Abstract
Summary
We present results of a randomized, placebo-controlled trial to examine the effect of 50 mg daily oral DHEA supplementation for one year on bone mineral density (BMD), bone metabolism and body composition in 225 healthy adults aged 55 to 85 years.
Introduction
Dehydroepiandrosterone (DHEA) levels decline dramatically with age, concurrent with the onset of osteoporosis, suggesting a role for DHEA supplementation in preventing age-related bone loss.
Methods
We conducted a randomized, placebo-controlled trial to examine the effect of 50 mg daily oral DHEA supplementation for one year on bone mineral density (BMD), bone metabolism and body composition in 225 healthy adults aged 55 to 85 years.
Results
DHEA treatment increased serum DHEA and DHEA sulfate levels to concentrations seen in young adults. Testosterone, estradiol and insulin-like growth factor (IGF-1) levels increased in women (all p<0.001), but not men, receiving DHEA. Serum C-terminal telopeptide of type-1 collagen levels decreased in women (p=0.03), but not men, whereas bone-specific alkaline phosphatase levels were not significantly altered in either sex. After 12 months, there was a positive effect of DHEA on lumbar spine BMD in women (p=0.03), but no effect was observed for hip, femoral neck or total body BMD, and no significant changes were observed at any site among men. Body composition was not affected by DHEA treatment in either sex.
Conclusion
Among older healthy adults, daily administration of 50 mg of DHEA has a modest and selective beneficial effect on BMD and bone resorption in women, but provides no bone benefit for men.
Keywords: Body composition, Bone metabolism, Bone mineral density (BMD), Dehydroepiandrosterone (DHEA) levels, Placebo-controlled trial, Testosterone
Introduction
Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) are abundant steroid hormones produced by the adrenal gland and are an important source of sex hormones in older adults. Levels of DHEA(S) decline markedly with age in parallel with the decrease in bone mineral density (BMD), suggesting a protective role for DHEA(S) in bone metabolism [1]. Osteogenic effects of DHEA treatment have been demonstrated in older people, consistent with the conversion of DHEA to active androgens and estrogens in local tissues such as bone [1]. Other mechanisms through which DHEA may exert an effect on bone include enhancement of insulin-like growth factor (IGF-1) bioactivity and regulation of bone-specific enzymatic activity [2-4]. Most studies evaluating the effect of exogenous DHEA on BMD and bone metabolism have had small sample sizes and/or short durations, or have been conducted in men and women with low levels of DHEA due to disease [4-9]. Large, long-term, placebo-controlled clinical trials of DHEA administration to older healthy individuals with the aim of preventing bone loss and osteoporosis are rare and results are mixed [10, 11]. Meanwhile, DHEA supplements are being widely used by the general public to prevent age-related diseases, including bone loss, without a careful examination of adverse effects versus possible benefits.
The purpose of this study was to determine the effect of 50 mg daily oral DHEA replacement for one year on BMD, bone metabolism and body composition in older healthy adults unselected for lower levels of DHEA.
Design and methods
The DHEA And Well-Ness (DAWN) study was a double-blind, placebo-controlled randomized trial designed to determine the effect of a 1-year course of 50 mg daily oral DHEA supplementation. Between June of 2001 and May of 2003, 110 men and 115 women aged 55 to 85 years who were not currently using any hormone therapy were enrolled. Participants were healthy, community-dwelling individuals, unselected on the basis of DHEA level at entry. Details of the rationale and design are published elsewhere [12]. This study was approved by the Human Subjects Protection Program of the University of California San Diego; all participants gave written informed consent prior to participation.
Body mass index (BMI) was calculated as weight (kg)/height (m)2. Bone mineral density (whole body, total hip, and spine) was measured at baseline, 6 and 12 months by a certified Hologic radiology technician using dual-energy x-ray absorptiometry (DXA) (Hologic QDR model 2000; Hologic, Inc., Waltham, Massachusetts). Body composition, including the proportion of abdominal fat, percent body fat, and lean body mass, was assessed using DXA. Blood samples at baseline and follow-up visits were obtained in the morning, after a requested 12-hour fast. Plasma was separated and stored at -70°C for later measurements of sex hormones, including DHEA(S) and other biomarkers.
