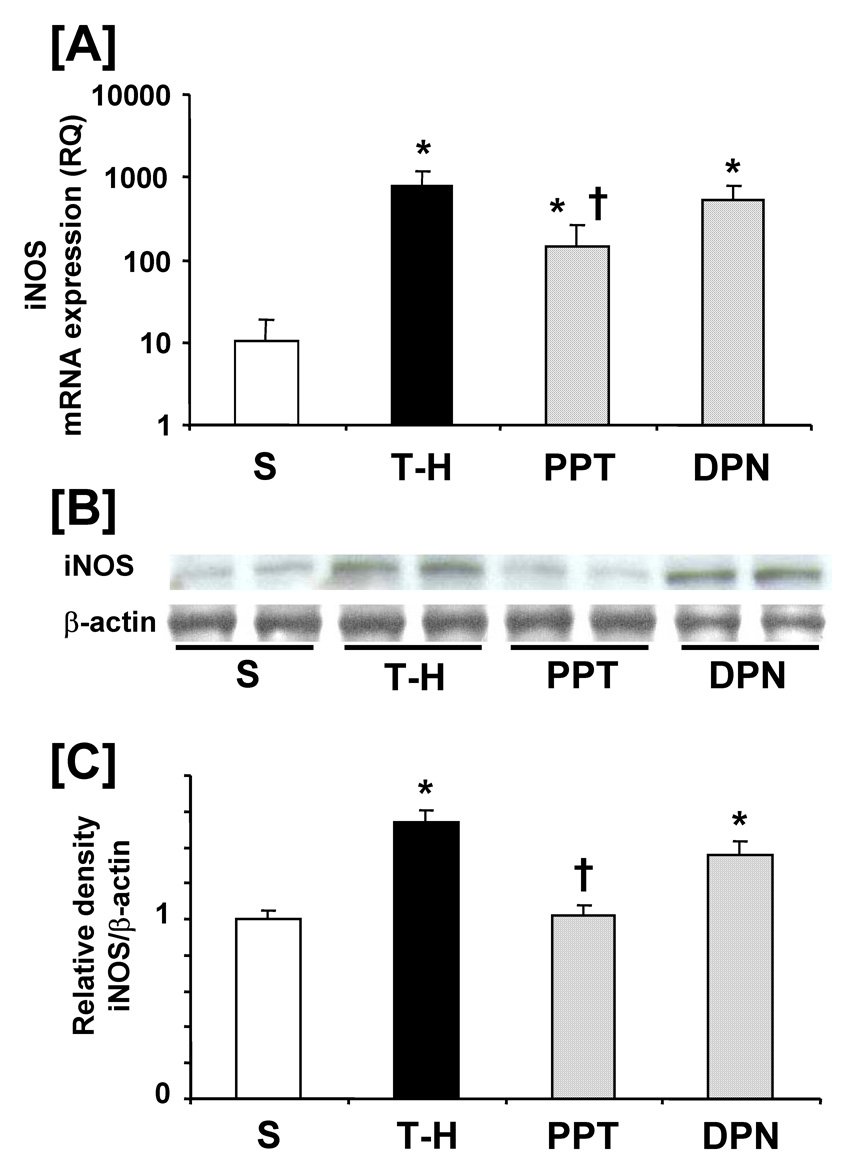
Effects of PPT and DPN on hepatic mRNA and protein expression of iNOS at 24 hrs after trauma-hemorrhage (T-H). [A] hepatic iNOS mRNA expression, [B] hepatic iNOS protein expression, [C] folds induction of hepatic iNOS protein expression compared to sham. Data are presented as mean ± SEM (n = 3–5 animals/group). *p<0.05 vs. sham, †p<0.05 vs. T-H, S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H, RQ: relative quantity.
