Abstract
The evidence for common genetic and environmental influences on conduct disorder (CD) and major depressive disorder (MDD) in adolescents was examined. A sample of 570 monozygotic twin pairs, 592 dizygotic twin pairs, and 426 non-twin siblings, aged 12−18 years, was recruited from the Colorado Twin Registry. For the past year data, there was a significant correlation between the genetic influences on MDD and CD and, for the lifetime data, there was a significant correlation between the genetic influences on MDD and CD, and a significant correlation between the nonshared environmental influences on MDD and CD. Our results suggest that some genetic factors will increase an individual's vulnerability to both MDD and CD in adolescence.
Keywords: major depressive disorder, conduct disorder, comorbidity
Knowledge regarding the magnitude of common genetic and/or environmental influences on MDD and CD has the potential of informing future research examining the etiology and development of both disorders. Finding evidence of common genetic influences should inform future studies examining quantitative trait loci influencing both disorders. For example, finding evidence that a candidate gene is a significant risk factor for one of the disorders would indicate that the same gene may be a risk factor for the other disorder also. Similarly, finding evidence of common environmental influences will inform future studies of specific environmental risk factors. Explaining why MDD and CD co-occur also could influence the clinical approach to both disorders, making prevention and treatment more effective. In the present study, we examined whether common genetic and/or environmental influences explain the co-occurrence between MDD and CD.
MDD is an internalizing disorder characterized by a persistent sad or irritable mood (Diagnostic and statistical manual of mental disorders, fourth edition, [DSM-IV], American Psychiatric Association [APA], 1994), and CD is an externalizing disorder characterized by a pattern of antisocial behavior that is repetitive and persistent and in which others’ basic rights or major societal norms are violated (DSM-IV, APA, 1994). There is evidence of a clear distinction between internalizing and externalizing disorders (e.g., Krueger, McGue, & Iacono, 2001). Internalizing disorders are characterized by a propensity to express distress inwards (Krueger et al.), and individuals with these disorders are prone to emotional withdrawal, sadness, low impulsivity, and low attentional regulation (Eisenberg et al., 2001). Externalizing disorders are characterized by a propensity to express distress outwards (Krueger et al.), and individuals with these disorders are prone to anger, impulsivity, and low attentional and behavioral regulation (Eisenberg et al.). However, there is also significant covariance between internalizing and externalizing behavior and between internalizing and externalizing disorders (e.g., Lilienfeld, 2003; Loeber & Keenan, 1994).
The prevalence of MDD in the U.S. has been reported as being as high as 2.5 percent in children and 8.3 percent of adolescents (Birmaher et al., 1996). Boys and girls are equally affected in childhood, but by adolescence, depression affects twice as many girls as boys. Some of the negative outcomes associated with adolescent depression include suicide, substance abuse, and poor psychosocial functioning (Birmaher, Brent, & Benson, 1998). In populations of individuals under 18 years of age, the estimated prevalence of CD ranges from 6% to 16% for males, and from 2% to 9% in females. Some of the risks associated with CD include substance abuse and antisocial personality disorder in adulthood (DSM-IV, APA, 1994).
Genetic and Environmental Influences on Depression and Conduct Disorder
Twin studies take advantage of the fact that monozygotic (MZ) twins share 100% of their genes, whereas dizygotic (DZ) twins, who are like ordinary sibling pairs genetically, share 50% of their genes identical by descent. Therefore, MZ twins should be more similar than DZ twins on a trait/disorder influenced by genes. Twin studies examine the extent to which observed individual differences are due to differences in genetic, shared environmental, or nonshared environmental influences. Heritability (“A” or “h2”), is the proportion of observed differences that can be explained by genetic differences. The two types of environmental influences are: 1) shared environmental influences (“C” or c2”), environmental influences that make family members similar to each other, and 2) nonshared environmental influences (“E” or “e2”), environmental influences that make family members different from each other. When examining the reasons for overlap between two traits or disorders, such as MDD and CD, the correlation between genetic factors is expressed as rg, the correlation between shared environmental factors is expressed as rc, and the correlation between nonshared environmental influences is expressed as re.
Rice, Harold, and Thapar (2002) reviewed the results from twin studies examining depressive symptoms in childhood and adolescence using either depression-specific questionnaires or the internalizing problem scale from the Child Behavior Checklist (CBCL; Achenbach, 1991; Achenbach & Edelbrock, 1983). Results of these studies are inconsistent, with heritability estimates varying widely across studies and heritability estimates being lower when child self-ratings were used (h2 = .15 to .80) than when parent ratings were used (h2 = .30 to .80). Most twin studies have estimated the heritability of depressive symptoms, not MDD. Recently, Glowinski, Madden, Bucholz, Lynskey, and Heath (2003) examined DSM-IV (APA, 1994) diagnoses of MDD in a population-based sample of female adolescent twins, and reported that variance in MDD is due to genes and nonshared environmental influences (h2= .40, c2=.00, e2=.60).
Twin studies have shown antisocial behavior is also heritable (e.g., Eaves et al., 1993; Hudziak, Rudiger, Neale, Heath, & Todd, 2000; Rowe, 1983). Rhee and Waldman (2002) conducted a meta-analysis of twin and adoption studies examining antisocial behavior, and reported that for the 5 studies that investigated CD, the best-fitting model included moderate additive genetic influences, modest shared environmental influences, and moderate nonshared environmental influences (h2= .50, c2=.11, e2=.39).
Co-occurrence of MDD and CD
The rate of co-occurrence between MDD and CD is higher than that expected by chance. Kovacs, Paulauskas, Gatsonis, and Richards (1988) reported that in a cohort of depressed children, 16% of cases displayed conduct-depressive disorder comorbidity, a figure that rose to 23% after a follow-up. They also found that the time-corrected cumulative risk of CD among depressed children was 36% by age 19. This prevalence is considerably higher than the prevalence of CD in the general population (2−16%; APA, 1994). Angold and Costello (1993) conducted a literature review that used DSM-III (APA, 1980) or DSM-III-R (APA, 1987) criteria and standardized diagnostic interviews. They found that reported rates of comorbidity of depression and CD/oppositional defiant disorder ranged from 21% to 83% and noted that the mechanisms for comorbidity were unknown.