Bone marker concentrations, estradiol, testosterone, IGF-1 and IGF binding protein 3 (IGFBP-3) levels were measured at 0, 3, 6 and 12 months. Serum levels of C-terminal telopeptide of type-1 collagen (CTx) were measured by ELISA (Serum Crosslaps® ELISA: Nordic Bioscience Diagnostic A/S); intra-assay CV=3.2%, inter-assay CV=7.2%. Serum bone-specific alkaline phosphatase (BSAP) was measured by EIA (enzyme immunoassay; Metra BAP EIA kit); intra assay CV=3.2%, inter-assay CV=2.8%. Steroid hormones were measured in the UCSD Reproductive Endocrinology laboratory. Levels of estradiol, testosterone, and DHEA were determined by radioimmunoassay after solvent extraction and celite column-chromatography [13]; DHEAS was measured by direct RIA. The sensitivity and the intra- and inter-assay coefficients of variation respectively were 11 pmol/L, 5.9% and 7.1% for estradiol; 0.07 nmol/L, 4.0% and 4.9% for testosterone; 0.14 nmol/L, 6.1% and 7.1% for DHEA; 0.07 μmol/L, 3.0% and 6.3% for DHEAS. IGF-1 and IGFBP-3 were measured by highly specific two-site IRMA kits from Diagnostics Systems Laboratory (Webster, TX). The sensitivity and intra- and inter-assay coefficients of variation, respectively, were 2 ng/ml, 4.9% and 5.1% for IGF-1; and 0.05 ng/ml, 3.0% and 1.0% for IGFBP-3. All samples for each participant were assayed side by side in the same assay minimizing both intra- and inter- assay variability; all analytes were measured in duplicate. In addition, potential adverse effects of DHEA administration were monitored by safety measures including chemistry and hematology panels to screen and serially monitor renal and liver function, blood counts and prostate-specific antigen (PSA) levels for men. An independent data safety and monitoring board met eight times during the 34 months of data collection to review potential side effects and to assure participant safety. The importance of any rise in serum PSA levels was arbitrated by an urologist who was independent of the study.
Statistical analyses
Analyses were performed on an intent to treat basis. Differences between groups were analyzed with t-tests for continuous variables and chi-square analysis for categorical variables. Baseline DHEA and testosterone levels were higher in women randomized to treatment than those randomized to placebo (p=0.003 and p=0.01, respectively). Analyses adjusted for baseline DHEA levels or baseline testosterone levels yielded similar results; only results of analysis adjusting for baseline DHEA levels are presented. Significance tests were two-sided with an alpha level of 0.05. Repeated-measures analyses were performed with linear mixed models to analyze cross-sectional and longitudinal comparisons of outcome measures between the treatment and placebo groups, the independent effect of time (0, 3, 6 and 12 months), and the interaction of time by group. Models were adjusted for potentially confounding covariates. Because there was a sex by treatment group interaction, results are presented stratified by sex. The DAWN trial was designed to examine DHEA effects by sex and by age group (55 to 70 years versus 71 to 85 years) [12]. A more detailed explanation of the statistical analyses utilized in this study can be found in our previous publication [12].
Results
At baseline, the mean (±SD) age for both men and women was 68.7±7.9 years (range 55 to 85), mean BMI was 27.0±3.3 for men and 26.6±4.3 for women. Comparisons of baseline characteristics by treatment status for men and women are shown in Table 1. Within each sex there were no differences between treatment and placebo groups in age, lifestyle variables, body composition, BMD, bone markers or IGF-1 levels (p>0.05). Baseline serum testosterone and DHEA levels were higher in women randomized to treatment than those randomized to placebo (median (IQR)= 0.70 (0.42) versus 0.54 (0.28) nmol/l, p=0.01 and 6.47 (5.99) versus 5.17 (3.58) nmol/l, p=0.003), respectively. There were no significant differences in PSA levels between men in the placebo versus DHEA treatment groups at baseline or at any follow-up visit; mean (SD) serum PSA was 1.38 ng/ml (0.97) at baseline and 1.46 ng/ml (1.16) at the 12-month follow-up visit.
Table 1.