Hepatic NF-κB and AP-1 DNA binding activities
Trauma-hemorrhage significantly increased NF-κB DNA binding activity (Fig 5, lanes 4–5) compared to shams. PPT treatment significantly normalized the NF-κB DNA binding activity to shams (lanes 6–7). DPN administration significantly reduced the increase in hepatic NF-κB DNA binding activity compared to vehicle administration; however, the levels remained higher than shams and PPT-treated rats (lanes 8–9). DNA binding activity was completely abolished in the presence of specific oligonucleotide cold probe (lane 10). Trauma-hemorrhage also produced a significant increase in AP-1 DNA binding activity (Fig 6) (lanes 4–5). PPT administration following trauma-hemorrhage prevented the increase in AP-1 DNA binding activity (lanes 6–7). However, DPN treatment further increased AP-1 DNA binding activity compared to vehicle-treated animals (lanes 8–9).
Figure 5.
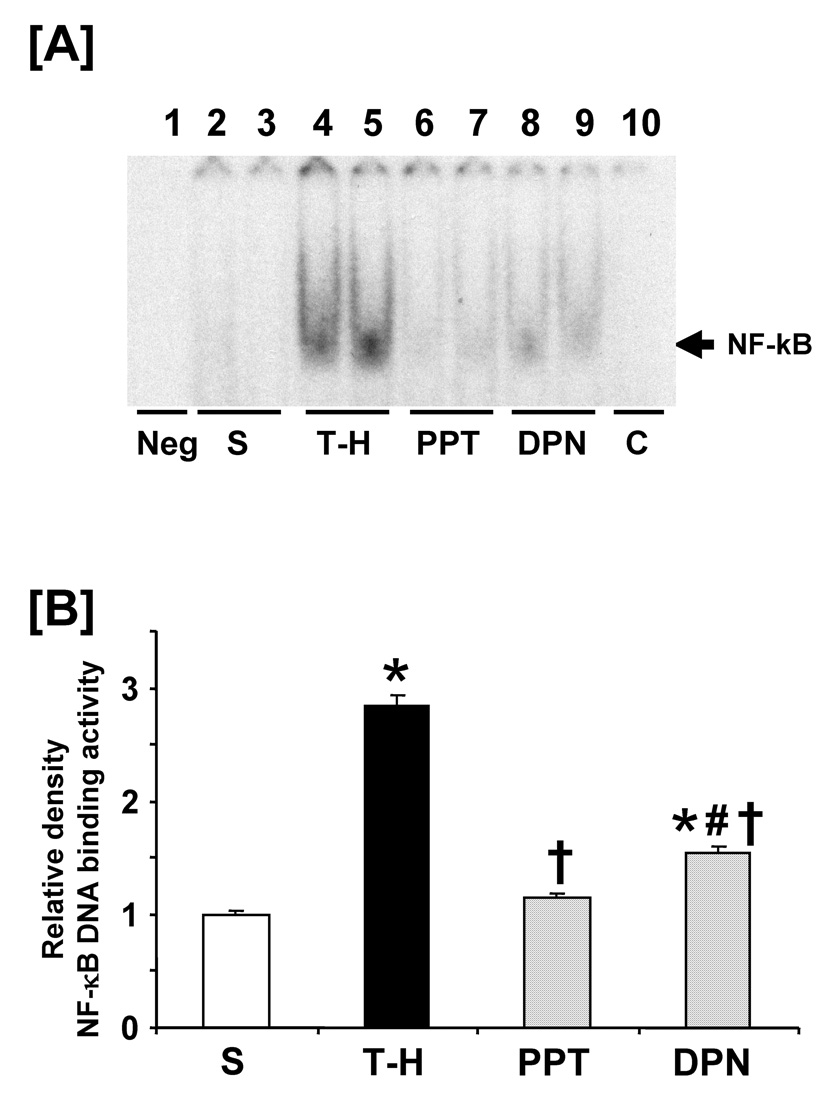
Effects of PPT and DPN on hepatic NF-κB DNA binding activity at 24 hrs after sham operation or trauma-hemorrhage (T-H). [A] NF-κB DNA binding activity, lane 1: negative (Neg. probe only), lanes 2 and 3: S (sham), lanes 4 and 5: T-H, lanes 6 and 7: PPT-treated T-H (PPT), lanes 8 and 9: DPN-treated T-H (DPN), lane 10: competitive assay (C) using 100 times higher concentration of excessive probe. [B] The index of NF-κB DNA binding activity. Data are presented as mean ± SEM (n = 3–5 animals/group): *p<0.05 vs. sham, †p<0.05 vs. T-H, #p<0.05 vs. PPT, S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H.