Three main hypotheses explaining the co-occurrence between MDD and CD have been discussed in the literature (e.g., Lilienfeld et al. 2003). First, early depressive symptoms predict conduct problems (e.g., Loeber, Russo, Stouthamer-Loeber, & Lahey 1994; Capaldi, 1992), suggesting the possibility that MDD may play a causal role in the development of CD. Second, early conduct problems predict later depressive symptoms (e.g., Capaldi, 1992), suggesting the possibility that CD plays a causal role in the development of MDD. Third, the co-occurrence between MDD and CD may be due to the same underlying causal factors. For example, several researchers have suggested the negative emotionality, or the increased tendency to experience negative emotions frequently and intensely, as a common feature that distinguishes both internalizing and externalizing disorders from normality (e.g., Keiley, Lofthouse, Bates, Dodge, & Pettit, 2003; Lilienfeld, 2003). Also, others have examined family characteristics such as parental negativity, parental bonding, and parent-adolescent communications as possible common risk factors for depression and conduct problems (e.g., Beyers & Loeber, 2003; Heaven, Newbury, & Mak, 2004; Pike, McGuire, Hetherington, Reiss, & Plomin, 1996).
Unfortunately, the ability to test causal hypotheses using cross-sectional family data is limited (e.g., Duffy & Martin, 1994; Heath et al., 1993). Twin studies examining the co-occurrence between MDD and CD or related constructs such as internalizing and externalizing behavior have focused on examining the degree to which the co-occurrence can be attributed to genetic, shared environmental, and nonshared environmental influences.
Common Genetic or Environmental Risk Factors for MDD and CD
Gjone and Stevenson (1997) examined the broader construct of internalizing and externalizing behavior problems assessed by the Child Behavior Checklist (CBCL), a parent questionnaire, in 1,529 child and adolescent twin pairs. They examined the proportions of variance for internalizing and externalizing behavior that could be attributed to genetic, shared environmental, and nonshared environmental influences and divided them into factors specific to either internalizing or externalizing behavior and factors common to both. In general, Gjone and Stevenson concluded that there are both common genetic and shared environmental influences on the covariance between internalizing and externalizing behavior, but that there is more consistent evidence for common shared environmental influences (although estimates of shared environmental influences may have been inflated by rater bias given that the same individual rated both twins in a pair), and little evidence of nonshared environmental influences common to both types of behavior.
O'Connor et al. (1998) examined a sample of 720 families with two target same-sex siblings that varied in genetic relatedness, including MZ twins, DZ twins, full siblings, half siblings, and unrelated siblings. They examined the covariation between depression and antisocial behavior assessed using a composite of mother, father, and self-report on questionnaires and observers’ report in adolescents. Genetic, shared environmental, and nonshared environmental influences were responsible for the covariation between antisocial and depressive symptoms (h2= .45, c2=.30, e2=.25).
Kendler, Prescott, Myers, and Neale (2003) examined the covariance between internalizing and externalizing disorders in an adult sample of 5600 twins. They examined 7 disorders: major depression, generalized anxiety disorder, phobia, alcohol dependence, drug abuse/dependence, adult antisocial behavior, and conduct disorder, using diagnostic criteria from DSM-III-R (APA, 1987) and DSM-IV (APA, 1994). They divided the disorders into internalizing (i.e. major depression, generalized anxiety disorder, and phobia), and externalizing (i.e. alcohol dependence, drug abuse/dependence, adult antisocial behavior, and conduct disorder), and then examined whether the genetic, shared environmental, and nonshared environmental factors influencing internalizing disorders also influenced externalizing disorders, and vice versa. They found that the genetic factors influencing externalizing disorders (.14 to .42) had little influence on internalizing disorders (.00 to .06), and that the genetic factors influencing internalizing disorders (.11 to .29) had little influence on externalizing disorders (.01 to .05). Their common externalizing genetic factor did have a positive, albeit small, loading on MDD, and their common internalizing genetic factor did have a positive, albeit small, loading on CD. Although they found a fairly clear distinction between genetic influences on externalizing disorders and genetic influences on internalizing disorders, they did not find a similarly clear distinction for shared environmental influences and nonshared environmental influences on externalizing versus internalizing disorders.
In summary, the evidence from these studies for common genetic or environmental influences on internalizing behavior/depressive symptoms and externalizing behavior/antisocial behavior is inconclusive. The studies varied in the construct examined (internalizing and externalizing behavior problems in Gjone and Stevenson, 1997, depression and antisocial behavior in O'Connor et al., 1998, and internalizing and externalizing disorders in Kendler et al., 2003), the assessment method used (parent report in Gjone and Stevenson, a composite of measures in O'Connor et al., and self report in Kendler et al.), and the age of the participants examined (children and adolescents in Gjone and Stevenson and O'Connor et al. and adults in Kendler et al.). The discrepancies in the results of these studies may be due to these methodological differences.
The goal of the present study was to determine the extent to which common genetic, shared environmental, and nonshared environmental influences explain the covariation between MDD and CD in adolescents. The few studies that have examined common genetic and environmental influences on broader constructs of depressive symptoms/internalizing behavior and antisocial behavior/externalizing behavior have yielded inconsistent results. We examined DSM-IV symptoms and diagnoses to examine the more severe end of the continuum of psychopathology that has been relatively neglected. However, the present study is an examination of diagnostic measures in a general population sample, possibly limiting the generalizability of the results to clinical samples.
Method
Participants
The participants were twin pairs and their siblings participating in the twin study component of the Center for the Genetics of Antisocial Drug Dependence (CADD). They were recruited from the Colorado Twin Registry, and include participants from two samples, the Longitudinal Twin Study and the Colorado Twin Study. Participants from the Longitudinal Twin Study have been studied since birth, and participated in the present study approximately at age 12. The Colorado Twin Study participants were assessed for the first time by CADD, and were age 12−18 at assessment.
Initial contact information for twin pairs was obtained from the Colorado Department of Health, Division of Vital Statistics (CDH) and participating school districts in Colorado. CDH mailed inquiry letters on behalf of the Colorado Twin Registry, and their attempt to collect contact information was not systematic and thorough in all years. Participating school districts used varying methods to provide contact information to the Colorado Twin Registry, with some districts sending the list of putative twins directly and other districts mailing inquiry letters on behalf of the Colorado Twin Registry. Therefore, it is not possible to calculate the exact response rate in the Colorado Twin Registry. More detailed information regarding the Colorado Twin Registry is available in Rhea, Gross, Haberstick, and Corley (in press).
The present study included 570 MZ twin pairs, 592 DZ twin pairs, of which 370 were same-sex twin pairs and 222 were opposite-sex twin pairs, and 426 non-twin siblings of twins. The age range of this sample was 12−18 years (born from 1979−1990), and 48% of participants were male and 52% were female. The ethnic distribution of the sample was 85% White, 2% African-American, 8% Hispanic, 3% Asian, 2% Native American, and 1% unknown, as reported by the participants on a questionnaire, and is similar to the ethnic distribution of live births in Colorado reported for the years 1980−1985 (approximately 80% White, 4% African-American, 14% Hispanic, 2% Asian, and less than 1% Native American).