Comparisons of sample characteristics in men and women by treatment status at baseline
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| DHEA (n=55) |
Placebo (n=55) |
P value | DHEA (n=57) |
Placebo (n=58) |
P value | |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | |||
| Age | 68.9±7.5 | 68.4±8.5 | 0.74 | 68.9±8.1 | 68.5±6.7 | 0.80 |
| Body composition | ||||||
| BMI (kg/m2) | 26.4±3.0 | 27.5±3.4 | 0.10 | 27.0±4.4 | 26.1±4.2 | 0.28 |
| Total body fat* (kg) | 24.3±8.0 | 22.6±8.8 | 0.29 | 31.5±10.2 | 30.1±9.9 | 0.47 |
| Lean mass* (kg) | 54.9±6.2 | 55.0±6.3 | 0.90 | 36.7±4.3 | 36.4±3.9 | 0.64 |
| Lean/fat Index | 2.83±1.31 | 2.60±1.35 | 0.37 | 1.31±0.53 | 1.33±0.45 | 0.82 |
| Percent body fat* | 27.5±7.3 | 29.0±6.9 | 0.25 | 43.7±8.0 | 43.0±7.6 | 0.60 |
| Waist (cm) | 94.2±9.5 | 97.0±9.9 | 0.13 | 88.2±12.1 | 86.5±11.1 | 0.45 |
| Waist/hip ratio | 0.92±0.04 | 0.93±0.04 | 0.51 | 0.82±0.06 | 0.81±0.06 | 0.44 |
| BMD (g/cm2) | ||||||
| Total body | 1.136±0.104 | 1.167±0.097 | 0.11 | 1.013±0.103 | 1.018±0.075 | 0.76 |
| Spine | 1.071±0.196 | 1.104±0.185 | 0.37 | 1.011±0.211 | 0.974±0.129 | 0.26 |
| Femoral neck | 0.758±0.103 | 0.777±0.093 | 0.29 | 0.712±0.089 | 0.724±0.089 | 0.47 |
| Total hip | 0.933±0.124 | 0.977±0.124 | 0.07 | 0.844±0.103 | 0.845±0.084 | 0.97 |
| Bone metabolism | ||||||
| CTX (ng/ml) | 0.44±0.27 | 0.40±0.26 | 0.35 | 0.52±0.23 | 0.54±0.23 | 0.58 |
| BSAP (U/L) | 24.8±8.47 | 24.9±9.94 | 0.96 | 25.7±10.12 | 25.9±12.6 | 0.95 |
| Endocrine outcomes | ||||||
| DHEA** (nmol/l) | 5.19 (3.31) | 5.92 (4.82) | 0.51 | 6.47 (5.99) | 5.17 (3.58) | 0.003 |
| DHEAS** (μmol/l) | 2.00 (1.17) | 2.14 (1.10) | 0.60 | 1.41 (1.33) | 1.05 (1.45) | 0.07 |
| Testost.** (nmol/l) | 16.81 (9.54) | 15.47 (6.69) | 0.15 | 0.70 (0.42) | 0.54 (0.28) | 0.01 |
| Estradiol (pmol/l) | 113.76±28.17 | 110.31±28.40 | 0.52 | 41.05±18.81 | 35.74±17.82 | 0.13 |
| IGF-1 (ng/ml) | 213.2±91.1 | 222.5±88.2 | 0.59 | 199.2±90.4 | 184.4±87.0 | 0.37 |
| IGFBP-3 (ng/ml) | 3720.2+822.3 | 3609.2±818.4 | 0.48 | 4211.9±1035.2 | 3981.2±896.8 | 0.20 |
| Life style (%) | ||||||
| Estrogen ever | - | - | - | 67.2 | 73.7 | 0.45 |
| Exercise | 38.2 | 29.1 | 0.31 | 20.7 | 8.9 | 0.08 |
| Alcohol | 51.0 | 40.0 | 0.25 | 52.7 | 47.3 | 0.23 |
Obtained from DXA scans.
Median and inter-quartile range is reported, p-values from Wilcoxon non-parametric; comparisons of means performed with t-tests
DHEA(S), testosterone and estradiol
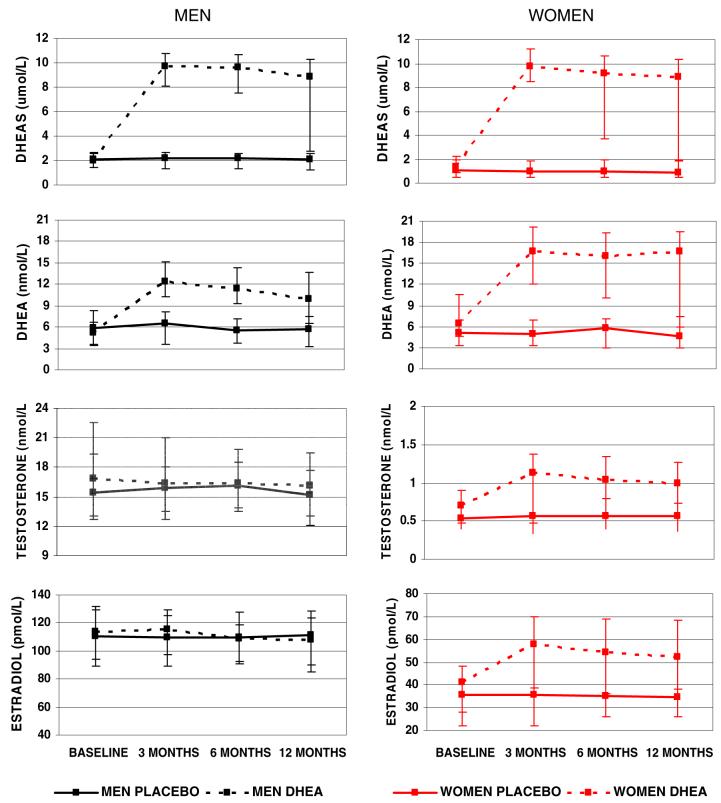
For both men and women, DHEA treatment restored DHEA and DHEAS levels to those of young adults at 3 months of treatment, and the two- to fourfold increase (P<0.001) was sustained throughout the 12 month treatment period. A significant increase in levels of testosterone and estradiol was observed in women on DHEA treatment, but no changes in these hormones were observed for men. The approximately 60% increase in testosterone and 40% increase in estradiol in women were significant at 3 months of treatment, and these higher levels were maintained throughout the trial. No changes in these hormones were seen in the placebo group at any sampling interval (Fig. 1).