Figure 6.
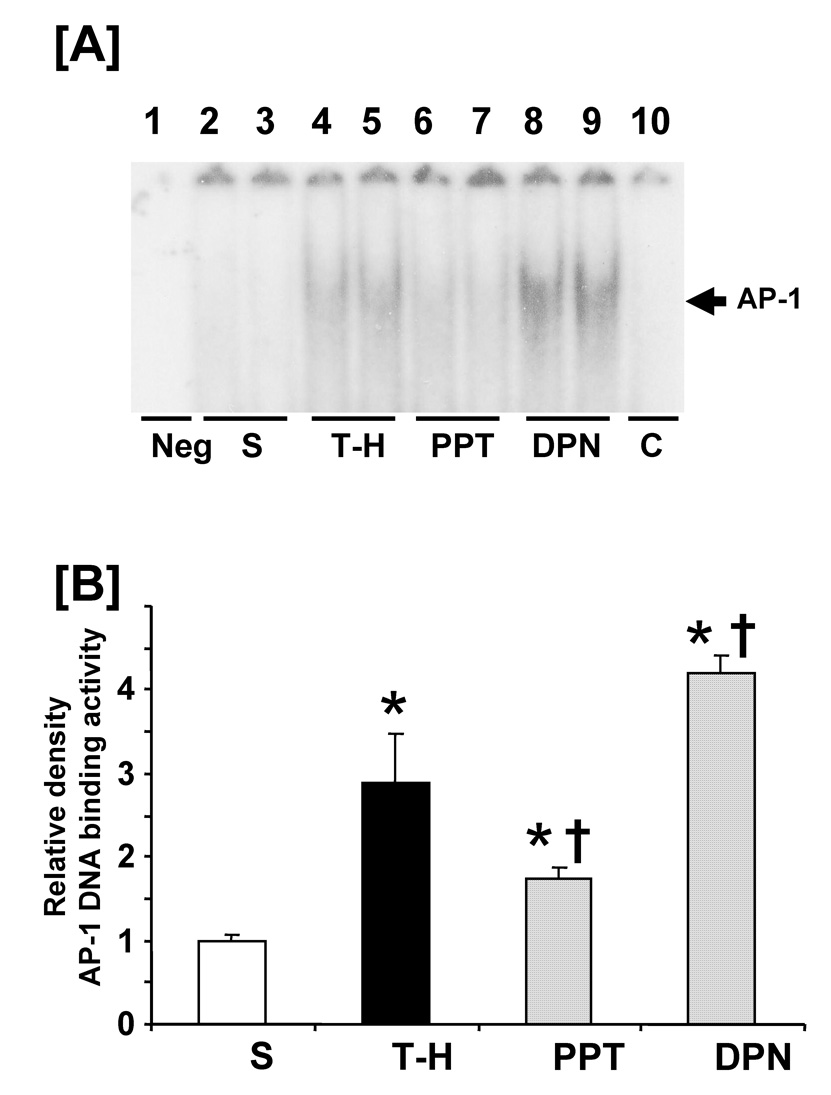
Effects of PPT and DPN on hepatic AP-1 DNA binding activity at 24 hrs after sham operation or trauma-hemorrhage (T-H). [A] AP-1 DNA binding activity, lane 1: negative (Neg. probe only), lanes 2 and 3: S (sham), lanes 4 and 5: T-H, lanes 6 and 7: PPT-treated T-H (PPT), lanes 8 and 9: DPN-treated T-H (DPN), lane 10: competitive assay (C) using 100 times higher concentration of excessive probe. [B] The index of AP-1 DNA binding activity. Data are presented as mean ± SEM (n = 3–5 animals/group): *p<0.05 vs. sham, †p<0.05 vs. T-H. S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H.
Kupffer cells NO production
No significant alteration in nitrate/nitrite production was observed among experimental groups without LPS stimulation. Trauma-hemorrhage significantly increased the levels of nitrate/nitrite in the supernatants of Kupffer cells culture. PPT treatment prevented the increase in Kupffer cells nitrate/nitrite production. DPN administration significantly reduced the increase in nitrate/nitrite production compared to vehicle-treatment; however, these levels remained higher than shams and PPT-treated rats (Fig. 7A). Kupffer cells iNOS mRNA expression increased significantly following trauma-hemorrhage. Although both PPT and DPN treatment significantly reduced the increase in iNOS mRNA expression, the levels remained significantly higher in the DPN-treated animals than shams (Fig. 7B).