Procedure
Participants received a written description of the study and signed written informed assent (if they were minors) or consent (if they were adult participants or guardians of minor participants). Two sources determined zygosity. First, interviewers completed a nine-item assessment of physical characteristics. Second, the concordance of the twin pairs’ genotype at 11 highly informative short tandem repeat polymorphisms was examined. Twin pairs who were physically similar and had matching genotypes were classified as MZ, whereas those who were physically dissimilar and did not have matching genotypes were classified as DZ. Any discrepancies between the results of the two sources of zygosity determination were re-examined and resolved.
Interviewers administered the youth informant version of the Diagnostic Interview Schedule for Children – IV (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), a structured psychiatric interview, which assesses DSM-IV symptoms and diagnoses for Axis I disorders, including MDD and CD. The adolescents answered questions regarding their own psychiatric symptoms. The DISC-IV includes questions about experiences over the past year, as well as over the lifetime. In accordance with instructions from the instrument's authors, computer algorithms were developed to determine the presence or absence of symptom/behavioral patterns for each disorder. Individuals were categorized as having no symptoms, one or more symptoms, or a diagnosis of a disorder for the past year and lifetime.
Schwab-Stone et al. (1996) examined the validity of an earlier version of the DISC (DISC 2.3) and reported kappas (i.e., agreement with clinicans’ ratings) of .77 for CD and .79 for major depression for youth reports. Shaffer et al. (1996) reported test-retest reliabilities of the youth informant DISC 2.3 of .92 for CD and .40 for major depression/dysthymia. More recently, Shaffer, Fisher, Lucas, Dulcan, and Schwab-Stone (2000) reported test-retest reliabilities of the youth informant DISC-IV of .65 for CD and .92 for major depressive episode.
Analyses
In twin studies, genetic influence is implied by the extent to which MZ twins are more similar to one another than DZ twins and siblings are to one another. Therefore, greater MZ than DZ or sibling pair correlations indicate the presence of genetic influences. Nonshared environmental influences are implied by the extent to which siblings, and particularly MZ twins, are dissimilar. Finally, shared environmental influences account for the remainder of observed variance and are implicated when the DZ twin and/or full sibling correlation exceeds half of the MZ twin correlation (Plomin, DeFries & McClearn, 1990). The addition of nontwin siblings increases power to detect genetic and shared environmental influences (Posthuma & Boomsma, 2000) and allows the estimation of the influences of special twin environment, t2. MZ twins are correlated 1.0 for additive genetic influence, whereas DZ twins and siblings are correlated 0.5. By definition, for all twin and sibling pairs, shared environmental variance is correlated 1.0 and nonshared environmental variance is correlated 0.0 (Plomin et al., 1990). Special twin environment is correlated 1.0 for MZ and DZ twins and is 0.0 for ordinary siblings.
The present study is a follow-up to Ehringer, Rhee, Young, Corley, and Hewitt's (2006) examination of genetic and environmental influences on a variety of adolescent disorders, including MDD and CD in a sample of twins and siblings. The univariate results for the data examined in this study are detailed in Ehringer et al. but will be described here briefly.
The results from the full model examining MDD suggested additive genetic, shared environmental, and nonhsared environmental influences, but no twin-specific environmental influences (past year – a2 = .16, c2 = .25, t2 = .00, e2 = .58; lifetime – a2 = .10, c2 = .20, t2 = .00, e2 = .71). Both the model including additive genetic influences and nonshared environmental influences (i.e., the AE model; past year – a2 = .47, e2 = .52; lifetime – a2 = .34, e2 = .66) and the model including shared environment and nonshared environment fit well (i.e., the CE model; past year – c2 = .37, e2 = .63; lifetime – c2 = .27, e2 = .73), although the latter model fit slightly better. In contrast, a model including only nonshared environment did not fit the data well. Thus, both genes and shared environment are probably influential, but there was not enough power to discriminate between these two sources of family resemblance. No sex differences were found in the univariate results for MDD.
The results from the full model examining CD suggested additive genetic, twin-specific environmental influences, and nonshared environmental influences, and little or no shared environmental influences (past year – a2 = .32, c2 = .06, t2 = .14, e2 = .48; lifetime – a2 = .40, c2 = .00, t2 = .16, e2 = .44). Shared environmental influences and twin-specific environmental influences could be dropped from the full model without a significant decrement in fit, but additive genetic influences could not be dropped from the full model, and the model including additive genetic influences and nonshared environmental influences (i.e., the AE model; past year – a2 = .53, e2 = .47; lifetime – a2 = .56, e2 = .44) was the best fitting model. No sex differences were found in the univariate results for CD.
The purpose of bivariate modeling is to partition the correlation between two traits, behaviors, or disorders (e.g., MDD and CD) into its genetic and environmental components. Information comes from cross-sibling, cross-trait correlations, i.e. one participant's score on one construct with his/her sibling's on the other construct (e.g. O'Connor et al., 1998). A genetic influence on the covariance between MDD and CD is suggested if MZ twins’ cross-sibling, cross-trait correlations are greater than those of DZ twins or full siblings.
Given that DSM-IV symptom counts for MDD and CD are highly skewed, the data were analyzed assuming that a normal continuous liability distribution underlies the ordinal assessments for all variables (i.e., 0 = no symptoms, 1 = one or more symptoms, and 2 = diagnosis). This is an optimum approach, because it retains the statistical advantages conferred by the normality assumptions for the underlying liability, retains an explicit mapping between the underlying liability and observed behavior, and correctly recovers the underlying correlations and parameter estimates (Stallings et al., 2001).
Polychoric correlations for MDD and CD and cross-sibling, cross-trait correlations were estimated for MZ and DZ twin pairs, and for twin-sibling pairs. The prevalence of these disorders increases with age and varies by sex (see Table 1), with MDD symptoms and MDD diagnoses being more prevalent in females than in males, and CD symptoms and CD diagnoses being more prevalent in males than in females. If correlations are calculated without addressing these age and sex differences in the prevalences, the results may be biased (e.g., Howell, 2004). Therefore, we estimated thresholds (i.e. prevalences) for each age (by year) and sex in the entire sample, then fixed the threshold to the age- and sex-specific threshold appropriate for each participant1. Here, we are assuming that the presence of symptoms or diagnoses of MDD and CD manifested at an earlier age and that of CD manifested in females and MDD manifested in males are indicative of a more severe underlying liability.