Fig. 1.
Mean (median) and 95%CI (inter-quartile range) for steroids at baseline and follow-up visits, by sex and treatment group
Bone markers
Serum concentrations of CTx, a marker of bone resorption, showed a slight but significant decrease in women in the treatment group, whereas levels were constant in the placebo group (p=0.03 for treatment effect); no effect was observed among men (Table 2). Serum concentrations of BAP, a marker of bone formation, did not vary by treatment status in either men or women (Table 2).
Table 2.
Comparisons of mean bone markers, IGF1, IGFPB-3 and IGF-1/IGFBP-3 ratio at baseline and follow-up visits by treatment status in men and women
| Parameter | Baseline | 3 Months | 6 Months | 12 Months | P value |
|||
|---|---|---|---|---|---|---|---|---|
| Time | Group | Time*Group | ||||||
| Men | BSAP (U/L) | |||||||
| Placebo | 25.2 | 24.5 | 25.0 | 22.5 | ||||
| DHEA | 24.8 | 24.7 | 24.8 | 24.1 | 0.01 | 0.86 | 0.37 | |
| CTx (ng/ml) | ||||||||
| Placebo | 0.39 | 0.34 | 0.33 | 0.34 | ||||
| DHEA | 0.43 | 0.43 | 0.42 | 0.43 | 0.10 | 0.05 | 0.28 | |
| IGF-1 (ng/ml) | ||||||||
| Placebo | 220.9 | 230.1 | 223.4 | 221.8 | ||||
| DHEA | 214.1 | 235.2 | 227.1 | 231.1 | 0.008 | 0.87 | 0.58 | |
| IGFBP-3 (ng/ml) | ||||||||
| Placebo | 3590.9 | 3699.3 | 3655.4 | 3653.3 | 0.004 | 0.30 | 0.86 | |
| DHEA | 3745.6 | 3882.4 | 3781.6 | 3760.3 | ||||
| IGF-1/IGFBP-3 | ||||||||
| Placebo | 0.061 | 0.062 | 0.060 | 0.060 | 0.19 | 0.59 | 0.68 | |
| DHEA | 0.056 | 0.059 | 0.059 | 0.060 | ||||
| Women | BSAP (U/L) | |||||||
| Placebo | 25.4 | 28.2 | 27.9 | 26.2 | ||||
| DHEA | 25.9 | 26.9 | 25.9 | 26.3 | 0.08 | 0.74 | 0.46 | |
| CTx (ng/ml) | ||||||||
| Placebo | 0.54 | 0.56 | 0.57 | 0.55 | 0.50 | 0.07 | 0.03 | |
| DHEA | 0.52 | 0.48 | 0.45 | 0.47 | ||||
| IGF-1 (ng/ml) | ||||||||
| Placebo | 195.3 | 199.3 | 196.4 | 185.0 | <0.001 | 0.25 | 0.008 | |
| DHEA | 186.9 | 226.8 | 220.9 | 216.0 | ||||
| IGFBP-3 (ng/ml) | ||||||||
| Placebo | 4044.3 | 4150.9 | 4073.6 | 4068.2 | 0.025 | 0.90 | 0.45 | |
| DHEA | 4119.7 | 4127.1 | 4024.5 | 3976.3 | ||||
| IGF-1/IGFBP-3 | ||||||||
| Placebo | 0.047 | 0.047 | 0.047 | 0.042 | <0.001 | 0.08 | <0.001 | |
| DHEA | 0.045 | 0.054 | 0.054 | 0.053 | ||||
Analyses performed with mixed models adjusting for age, exercise, weight and baseline levels of DHEA
Time*Group=time by group interaction
IGF-1, IGFBP-3 and IGF-1/IGFBP-3
A significant increase in IGF-1 and the IGF-1/IGFBP-3 ratio over time was observed in women on DHEA treatment as compared to placebo (p<0.001 for both). Both IGF-1 levels and the IGF-1/IGFBP-3 ratio increased by more than 20% and these increases were maintained throughout the 12 month treatment. There was no significant effect of treatment on IGFBP-3 levels in either sex, and no significant difference in IGF-1 or the IGF-1/IGFBP-3 ratio by treatment status in men (Table 2).