Figure 7.
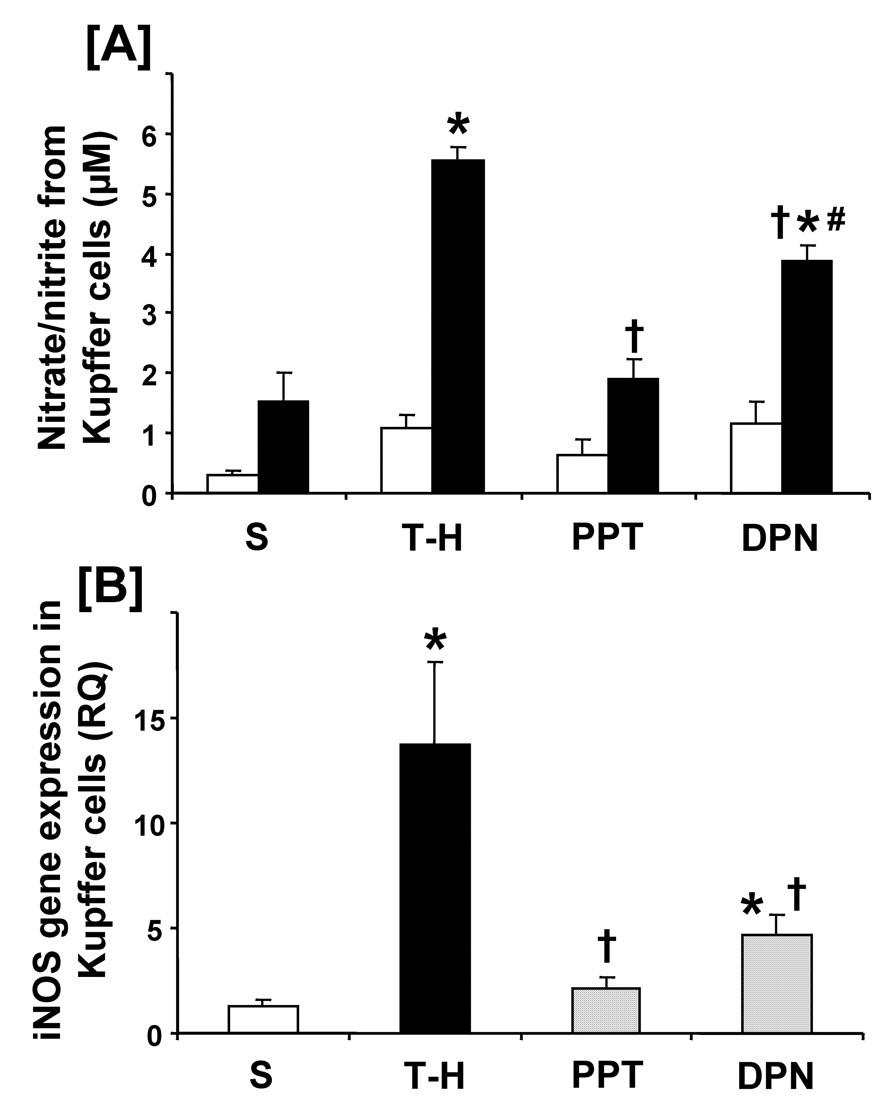
Effects of PPT and DPN on nitric oxide production from Kupffer cells at 24 hrs after sham operation or trauma-hemorrhage (T-H). [A] Nitrate/nitrite production in the supernatant from Kupffer cells. Kupffer cells were cultured without or with LPS (1 µg/mL) for 24 h. The white and black bar graphs indicate without and with LPS stimulation, respectively. [B] iNOS gene protein expression in the Kupffer cells. Data are presented as mean ± SEM (n = 3–6 animals/group). *p<0.05 vs. sham, †p<0.05 vs. T-H, #p<0.05 vs. PPT. S; sham, T-H; trauma-hemorrhage, PPT; PPT-treated T-H, DPN; DPN-treated T-H, RQ: relative quantity.