Table 1.
Prevalence of Conduct Disorder (CD) and Major Depressive Disorder (MDD) Symptoms and Diagnosis by Age and Sex
| Sample Size | CD Symptoms | CD Diagnosis | MDD Symptoms | MDD Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females |
| Past Year | ||||||||||
| 12 | 285 | 311 | .14 | .07 | .01 | .01 | .01 | .01 | .00 | .00 |
| 13 | 123 | 113 | .24 | .12 | .05 | .01 | .02 | .04 | .00 | .02 |
| 14 | 213 | 217 | .24 | .12 | .04 | .01 | .02 | .03 | .01 | .03 |
| 15 | 168 | 197 | .25 | .20 | .10 | .02 | .02 | .10 | .02 | .03 |
| 16 | 170 | 201 | .25 | .20 | .05 | .02 | .04 | .08 | .01 | .05 |
| 17 | 193 | 186 | .28 | .17 | .08 | .01 | .05 | .06 | .02 | .05 |
| 18 | 163 | 210 | .34 | .19 | .03 | .01 | .04 | .08 | .01 | .06 |
| Lifetime | ||||||||||
| 12 | 285 | 311 | .26 | .25 | .05 | .01 | .02 | .04 | .01 | .00 |
| 13 | 123 | 113 | .36 | .32 | .15 | .03 | .04 | .04 | .01 | .05 |
| 14 | 213 | 217 | .45 | .34 | .10 | .03 | .03 | .05 | .02 | .04 |
| 15 | 168 | 197 | .44 | .43 | .20 | .04 | .06 | .15 | .04 | .07 |
| 16 | 170 | 201 | .51 | .39 | .14 | .10 | .05 | .13 | .03 | .13 |
| 17 | 193 | 186 | .45 | .45 | .27 | .06 | .09 | .09 | .07 | .12 |
| 18 | 163 | 210 | .48 | .44 | .26 | .12 | .09 | .12 | .09 | .17 |
We conducted model-fitting on raw data by using the statistical package Mx (Neale, Boker, Xie & Maes, 1999). Given the significant sex difference in the prevalence of MDD and CD, we tested whether there are sex differences in the magnitude of genetic and environmental influences on MDD and CD and sex differences in the magnitude of the correlation between genetic and environmental influences on MDD and CD (Neale & Cardon, 1992). Sex and age differences in the prevalence of MDD and CD were addressed by using age- and sex-dependent thresholds in all analyses2. The homogeneity model assumes that the same genetic and environmental influences affect males and females to the same extent, and constrains the parameter estimates to be equal in males and females. The heterogeneity model assumes that the same genetic and environmental influences affect males and females, but that the magnitude of genetic and environmental influences and the magnitude of the correlation between genetic and environmental influences on MDD and CD differ for males and females, and the parameter estimates are free to vary in males and females. If these parameter estimates can be constrained to be equal in males and females, the results would suggest that the magnitude of genetic and environmental influences on MDD and CD and the magnitude of the correlation between genetic and environmental influences on MDD and CD are similar for males and females.
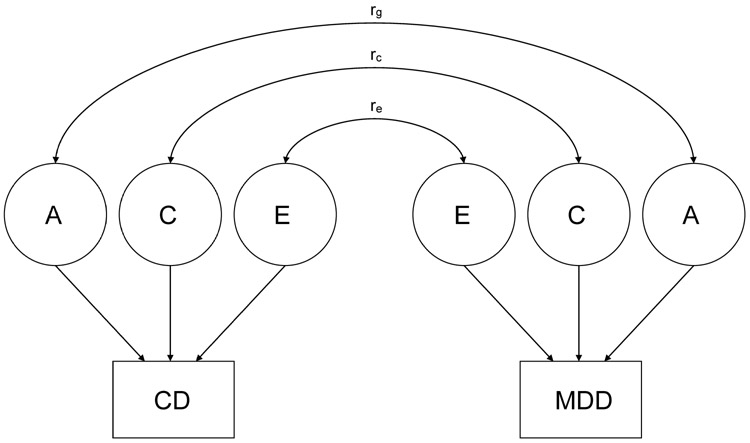
We began with a full ACE model (see Figure 1) that included additive genetic influences (A), environmental influences shared by all siblings (C), and nonshared environmental influences (E) for both MDD and CD, given evidence of genetic (A), shared environmental influences (C) and nonshared environmental influences (E) on MDD in Ehringer et al. (2006). Models including nonadditive genetic influences (D) and special twin environment (T) were not fit, because D and T were not statistically significant for both MDD and CD in univariate analyses (Ehringer et al., 2006). The model also contained rg, a correlation between genetic influences on MDD and CD, rc, a correlation between shared environmental influences on MDD and CD, and re, a correlation between nonshared environmental influences on MDD and CD. We then examined models in which rg, rc, or re were constrained to be zero and tested whether these parameters could be dropped without a significant worsening of fit. If dropping rg, rc, or re leads to significant worsening of fit, the result suggests that there is a significant correlation between genetic influences, shared environmental influences, or nonshared environmental influences, respectively, on MDD and CD.
Figure 1.
Full ACE model. A = genetic influences; C = shared environmental influences; E = nonshared environmental influences; CD = conduct disorder; MDD = major depressive disorder; rg = correlation between genetic influences; rc = correlation between shared environmental influences; re = correlation between nonshared environmental influences.
Results
Prevalence of MDD and CD Symptoms and Diagnosis
During the past year, 19% of the sample had CD symptoms (but did not meet diagnosis) and 3% of the sample had a CD diagnosis, whereas 4% of the sample had MDD symptoms (but did not meet diagnosis) and 2% of the sample had a MDD diagnosis. During their lifetime, 39% of the sample had CD symptoms (but did not meet diagnosis) and 10% of the sample had a CD diagnosis, whereas 7% of the sample had MDD symptoms (but did not meet diagnosis) and 6% of the sample had an MDD diagnosis. Table 1 presents the prevalence of CD and MDD symptoms and diagnosis by age and sex.
Correlations
The phenotypic correlation between MDD and CD was .29 for past year MDD and CD and .27 for lifetime MDD and CD. The MZ, DZ, and twin-sib correlations for MDD, CD, and the cross-trait cross-twin/sibling correlations are presented in Table 2. There were 570 MZ twin pairs and 592 DZ twin pairs. For 426 of these twin pairs, an additional non-twin sibling was assessed (i.e., there were 426 trios of twin 1, twin 2, and a non-twin sibling). The twin-sib correlation is the correlation between each of the twins and the non-twin sibling (i.e., the correlation between ordinary sibling pairs). The twin 1-sibling correlation and the twin 2-sibling correlation were constrained to be equal. Also, the twin-sib correlation was constrained to be equal across the MZ and DZ groups, given that the degree of sharing of genetic and environmental influences is the same between one of the twins and the non-twin sibling whether the twin is part of a MZ or DZ twin pair.