BMD
After 12 months, there was a slight increase in BMD of the lumbar spine in women on DHEA treatment and a slight decrease in women on placebo (p= 0.03 for treatment effect). These differences were significant before and after adjusting for weight, physical activity and baseline values of DHEA. DHEA treatment did not significantly affect BMD of the femoral neck, hip or total body in women, and no significant changes in BMD were observed at any bone site among men. Stratification by age group or by tertile of baseline DHEA yielded similar results (data not shown) (Table 3).
Table 3.
Comparisons of mean BMD at baseline and follow-up visits and change over by treatment status time in men and women
| Parameter | Baseline | 6 months | 12 months | P value |
|||
|---|---|---|---|---|---|---|---|
| Time | Group | Time*Group | |||||
| Men | Total body BMD (g/cm2) | ||||||
| Placebo | 1.163 | 1.164 | 1.169 | ||||
| DHEA | 1.131 | 1.135 | 1.134 | 0.14 | 0.09 | 0.07 | |
| Femoral neck (g/cm2) | |||||||
| Placebo | 0.777 | 0.780 | 0.780 | 0.43 | 0.27 | 0.97 | |
| DHEA | 0.755 | 0.759 | 0.760 | ||||
| Total hip (g/cm2) | |||||||
| Placebo | 0.975 | 0.975 | 0.976 | ||||
| DHEA | 0.929 | 0.932 | 0.929 | 0.92 | 0.05 | 0.25 | |
| Lumbar spine (g/cm2) | |||||||
| Placebo | 1.106 | 1.104 | 1.104 | ||||
| DHEA | 1.061 | 1.061 | 1.066 | 0.81 | 0.23 | 0.75 | |
| Women | Total body BMD (g/cm2) | ||||||
| Placebo | 1.023 | 1.022 | 1.020 | ||||
| DHEA | 1.007 | 1.003 | 1.005 | 0.39 | 0.34 | 0.51 | |
| Femoral neck (g/cm2) | |||||||
| Placebo | 0.727 | 0.726 | 0.717 | 0.009 | 0.41 | 0.13 | |
| DHEA | 0.707 | 0.710 | 0.708 | ||||
| Total hip (g/cm2) | |||||||
| Placebo | 0.852 | 0.847 | 0.842 | ||||
| DHEA | 0.837 | 0.838 | 0.834 | 0.004 | 0.58 | 0.34 | |
| Lumbar spine (g/cm2) | |||||||
| Placebo | 0.989 | 0.979 | 0.971 | ||||
| DHEA | 0.997 | 0.995 | 1.000 | 0.19 | 0.60 | 0.03 | |
Analyses performed with mixed models adjusting for age, exercise, weight and baseline levels of DHEA
Time*Group=time by group interaction
Body composition
Body composition, whether assessed by BMI, total fat mass, abdominal fat mass, lean body mass, lean/fat index, or waist to hip ratio, was not affected by DHEA treatment in either sex (data not shown).
Compliance and adverse effects
At the 12-month follow-up, overall mean compliance was 95% in the treatment group and 94% in the placebo group. During the study, 23 participants who received DHEA replacement and ten participants who received placebo experienced adverse events that led to treatment discontinuation (Table 4). Events that contributed most frequently to treatment discontinuation were chest pain (three participants receiving DHEA and one placebo) and palpitations (four participants receiving DHEA and one placebo) and a greater than 1.4 ng/ml increase in PSA levels in men (five receiving DHEA and two placebo). PSA levels returned to normal in all but one man after discontinuing DHEA. One man was diagnosed with prostate cancer after the 3-month follow-up; it was determined that his cancer had started prior to his participation in the study.
Table 4.