DISCUSSION
The present study showed that both PPT and DPN significantly improved hepatic injury following trauma-hemorrhage; however, the salutary effects of ER-α agonist, PPT, are more significant than those of ER-β agonist, DPN. Our findings suggest that as compared to ER-β, ER-α agonist may predominantly downregulate pro-inflammatory cascade by reducing hepatic NF-κB and AP-1 DNA binding activity following trauma-hemorrhage. Furthermore, the elevated iNOS mRNA levels and NO production in Kupffer cells were reduced by both PPT and DPN administration; however, these reductions are more effective in the PPT-treated group than those in the DPN-treated group.
Several studies have supported the concept that maintenance of hepatic circulation following hemorrhagic shock is essential for preventing hepatocellular dysfunction (4;6;30). Studies have shown that Hsp32 contributes to maintenance of hepatic perfusion under stressful conditions (12). Our findings suggest that the maintenance of liver function in proestrus females and in males treated with E2 following trauma-hemorrhage is due in part to Hsp32 upregulation (2;6). Furthermore, both ER-α- and ER-β are likely to play roles in estrogen-mediated Hsp32 upregulation following trauma-hemorrhage.
Hsp70 is shown to protect myocardial depression associated with ischemia-reperfusion or sepsis (32). It has also been shown that either Hsp70 upregulation or iNOS downregulation can limit tissue injury caused by ischemia/reperfusion or hemorrhage/resuscitation (14). We have shown that preinduction of Hsp70 protects cardiovascular/hepatocellular functions following trauma-hemorrhage (19). Furthermore, estrogen has been reported to upregulate Hsp70 in the heart and endothelium (33;34). We found that E2 administration in males further increased hepatic Hsp70 mRNA levels and prevented hepatic damage at 5 hrs after trauma-hemorrhage (18). In this study, we did not find any increase in Hsp70 mRNA/protein expression at 24 hrs after trauma-hemorrhage. It is likely that the peak Hsp70 expression may occur at a time point earlier than 24 hrs. Nonetheless, administration of ER-β agonist, DPN, following trauma-hemorrhage increased both Hsp70 mRNA/protein expression in liver. ER-α agonist, PPT on the other hand was able to increase Hsp70 mRNA expression only. Further studies are needed to clarify the precise mechanisms of ER-α and ER-β agonist-mediated induction of Hsps following trauma-hemorrhage.
The iNOS is significantly upregulated after hemorrhagic shock in liver and is thought to be one of the major contributors of hepatic injury following hemorrhagic shock or sepsis (30;35–37). Our present study also corroborates that there is a positive correlation between hepatic injury and iNOS expression. Estrogen is reported to modulate iNOS expression in various tissues; however, the results of those studies are somewhat controversial. For instance, estrogen attenuates NF-κB activation and iNOS over-expression by transient cerebral ischemia (38) and reduces NO production or iNOS expression induced by various conditions in macrophage (39) and in smooth muscle cells (40). In contrast others have demonstrated that estrogen induces iNOS expression or increases NO release in macrophages (41) and in the ovine coronary artery (42). Thus the effects of E2 on NO and iNOS are tissue- or cell-specific. Baker et al. demonstrated that estrogen modulates NO production from iNOS via interactions with ER-β in microglial cells (43). Furthermore ER-β is reported to activate eNOS and iNOS transcription in cardiac myocyte (44). The present study demonstrated that ER-α agonist downregulated hepatic iNOS levels, especially NO production from Kupffer cells. A recent study also reported tissue-specific action of PPT and DPN on neutrophil accumulation following trauma-hemorrhage (45). Therefore, the regulation in iNOS induction and NO production by PPT and DPN may also be tissue- or cell-specific. Furthermore, the protective effect afforded in female mice after hepatic ischemia-reperfusion injury has been reported to be due to the activation of hepatic eNOS activity (46). Thus, eNOS may also play a role in ER-α-mediated attenuation of hepatic injury following trauma-hemorrhage. Additional studies are, however, needed to clarify the role of eNOS following trauma-hemorrhage.