Table 2.
Polychoric Correlations for Past Year and Lifetime Measures of Conduct Disorder (CD) and Major Depressive Disorder (MDD)
| Sample Size | CD | MDD | Cross-Twin Cross-Trait | |
|---|---|---|---|---|
| Past Year | ||||
| MZ | 570 pairs | .51 | .41 | .22 |
| DZ | 592 pairs | .32 | .11 | .17 |
| Twin-Sib | 426 trios | .21 | .36 | .16 |
| Lifetime | ||||
| MZ | 570 pairs | .56 | .31 | .19 |
| DZ | 592 pairs | .31 | .07 | .05 |
| Twin-Sib | 426 trios | .21 | .30 | .10 |
For both past year and lifetime data, the MZ correlations for MDD and CD were higher than the DZ and twin-sib correlations for MDD and CD, suggesting genetic influences on these disorders. Also, for both past year and lifetime data, the MZ cross-trait cross-twin correlation was higher than the DZ cross-trait cross-twin correlation or the twin-sib cross-trait cross-sibling correlation, suggesting common genetic influences on MDD and CD.
Biometrical modeling
Table 3 shows the results of the heterogeneity model, which allows sex differences in the magnitude of genetic and environmental influences on MDD and CD and the magnitude of correlations between the genetic and environmental influences on MDD and CD, and the homogeneity model, where these parameters are constrained to be equal for the two sexes. Constraining these parameters to be equal in the homogeneity model did not lead to a significant decrement in the fit of the model, suggesting that there are no significant sex differences in the magnitude of genetic and environmental influences on MDD and CD or the magnitude of the correlation between genetic and environmental influences on MDD and CD. Therefore, the data for males and females were combined for all analyses. Sex differences in the prevalence of MDD and CD were addressed by using sex-dependent thresholds in all analyses.
Table 3.
Model Fitting Results Examining Sex Differences
| −2 ln L | df | Δχ2 | df | p | |
|---|---|---|---|---|---|
| Past Year | |||||
| Homogeneity | 4577.67 | 5493 | |||
| Heterogeneity | 4569.22 | 5486 | 8.45 | 7 | .29 |
| Lifetime | |||||
| Homogeneity | 7083.51 | 5493 | |||
| Heterogeneity | 7082.82 | 5486 | 0.69 | 7 | .99 |
Note. −2 ln L = −2 log likelihood; df = degrees of freedom; Δχ2 = difference in chi square from homogeneity model; p = probability.
Table 4 presents bivariate model fitting results from alternative biometrical models. The full ACE model includes A (genetic influences), C (shared environmental influences), and E (nonshared environmental influences) for both disorders and rg (the correlation between genetic influences on each disorder), rc (the correlation between shared environmental influences on each disorder), and re (the correlation between environmental influences on each disorder).
Table 4.
Model Fitting Results for Past Year and Lifetime Data
| −2 ln L | df | AIC | Δχ2 | df | p | |
|---|---|---|---|---|---|---|
| Past Year | ||||||
| 1. Full ACE model | 4576.64 | 5493 | −6409.36 | -- | -- | -- |
| 2. rg dropped | 4596.32 | 5494 | −6391.68 | 19.68 | 1 | <.01 |
| 3. rc dropped | 4577.36 | 5494 | −6410.64 | 0.72 | 1 | 0.40 |
| 4. re dropped | 4578.17 | 5494 | −6409.83 | 1.53 | 1 | 0.22 |
| 5. rc and re dropped | 4578.17 | 5495 | −6411.83 | 1.53 | 2 | 0.79 |
| 6. rc, re, and C for CD dropped | 4578.17 | 5496 | −6413.83 | 1.53 | 3 | 0.68 |
| 7. rc, re, C for CD and C for MDD dropped* | 4578.17 | 5497 | −6415.83 | 1.53 | 4 | 0.82 |
| Lifetime | ||||||
| 1. Full ACE model | 7082.96 | 5493 | −3903.04 | -- | -- | -- |
| 2. rg dropped | 7101.22 | 5494 | −3886.78 | 18.26 | 1 | <.01 |
| 3. rc dropped | 7083.50 | 5494 | −3904.50 | 0.54 | 1 | 0.46 |
| 4. re dropped | 7087.89 | 5494 | −3900.11 | 4.93 | 1 | 0.03 |
| 5. rc and C for CD dropped | 7083.50 | 5495 | −3906.50 | 0.54 | 2 | 0.76 |
| 6. rc, C for CD, and C for MDD dropped* | 7083.50 | 5496 | −3908.50 | 0.54 | 3 | 0.91 |
Note. Full ACE model includes rg, rc and re. Best-fitting model is indicated by an asterisk (*). −2 ln L = fit statistic, negative two times the log-likelihood; df = degrees of freedom, Δχ2 = difference in chi square from full ACE model; p = probability; AIC = fit statistic, Akaike Information Criterion; A = genetic influence; C = shared environmental influence; E = nonshared environmental influence; rg = correlation between genetic influences on each disorder; rc = correlation between shared environmental influences on each disorder; re = correlation between nonshared environmental influences on each disorder; CD = conduct disorder; MDD = major depressive disorder.
According to the full model, there are moderate genetic influences (h2 = .23 for past year and .23 for lifetime), moderate shared environmental influences (c2 = .16 for past year and .09 for lifetime), and substantial nonshared environmental influences (e2 = .61 for past year and .68 for lifetime) on MDD, and moderate genetic influences (h2 = .47 for past year and .55 for lifetime), modest shared environmental influences (c2 = .03 for past year .00 for lifetime), and moderate nonshared environmental influences (e2 = .50 for past year .45 for lifetime) on CD. The correlation between the genetic influences on MDD and CD was .47 for past year and .51 for lifetime. Given that the c2 estimates were very low for CD (.00 to .03), the correlation between the shared environmental influences on MDD and CD (.99 for past year and .11 for lifetime) are not meaningful (and can be dropped from the model without a significant decrement in the fit of the model; see Model 3 in Table 4). The correlation between the nonshared environmental influences on MDD and CD was .11 for past year and .17 for lifetime.