Summary of adverse events leading to treatment discontinuation
| Sex | DHEA | Placebo | |
|---|---|---|---|
| Adverse events | Male/Female | N (%) | N (%) |
| Blood pressure elevations | 1/1 | - | 2 (1.8) |
| Chest pain | 3/1 | 3 (2.7) | 1 (0.9) |
| Palpitations | 3/2 | 4 (3.6) | 1 (0.9) |
| Headaches | 0/3 | 1 (0.9) | 2 (1.8) |
| Elevated liver function enzymes | 0/3 | 3 (2.7) | - |
| Anxiety | 1/0 | 1 (0.9) | - |
| Facial hair | 0/2 | 2 (1.8) | - |
| Elevated cholesterol | 0/2 | 2 (1.8) | - |
| Elevated BUN | 1/0 | - | 1 (0.9) |
| Joint pain | 0/1 | 1 (0.9) | - |
| Muscle pain | 0/1 | 1 (0.9) | - |
| Dry mouth | 1/0 | - | 1 (0.9) |
| PSA elevations (men only) | 7/0 | 5 (9.1) | 2 (3.6) |
| Total | 17/16 | 23 (20.5) | 10 (8.8) |
Discussion
Results of this clinical trial suggest a small beneficial effect of DHEA treatment on bone metabolism and bone mineral density, but only for women. Treatment with a 50 mg daily dose of DHEA for 12 months had a small but significant positive effect on BMD of the lumbar spine in women, as well as a beneficial effect on CTx, a marker of bone resorption. These results were independent of known confounders or baseline levels of DHEA. Similar positive bone effects were not observed in men and no beneficial (or adverse) effect of DHEA treatment on body composition was found in either sex. The administration of 50 mg of DHEA was safe and no major adverse effects were observed.
Unlike many published studies, men and women were recruited for the present study without selecting for lower levels of DHEA, enabling an examination of the effects of DHEA in the general population. DHEA is available over-the-counter in the United States as well as other countries, and is largely used as a preventive agent for age related diseases without a thorough understanding or careful examination of adverse effects versus possible benefits. The present study addresses the effect of DHEA in an older sample of relatively healthy community-dwelling men and women.
Evidence from previous studies indicates that DHEA supplementation may increase levels of BMD and have a beneficial effect on bone turnover markers in older adults with low levels of endogenous DHEA [5, 18], in men and women with adrenal insufficiency [5, 10, 14], and in women with anorexia nervosa [6]. A recent clinical trial with 87 men and 57 women aged 60 and older selected for low DHEAS levels, found that 75 mg of DHEA daily for men and 50 mg of DHEA daily for women administered for 2 years was associated with a small but significant increase in femoral neck BMD in men and a small but significant increase in BMD at the ultradistal radius in women when compared to participants receiving placebo [14].
The case for a beneficial effect in older individuals unselected for low levels of DHEA is less clear, with small studies using varying dosages and reporting contradictory results. For example, Labrie and colleagues [8] conducted a cross-over clinical trial with 14 women aged 60-70, and reported that use of a 10% DHEA cream for 16 months led to a significant decrease in markers of bone resorption (hydroxyproline/creatinine ratio) and bone formation (BSAP), and an increase in hip BMD, but had no effect on lumbar spine. In a clinical trial of 86 men with osteoporosis [15], daily use of 100 mg of DHEA for 6 months increased BMD at both the lumbar spine and femoral neck. In contrast, a small study of ten women and nine men aged 50 to 65 years using a 100 mg dose of DHEA and a cross-over design, reported no effect on BMD at the hip, lumbar spine or total body, and no change in urinary pyridinoline, a marker of bone resorption [9].
The only other relatively large clinical trial conducted in a sample non-selected for low DHEA was done by Baulieu and colleagues [11], who recruited 280 older French men and women attending a geriatric clinic for a variety of minor age-related symptoms, such as asthenia, memory complaints and anxiety. After 12 months of supplementation with 50 mg of DHEA versus placebo, a significant increase was found in BMD at the femoral neck and Ward’s triangle (trabecular bone) in women less than 70yrs old, and at the total radius in women 70 years and older. As in our study, no effect of DHEA was found among the men. The results from Baulieu and colleagues along with those observed in the present study suggest a sex-specific effect of DHEA on bone metabolism in healthy older adults.
Body mass index is recognized as one of the strongest predictors for BMD in both men and women [16, 17], and recent studies suggest that body composition has a stronger contribution as a determinant of BMD than body size [18-21]. However, in accord with most previous studies [5, 10, 14, 22, 23], DHEA supplementation in this trial did not alter measures of body size and body composition in either men or women, suggesting that the small effect of DHEA on bone was not mediated by an effect on body composition.