The production of proinflammatory mediators is regulated by NF-κB and AP-1 (36;47). The activation of NF-κB plays a pivotal role in the expression of proinflammatory mediators (48). One recent study demonstrated cross-talk between ER and NF-κB activity at several levels (49;50). AP-1 is also activated by hemorrhagic shock (30) via. ER activates transcription at alternative elements such as AP-1 sites (51). Our present study demonstrated that while PPT attenuated liver AP-1 activity, DPN, further increased hepatic AP-1 DNA binding activity following trauma-hemorrhage. However, studies have reported that ER-α stimulates AP-1 in various cancer cell transfected with ER-α and ER-β (52). ER-α and ER-β have different effects on cellular AP-1 activity (53) and NF-κB activity (54), in some cases producing opposite effects even within the same cell line (44). The responses of PPT and DPN on hepatic AP-1 activity remains controversial and further studies are needed to resolve this controversy.
We have recently found that ER-α mRNA expression was highest in the liver, whereas ER-β mRNA expression was greatest in the lung (41). Other investigators have also shown tissue specific ER expression (55). Thus, it is also likely that the predominant expression of ER-α in the liver may contribute to the selective action of ER-α agonist and thereby to the different regulation of the mechanism observed in response to PPT and DPN following trauma-hemorrhage.
It could be argued that the present study utilized measurement at a single time point, i.e., at 24 hours after treatment and thus it remains unclear whether the salutary effects of E2 or PPT are evident at time periods earlier than 24 hours or the salutary effects are sustained for periods of time longer than 24 hours after treatment. However, our previous studies have shown that if the improvement in organ functions by any pharmacological agent is evident at 2, 5 or 24 hours after treatment that those effects are sustained for prolonged intervals and also improve the survival of animals (56). Thus, although a time point other than 24 hours was not examined in this study, it would appear that the salutary effects of E2 or PPT on the measured parameters in different organs would be evident even if one measured those effects at another time point following trauma-hemorrhage.
In conclusion, since PPT and DPN improve hepatic injury following trauma-hemorrhage, both ER-α and ER-β may be responsible for the E2-mediated reduction in hepatic injury. However as compared to DPN, PPT appeared to be more effective in reducing hepatic injury as well as in normalizing other parameters such as NF-κB/AP-1 DNA binding activity, iNOS induction and nitrate/nitrite production. Nonetheless, additional studies utilizing ER-α and ER-β knockouts are needed to further delineate the role of ER-α and ER-β in ameliorating liver damage following trauma-hemorrhage.
Acknowledgments
GRANTS This investigation was supported by NIH grant R37 GM39519.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review) Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 2.Szalay L, Shimizu T, Schwacha MG, Choudhry MA, Rue LW, III, Bland KI, Chaudry IH. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol. 2005;289:H92–H98. doi: 10.1152/ajpheart.01247.2004. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T, Szalay L, Choudhry MA, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Mechanism of salutary effects of androstenediol on hepatic functions following trauma-hemorrhage: The role of endothelial and inducible nitric oxide synthase.”. Am J Physiol Gastrointest Liver Physiol. 2005;288:G244–G250. doi: 10.1152/ajpgi.00387.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Ba ZF, Burkhardt J, Chaudry IH. Measurement of hepatic blood flow after severe hemorrhage: lack of restoration despite adequate resuscitation. Am J Physiol. 1992;262:G92–G98. doi: 10.1152/ajpgi.1992.262.1.G92. [DOI] [PubMed] [Google Scholar]
- 5.Harbrecht BG, Doyle HR, Clancy KD, Townsend RN, Billiar TR, Peitzman AB. The impact of liver dysfunction on outcome in patients with multiple injuries. Am Surg. 2001;67:122–126. [PubMed] [Google Scholar]