The fit statistic used was negative two times the log-likelihood of data (−2 ln L). The difference in the −2 ln L of two nested models is distributed as chi-square, so the −2 ln L provides a relative measure of fit; a lower −2 ln L relative to its degrees of freedom indicates a better fit. We also used Akaike's Information Criterion (AIC); this fit statistic, chi-square minus twice the degrees of freedom (χ2 − 2 df), takes into account model fit and parsimony. A lower AIC indicates a better fit.
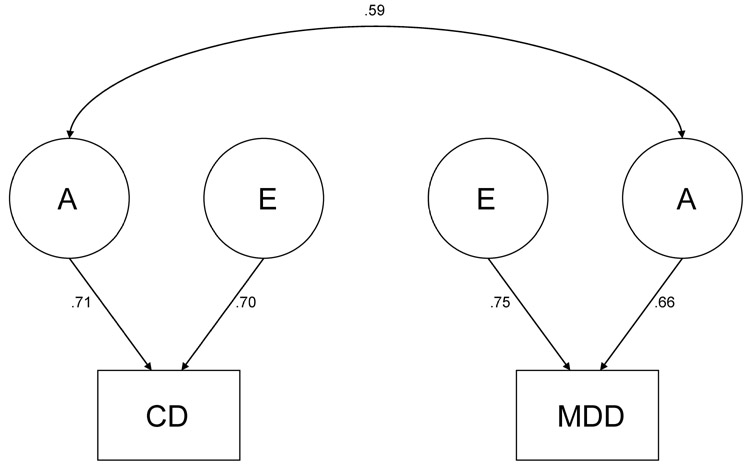
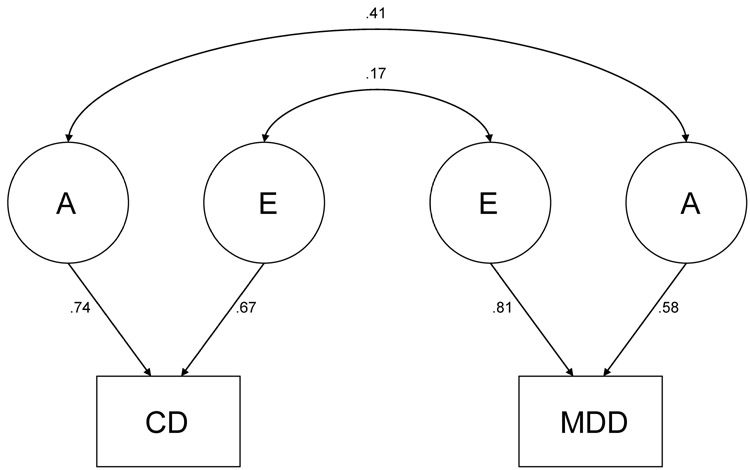
Also, in comparing the restricted models to the full ACE model, a significant change in χ2 (i.e., the difference between the −2 ln L of the full ACE model and the reduced model) indicates that dropping the parameter in question leads to a significant decrement in the fit of the model compared to the full ACE, whereas a non-significant change in χ2 indicates that the parameter tested can be dropped from the model. For past year data, dropping rg led to a significant decrement in fit in comparison to the full ACE model (Model 2), whereas rc (Model 3) and re (Model 4) could be dropped. The best-fitting model for the past year data (Model 7, indicated with an asterisk, *) was an AE model with rc, re, c2 for CD, and c2 for MDD dropped, but still includes rg. For lifetime data, dropping rg (Model 2) and re (Model 4) led to a significant decrement in the fit of the model compared to the full model, whereas rc (Model 3) could be dropped. The best-fitting model for the lifetime data (Model 6, indicated with an asterisk, *) was an AE model with rc, c2 for CD, and c2 for MDD dropped, but still includes rg and re. Figures 2 and 3 show the parameter estimates from the best-fitting models.
Figure 2.
Best fitting model for past year data. A = genetic influences; E = nonshared environmental influences; CD = conduct disorder; MDD = major depressive disorder.
Figure 3.
Best fitting model for lifetime data. A = genetic influences; E = nonshared environmental influences; CD = conduct disorder; MDD = major depressive disorder.
Discussion
We conducted a twin-sibling study to examine genetic and environmental influences on the covariation between DSM-IV MDD and CD in adolescents. Previous studies have examined related questions about the covariation between externalizing and internalizing behavior (Gjone & Stevenson, 1997), externalizing and internalizing disorders (Kendler et al., 2003) and antisocial behavior and depressive symptoms (O'Connor et al., 1998), and these studies have yielded conflicting results.
For either the lifetime or past year data, the heterogeneity model, which allowed the magnitude of genetic and environmental influences MDD and the magnitude of the correlations between the genetic and environmental influences on MDD and CD to differ between males and females, did not fit the data better than the homogeneity model, which constrained these parameters to be equal between the two sexes. This result suggests that the magnitude of genetic and environmental influences and the magnitude of the correlation between genetic and environmental influences on MDD and CD are similar for males and females (after correcting for the sex differences in the prevalence of MDD and CD).
The full model suggested moderate genetic and shared environmental and substantial nonshared environmental influences on MDD (h2 = 23, c2 = .09−.16, e2 = .61−68), and moderate genetic and nonshared environmental influences and little or no shared environmental influences on CD (h2 = .47−.55, c2 = .00−.03, e2 = .45−.50). The correlation between the genetic influences on MDD and CD was statistically significant for the past year (rg = .59) and lifetime (rg = .41). There was also a significant correlation between the nonshared environmental influences on lifetime CD and lifetime MDD (re = .17). There was no evidence of a significant correlation between shared environmental influences on MDD and CD. This was not surprising, as the magnitude of shared environmental influences on CD was zero or modest. Although the lack of evidence for shared environmental influences on CD is not consistent with the results of other studies examining CD (e.g., Rhee & Waldman, 2002), the difference in the conclusions may be due to the use of self-report and assessment of more severe symptoms in the present study.
Overall, the results for the past year and lifetime data were very similar. The only difference in the conclusion was that the correlation between the nonshared environmental influences could be dropped for past year MDD and CD, whereas it could not be dropped for lifetime MDD and CD. However, the re in the full model was similar in magnitude for past year (.11) and lifetime (.17) MDD and CD. The similarity between the results for the past year and lifetime data is not surprising, given that the sample examined here is an adolescent sample, and adolescents who had symptoms or diagnoses during their lifetime also were likely to have symptoms or diagnoses during the past year. For MDD, 52% of adolescents who had symptoms during their lifetime had at least one symptom during the past year, and among adolescents who had a lifetime diagnosis, 9% had at least one symptom during the past year and 36% had a past year diagnosis. For CD, 37% of adolescents who had symptoms during their lifetime had at least one symptom during the past year, and among adolescents who had a lifetime diagnosis, 48% had at least one symptom during the past year and 30% had a past year diagnosis.