The sex-specific biotransformation of exogenously administered DHEA might help explain the sex differences observed in this study. Both testosterone and estradiol levels were increased in women receiving DHEA treatment as seen in other studies [11, 14, 24, 25], while no changes were observed among the men. The apparent absence of any discernible bioconversion of DHEA to active sex hormones among men, despite a two- to fourfold increase in circulating DHEA levels was unexpected. While most studies using 50 to 100 mg of DHEA also reported no change in testosterone levels in men [4, 9, 11, 14, 22, 26-28], most have described a change in estradiol. An increase in estradiol levels was reported by Kahn and colleagues after 6 months of daily DHEA supplementation (90 mg) in 43 healthy men aged 56 to 80 years old [4], and by Baulieu and colleagues after administering 50 mg of DHEA daily for 12 months to 140 healthy men 60 to 79 years old [11]. Recently, Nair and colleagues reported an increase in estradiol levels after 24 months using 75 mg/daily in 87 men aged 60 years and older [14]. However, no effect was reported by Morales and colleagues in two different studies using doses of 50 and 100 mg DHEA daily for 6 months [9, 26], or by Villareal and colleagues using a daily dose of 50 mg for 6 months [29]. It is possible that a longer duration or higher dose than that used in the present study is necessary for DHEA supplementation to augment estrogen levels in men.
In accord with previous studies [26, 30], we found that DHEA increased the concentration of circulating IGF-1 in women, but unlike the same studies, we did not find any effect in men. Insulin-like growth factors are growth-promoting polypeptides that have an important role in regulating osteoblastic and osteoclastic functions, and maintaining bone mass [31]. Previous animal and human studies provide indirect support for the hypothesis that circulating IGF-1 plays an important role in the acquisition of bone mass [32, 33]. Cross-sectional studies show positive correlations between serum IGF-1 and BMD in areas with a high proportion of trabecular bone and high bone turnover such as the spine [34]. Langlois et al. [35] reported a relatively strong correlation between serum IGF-1 levels and BMD at several sites in women from the Framingham cohort, and low serum levels of IGF-1 were also associated with greater risk of hip fractures among a large cohort of older postmenopausal women in France [36]. Systemic and local effects of IGF-1 are modulated by a group of proteins, the insulin-like growth factor binding proteins (IGFBPs), which have high affinity and specificity for the IGFs. Approximately 75% of the IGF-1 in the circulation is carried in a complex with IGFBP-3 [37], and IGFBP-3 is believed to play a significant role in regulation of the bone remodeling process in humans by enhancing the anabolic effects of IGF-1 on bone [31]. IGFBP-3 levels were not changed in this trial, but the 20% increase in IGF-1 in women resulted in a significant elevation of the IGF-1/IGFBP-3 ratio. The selective increase in both IGF-1 and lumbar spine BMD, a site with abundant trabecular bone among the women randomized to DHEA suggests a causal link.
The observed beneficial changes in BMD and bone markers in women may reflect the combined effects of peripheral conversion of DHEA to sex steroids and a DHEA-induced increase in circulating IGF-1. Similar results were not observed in men, and the clinical significance of DHEA in preventing or treating osteoporosis in women is questionable. Longer trials may be necessary to allow for a positive and clinically significant effect of DHEA on bone mineral density. Although there were no serious adverse events related to DHEA treatment during this study, other more effective and as well tolerated treatments for osteoporosis are available.
Acknowledgments
The DAWN study was funded by the National Institute on Aging, grant AG018339 and the National Institute of Health, grant M01 RR00827.
Footnotes
Conflicts of interest
None.