- 6.Toth B, Yokoyama Y, Kuebler JF, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch Surg. 2003;138:1375–1382. doi: 10.1001/archsurg.138.12.1375. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama Y, Toth B, Kitchens WC, Schwacha MG, Bland KI, Chaudry IH. Role of thromboxane in producing portal hypertension following trauma-hemorrhage. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1293–G1299. doi: 10.1152/ajpgi.00268.2003. [DOI] [PubMed] [Google Scholar]
- 8.Watanakunakorn C. Staphylococcus aureus endocarditis at a community teaching hospital, 1980 to 1991. an analysis of 106 cases. Arch Int Med. 1994;154:2330–2335. [PubMed] [Google Scholar]
- 9.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodovotz Y, Kim PK, Bagci EZ, Ermentrout GB, Chow CC, Bahar I, Billiar TR. Inflammatory modulation of hepatocyte apoptosis by nitric oxide: in vivo, in vitro, and in silico studies. Curr Mol Med. 2004;4:753–762. doi: 10.2174/1566524043359944. [DOI] [PubMed] [Google Scholar]
- 11.De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rensing H, Bauer I, Zhang JX, Paxian M, Pannen BH, Yokoyama Y, Clemens MG, Bauer M. Endothelin-1 and heme oxygenase-1 as modulators of sinusoidal tone in the stress-exposed rat liver. Hepatology. 2002;36:1453–1465. doi: 10.1053/jhep.2002.36934. [DOI] [PubMed] [Google Scholar]
- 13.Tamion F, Richard V, Bonmarchand G, Leroy J, Lebreton JP, Thuillez C. Induction of heme-oxygenase-1 prevents the systemic responses to hemorrhagic shock. Am J Respir Crit Care Med. 2001;164:1933–1938. doi: 10.1164/ajrccm.164.10.2010074. [DOI] [PubMed] [Google Scholar]
- 14.Kiang JG. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell Res. 2004;14:450–459. doi: 10.1038/sj.cr.7290247. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Preinduction of heat shock proteins protects cardiac and hepatic functions following trauma and hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2000;278:R352–R359. doi: 10.1152/ajpregu.2000.278.2.R352. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama Y, Nimura Y, Nagino M, Bland KI, Chaudry IH. Current understanding of gender dimorphism in hepatic pathophysiology. J Surg Res. 2005;128:147–156. doi: 10.1016/j.jss.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24 Suppl 1:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 18.Szalay L, Shimizu T, Suzuki T, Yu HP, Choudhry MA, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Estradiol improves cardiac and hepatic function after trauma-hemorrhage: role of enhanced heat shock protein expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R812–R818. doi: 10.1152/ajpregu.00658.2005. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg. 2000;232:673–679. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–H2925. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 21.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 22.Harada H, Bharwani S, Pavlick KP, Korach KS, Grisham MB. Estrogen receptor-alpha, sexual dimorphism and reduced-size liver ischemia and reperfusion injury in mice. Pediatr Res. 2004;55:450–456. doi: 10.1203/01.PDR.0000110524.88784.DD. [DOI] [PubMed] [Google Scholar]
- 23.Hughes VF, Trull AK, Gimson A, Friend PJ, Jamieson N, Duncan A, Wight DG, Prevost AT, Alexander GJ. Randomized trial to evaluate the clinical benefits of serum alpha-glutathione S-transferase concentration monitoring after liver transplantation. Transplantation. 1997;64:1446–1452. doi: 10.1097/00007890-199711270-00013. [DOI] [PubMed] [Google Scholar]
- 24.Redl H, Schlag G, Paul E, Davies J. Plasma glutathione S-transferase as an early marker of posttraumatic hepatic injury in non-human primates. Shock. 1995;3:395–397. [PubMed] [Google Scholar]
- 25.Sherman M, Campbell JA, Titmuss SA, Kew MC, Kirsch RE. Glutathione S-transferase in human hepatocellular carcinoma. Hepatology. 1983;3:170–176. doi: 10.1002/hep.1840030206. [DOI] [PubMed] [Google Scholar]
- 26.Beckett GJ, Hayes JD. Glutathione S-transferases: biomedical applications. Adv Clin Chem. 1993;30:281–380. doi: 10.1016/s0065-2423(08)60198-5. [DOI] [PubMed] [Google Scholar]
- 27.Harbrecht BG, Billiar TR. The role of nitric oxide in Kupffer cell-hepatocyte interactions. Shock. 1995;3:79–87. [PubMed] [Google Scholar]
- 28.Shimizu T, Choudhry MA, Szalay L, Rue LW, III, Bland KI, Chaudry IH. Salutary effects of androstenediol on cardiac function and splanchnic perfusion after trauma-hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2004;287:R386–R390. doi: 10.1152/ajpregu.00214.2004. [DOI] [PubMed] [Google Scholar]