In comparison to previous twin studies examining the co-occurrence between MDD and CD or related constructs, our results are most similar to those of O'Connor et al.'s (1998), who found that common genetic influences explained more of the covariance between antisocial and depressive behavior than other sources of variance. They are less similar to those of Gjone and Stevenson (1997), who found stronger evidence for common shared environmental influences, and those of Kendler et al. (2003), who found that the genetic influences on internalizing disorders had little influence on externalizing disorders, and that the genetic influences on externalizing disorders had little influence on internalizing disorders. Our study also had more methodological similarities to those of O'Connor et al. than those of Gjone and Stevenson or Kendler et al. Although O'Connor et al. did not examine DSM-IV MDD and CD, the constructs they examined were more specific than the ones examined by Kendler et al. and Gjone and Stevenson (i.e., antisocial behavior and depression rather than externalizing and internalizing behavior/disorders) and closer to the constructs we examined. Another similarity to our study was their inclusion of non-twin sibling pairs as well as MZ and DZ twin pairs. Unfortunately, it is not possible to resolve why the results of the past studies examining this issue have been inconsistent or why the present study's results are most consistent with those of O'Connor et al.
A limitation of the present study is the reliance on retrospective recall in examining lifetime MDD and CD. Also, although the present study examined a more severe end of the continuum of psychopathology (i.e., DSM-IV symptoms and diagnoses of MDD and CD) than that examined in most previous studies, the sample examined in the present study was a general population sample. Therefore, it is possible that the results of the present study will not generalize to clinical samples. Third, the current results should be interpreted while recognizing that although we examined a large sample, our statistical power is still relatively low. As mentioned above, either genetic influences or shared environmental influences, but not both, could be dropped from the univariate model for MDD without a significant worsening of fit (Ehringer et al., 2006). However, there was power to distinguish between models dropping shared environmental influences vs. genetic influences in the bivariate analyses of MDD and CD, which takes into account additional information from their covariation. Shared environmental influences could be dropped from the model, whereas models dropping the genetic covariation between the two disorders did not fit the data well.
As with other twin studies examining the co-occurrence of MDD and CD and related constructs, our study is a cross-sectional study that is limited in the ability to distinguish the correct cause of co-occurrence among the three main hypotheses for the causes of co-occurrence between MDD and CD (i.e., MDD causes CD, CD causes MDD, and common causal factors between MDD and CD). Results from longitudinal studies examining the development of MDD and CD will be more informative regarding this question. For example, Beyers and Loeber (2003) examined the trajectories for delinquency and depressed mood from age 13.5 to 17.5. In their analyses, they controlled for several common risk factors for delinquency and depressed mood, such as poor parent-adolescent communication, family history of offending, family socioeconomic status, and peer delinquency. They found that depressed mood predicted concurrent delinquency and delinquency predicted concurrent depressed mood. However, depressed mood had a more robust effect on delinquency trajectories than delinquency on depressed mood trajectories. Additional longitudinal research examining the development of MDD and CD is needed. We are currently collecting five-year follow-up data on the same sample of twins and siblings.
These findings inform future research regarding both genetically and environmentally mediated risk factors on MDD and CD. Future genetic research may investigate common biological processes, such as neurotransmitter systems, that underlie the co-occurrence of MDD and CD. Molecular genetic studies may eventually identify specific genes that are responsible for symptoms of both depression and conduct disorder. There is preliminary evidence suggesting that some specific genetic and nonshared environmental influences may be shared by MDD and CD. For example, although the results in the literature have not been consistent, several researchers have found evidence suggesting that the short allele of the serotonin transporter gene is a risk factor for both depression (e.g., Collier et al., 1996; Gutierrez et al., 1998) and antisocial behavior (e.g., Retz et al., 2004; Sakai et al., in press).
As discussed in the introduction section, several researchers hypothesize that MDD and CD may share common precursors, such as negative emotionality (e.g., Keiley, Lofthouse, Bates, Dodge, & Pettit, 2003; Lilienfeld, 2003). As more solid evidence for specific genetic influences shared by MDD and CD develops, research examining how common genes, common precursors, and MDD and CD are related should increase our understanding of the etiology of MDD and CD. For example, it is possible that the influence of common genes on the more complex psychiatric disorders such as MDD and CD is mediated via common precursors (e.g., Lahey & Waldman, 2003). Research examining whether the common genes and precursors interact with the environment to lead to the different psychiatric outcomes also should be useful.
These data also demonstrate the importance of nonshared environment in the development of adolescent psychopathology, and suggest the importance of investigating factors other than family environment that may lead to the development of these disorders. An example of a study examining potential common nonshared environmental influences MDD and CD is Pike et al.'s (1996) examination of negative family interactions in twin and sibling pairs recruited from the Nonshared Environment Adolescent Development project. They found that there were significant nonshared environmental links between mothers’ negativity and both depressive symptoms and antisocial behavior, although there were significant nonshared environmental links between fathers’ negativity and antisocial behavior only. The results of the present study suggest that such efforts at identifying specific genetic and nonshared environmental influences shared by MDD and CD should prove productive.
Acknowledgments
This work was supported by National Institutes of Health Grants MH43899, MH16880, HD10333, DA11015, and DA13956. We thank Michael C. Stallings and Sally-Ann Rhea for helpful comments regarding the manuscript.
Footnotes
The overall pattern of results was the same when analyses were repeated using the same threshold for the entire sample.
The overall pattern of results was the same when analyses were repeated using the same threshold for the entire sample.