References
- 1.Villareal DT. Effects of dehydroepiandrosterone on bone mineral density: what implications for therapy? Treat Endocrinol. 2002;1:349–357. doi: 10.2165/00024677-200201060-00001. [DOI] [PubMed] [Google Scholar]
- 2.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 3.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6 in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 4.Kahn AJ, Halloran B, Wolkowitz O, Brizendine L. Dehydroepiandrosterone supplementation and bone turnover in middle-aged to elderly men. J Clin Endocrinol Metab. 2002;87:1544–1549. doi: 10.1210/jcem.87.4.8396. [DOI] [PubMed] [Google Scholar]
- 5.Callies F, Fassnacht M, van Vlijmen JC, Koehler I, Huebler D, Seibel MJ, Arlt W, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab. 2001;86:1968–1972. doi: 10.1210/jcem.86.5.7483. [DOI] [PubMed] [Google Scholar]
- 6.Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, Rosen CJ, Gundberg CM, LeBoff MS. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 7.Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandroster-one supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001;86:4686–4692. doi: 10.1210/jcem.86.10.7974. [DOI] [PubMed] [Google Scholar]
- 8.Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3498–3505. doi: 10.1210/jcem.82.10.4306. [DOI] [PubMed] [Google Scholar]
- 9.Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49:421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006;91:2986–2993. doi: 10.1210/jc.2005-2484. [DOI] [PubMed] [Google Scholar]
- 11.Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharriere O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Muhlen D, Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. The Dehydroepiandrosterone and WellNess (DAWN) study: research design and methods. Contemp Clin Trials. 2007;28:153–168. doi: 10.1016/j.cct.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 14.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, III, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Mao M, Sun L, Feng Y, Yang J, Shen P. Treatment of osteoporosis in men using dehydroepiandrosterone sulfate. Chin Med J (Engl) 2002;115:402–404. [PubMed] [Google Scholar]
- 16.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 17.van der Voort DJ, Geusens PP, Dinant GJ. Risk factors for osteoporosis related to their outcome: fractures. Osteoporos Int. 2001;12:630–638. doi: 10.1007/s001980170062. [DOI] [PubMed] [Google Scholar]
- 18.Cui LH, Shin MH, Kweon SS, Park KS, Lee YH, Chung EK, Nam HS, Choi JS. Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab. 2007;25:165–171. doi: 10.1007/s00774-006-0747-3. [DOI] [PubMed] [Google Scholar]
- 19.Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res. 2001;16:2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 21.Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003;6:151–156. [PubMed] [Google Scholar]
- 23.Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, Allen S, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84:1527–1533. doi: 10.1210/jcem.84.5.5672. [DOI] [PubMed] [Google Scholar]
- 24.Genazzani AD, Stomati M, Bernardi F, Pieri M, Rovati L, Genazzani AR. Long-term low-dose dehydroepiandroster-one oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril. 2003;80:1495–1501. doi: 10.1016/j.fertnstert.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Stomati M, Rubino S, Spinetti A, Parrini D, Luisi S, Casarosa E, Petraglia F, Genazzani AR. Endocrine, neuroendocrine and behavioral effects of oral dehydroepiandrosterone sulfate supplementation in postmenopausal women. Gynecol Endocrinol. 1999;13:15–25. doi: 10.1080/09513599909167527. [DOI] [PubMed] [Google Scholar]
- 26.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 27.Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VK. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656. doi: 10.1210/jcem.85.12.7022. [DOI] [PubMed] [Google Scholar]
- 28.Libe R, Barbetta L, Dall’Asta C, Salvaggio F, Gala C, Beck-Peccoz P, Ambrosi B. Effects of dehydroepiandrosterone (DHEA) supplementation on hormonal, metabolic and behavioral status in patients with hypoadrenalism. J Endocrinol Invest. 2004;27:736–741. doi: 10.1007/BF03347515. [DOI] [PubMed] [Google Scholar]
- 29.Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 2000;53:561–568. doi: 10.1046/j.1365-2265.2000.01131.x. [DOI] [PubMed] [Google Scholar]
- 30.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- 31.Rosen CJ, Donahue LR, Hunter SJ. Insulin-like growth factors and bone: the osteoporosis connection. Proc Soc Exp Biol Med. 1994;206:83–102. doi: 10.3181/00379727-206-43726. [DOI] [PubMed] [Google Scholar]
- 32.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen CJ, Dimai HP, Vereault D, Donahue LR, Beamer WG, Farley J, Linkhart S, Linkhart T, Mohan S, Baylink DJ. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone. 1997;21:217–223. doi: 10.1016/s8756-3282(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 34.Vestergaard P, Hermann AP, Orskov H, Mosekilde L. Effect of sex hormone replacement on the insulin-like growth factor system and bone mineral: a cross-sectional and longitudinal study in 595 perimenopausal women participating in the Danish Osteoporosis Prevention Study. J Clin Endocrinol Metab. 1999;84:2286–2290. doi: 10.1210/jcem.84.7.5865. [DOI] [PubMed] [Google Scholar]
- 35.Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab. 1998;83:4257–4262. doi: 10.1210/jcem.83.12.5308. [DOI] [PubMed] [Google Scholar]
- 36.Gamero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355:898–899. doi: 10.1016/s0140-6736(99)05463-x. [DOI] [PubMed] [Google Scholar]
- 37.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]