- 29.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu T, Szalay L, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Salutary effects of androstenediol on hepatic function after trauma-hemorrhage are mediated via peroxisome proliferators-activated receptor gamma. Surgery. 2005;138:204–211. doi: 10.1016/j.surg.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama Y, Kitchens WC, Toth B, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Role of IL-10 in regulating proinflammatory cytokine release by Kupffer cells following trauma-hemorrhage. Am J Physiol Gastrointest Liver Physiol. 2004;286:G942–G946. doi: 10.1152/ajpgi.00502.2003. [DOI] [PubMed] [Google Scholar]
- 32.Mavier P, Preaux A-M, Guigui B, Lescs M-C, Zafrani E-S, Dhumeaux D. In vitro toxicity of polymorphonuclear neutrophils to rat hepatocytes: Evidence for a proteinase-mediated mechanism. Hepatology. 1988;8:254–258. doi: 10.1002/hep.1840080211. [DOI] [PubMed] [Google Scholar]
- 33.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285:H687–H692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, heat shock proteins, and NFkappaB in human vascular endothelium. Arterioscler Thromb Vasc Biol. 2004;24:1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- 35.Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Curr Mol Med. 2003;3:519–526. doi: 10.2174/1566524033479582. [DOI] [PubMed] [Google Scholar]
- 36.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–18. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi T, Yamada K, Esaki T, Muto E, Chaudhuri G, Iguchi A. Physiological concentrations of 17beta-estradiol inhibit the synthesis of nitric oxide synthase in macrophages via a receptor-mediated system. J Cardiovasc Pharmacol. 1998;31:292–298. doi: 10.1097/00005344-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Kauser K, Sonnenberg D, Diel P, Rubanyi GM. Effect of 17beta-oestradiol on cytokine-induced nitric oxide production in rat isolated aorta. Br J Pharmacol. 1998;123:1089–1096. doi: 10.1038/sj.bjp.0701715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You HJ, Kim JY, Jeong HG. 17 beta-estradiol increases inducible nitric oxide synthase expression in macrophages. Biochem Biophys Res Commun. 2003;303:1129–1134. doi: 10.1016/s0006-291x(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 42.Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283:H1169–H1180. doi: 10.1152/ajpheart.00397.2000. [DOI] [PubMed] [Google Scholar]
- 43.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 44.Nuedling S, Karas RH, Mendelsohn ME, Katzenellenbogen JA, Katzenellenbogen BS, Meyer R, Vetter H, Grohe C. Activation of estrogen receptor beta is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- 45.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 46.Harada H, Pavlick KP, Hines IN, Lefer DJ, Hoffman JM, Bharwani S, Wolf RE, Grisham MB. Sexual dimorphism in reduced-size liver ischemia and reperfusion injury in mice: role of endothelial cell nitric oxide synthase. Proc Natl Acad Sci U S A. 2003;100:739–744. doi: 10.1073/pnas.0235680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meldrum DR, Shenkar R, Sheridan BC, Cain BS, Abraham E, Harken AH. Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol. 1997;29:2849–2854. doi: 10.1006/jmcc.1997.0506. [DOI] [PubMed] [Google Scholar]
- 48.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–S111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 49.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushner P, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 52.Cheung E, Acevedo ML, Cole PA, Kraus WL. Altered pharmacology and distinct coactivator usage for estrogen receptor-dependent transcription through activating protein-1. Proc Natl Acad Sci U S A. 2005;102:559–564. doi: 10.1073/pnas.0407113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 54.Bhat R, Harnish DC, Stevis PE. A novel human estrogen receptor beta: identification and functional analysis of additional N-terminal amino acids. J Steroid Biochem Mol Biol. 1998;67:233–240. doi: 10.1016/s0960-0760(98)00115-0. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 55.Arts J, Kuiper GG, Janssen JM, Gustafsson JA, Lowik CW, Pols HA, van Leeuwen JP. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology. 1997;138:5067–5070. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- 56.Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, III, Bland KI. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]