References
- Achenbach TM. Manual for the Child Behavior Checklist/ 4−18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington: 1991. [Google Scholar]
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. University of Vermont, Department of Psychiatry; Burlington: 1983. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed. Author; Washington, D.C.: 1980. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Author; Washington, D.C.: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, D.C.: 1994. [Google Scholar]
- Angold A, Costello EJ. Depressive comorbidity in children and adolescents: Empirical, theoretical and methodological Issues. American Journal of Psychiatry. 1993;150:1779–1791. doi: 10.1176/ajp.150.12.1779. [DOI] [PubMed] [Google Scholar]
- Beyers JM, Leober R. Untangling developmental relations between depressed mood and delinquency in male adolescents. Journal of Abnormal Child Psychology. 2003;31:247–266. doi: 10.1023/a:1023225428957. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Benson RS. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1234–1238. doi: 10.1097/00004583-199811000-00029. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B. Childhood and adolescent depression: a review of the past 10 years. Part I. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1427–39. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Capaldi DM. Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: II. A 2-year follow-up at grade 8. Development and Psychopathology. 1992;4:125–144. doi: 10.1017/s0954579499001959. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stöber G, Li T, Catalano M, Di Bella D, Arranz MJ, et al. A novel functional polymorphism within the promotor of the serotonin transporter gene: possible role in susceptibility to affective disorders. Molecular Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: Theoretical and empirical considerations. Genetic Epidemiology. 1994;11:483–502. doi: 10.1002/gepi.1370110606. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Hewitt JK, Rutter M, Meyer JM, Neale MC, Pickles A. Analyzing twin resemblance in multisymptom data: Genetic applications of a latent class model for symptoms of conduct disorder in juvenile boys. Behavior Genetics. 1993;23:5–19. doi: 10.1007/BF01067550. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Rhee SH, Young S, Corley R, Hewitt JK. Genetic and environmental contributions to common psychopathologies of childhood and adolescence: a study of twins and their siblings. Journal of Abnormal Child Psychology. 2006;34:1–17. doi: 10.1007/s10802-005-9000-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Murphy BC, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Gjone H, Stevenson J. The association between internalizing and externalizing behavior in childhood and early adolescence: Genetic or environmental common influences? Journal of Abnormal Child Psychology. 1997;25:277–286. doi: 10.1023/a:1025708318528. [DOI] [PubMed] [Google Scholar]
- Glowinski AL, Madden PAF, Bucholz KK, Lynskey MT, Heath AC. Genetic epidemiology of self-reported lifetime DSM-IV major depressive disorder in a population-based twin sample of female adolescents. Journal of Child Psychology and Psychiatry. 2003;44:988–996. doi: 10.1111/1469-7610.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez B, Pintor L, Gasto C, Rosa A, Bertranpetit J, Vieta E, et al. Variability in the serotonin transporter gene and increased risk for major depression with melancholia. Human Genetics. 1998;103:319–322. doi: 10.1007/s004390050823. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behavior Genetics. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Heaven PCL, Newbury K, Mak A. The impact of adolescent and parental characteristics on adolescent levels of delinquency and depression. Personality and Individual Differences. 2004;36:173–185. [Google Scholar]
- Howell DC. Fundamental Statistics for the Behavioral Sciences. 5th ed. Brooks/Cole-Thomson Learning; Belmont, CA: 2004. [Google Scholar]
- Hudziak JJ, Rudiger LP, Neale MC, Heath AC, Todd RD. A twin study of inattentive, aggressive, and anxious/depressed behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:469–476. doi: 10.1097/00004583-200004000-00016. [DOI] [PubMed] [Google Scholar]
- Keiley MK, Lofthouse N, Bates JE, Dodge KA, Pettit GS. Differential risks of covarying and pure components in mother and teacher reports of externalizing and internalizing behavior across ages 5 to 14. Journal of Abnormal Child Psychology. 2003;31:267–283. doi: 10.1023/a:1023277413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Paulauskas S, Gatsonis C, Richards C. Depressive disorders in childhood: III. A longitudinal study of comorbidity with and risk for conduct disorders. Journal of Affective Disorders. 1988;15:205–217. doi: 10.1016/0165-0327(88)90018-3. [DOI] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30:1245–1259. [Google Scholar]
- Lahey BB, Waldman ID. A developmental propensity model of the origins of conduct problems during childhood and adolescence. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. The Guilford Press; New York: 2003. pp. 76–117. [Google Scholar]
- Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: reflections and directions. Journal of Abnormal Child Psychology. 2003;31:285–291. doi: 10.1023/a:1023229529866. [DOI] [PubMed] [Google Scholar]
- Loeber R, Keenan K. Interaction between conduct disorder and its comorbid conditions: effects of age and gender. Clinical Psychological Review. 1994;14:497–523. [Google Scholar]
- Loeber R, Russo MF, Stouthamer-Loeber M, Lahey BB. Internalizing problems and their relation to the development of disruptive behaviors in adolescence. Journal of Research on Adolescence. 1994;4:615–637. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 6th ed. Department of Psychiatry, Virginia Commonwealth University; Richmond, VA: 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1992. [Google Scholar]
- O'Connor TG, McGuire S, Reiss D, Hetherington EM, Plomin R. Co-occurrence of depressive symptoms and antisocial behavior in adolescence: A common genetic liability. Journal of Abnormal Psychology. 1998;107:27–37. doi: 10.1037//0021-843x.107.1.27. [DOI] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behavior: A multivariate genetic analysis. Developmental Psychology. 1996;32:590–603. [Google Scholar]
- Plomin R, DeFries JC, McClearn GE. Behavioral genetics: A primer. 2nd ed. W.H. Freeman and Company; New York: 1990. [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behavior Genetics. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Retz W, Retz-Junginger P, Supprian T, Thome J, Rösler M. Association of serotonin transporter promoter gene polymorphism with violence: Relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behavioral Sciences and the Law. 2004;22:415–425. doi: 10.1002/bsl.589. [DOI] [PubMed] [Google Scholar]
- Rhea S-A, Gross AA, Haberstick BC, Corley RP. Colorado twin registry. Twin Research and Human Genetics. doi: 10.1375/183242706779462895. (in press) [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rice F, Harold G, Thapar A. The genetic aetiology of depression: A review. Journal of Child Psychology and Psychiatry. 2002;43:65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Rowe DC. Biometric genetic models of self-reported delinquent behavior: A twin study. Behavior Genetics. 1983;13:473–489. doi: 10.1007/BF01065923. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Young SE, Stallings MC, Timberlake D, Smolen A, Stetler GL, Crowley TJ. Case-control and within-family tests for an association between conduct disorder and 5HTTLPR. American Journal of Medical Geentics. doi: 10.1002/ajmg.b.30278. (in press) [DOI] [PubMed] [Google Scholar]
- Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, Goodman SH, Lahey BB, Lichtman JH, Canino G, Rubio-Stipec M, Rae DS. Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3). Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:878–888. doi: 10.1097/00004583-199607000-00013. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Lahey BB, Bourdon K, Jensen PS, Bird HR, Canino G, Regier DA. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan MK, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children, Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Lessem JM, Young SE, Corley RP, Mikulich SK, Crowley TJ. Modeling the familial transmission of alcohol dependence symptom counts in clinical and control family pedigrees [Abstract]. Behavior Genetics. 2001;31:470. [Google Scholar]