Abstract
Purpose
To characterize structural and functional injuries following a single dose of whole-thorax irradiation that might be survivable after a nuclear attack/accident.
Methods
Rats were exposed to 5 or 10 Gy of X-rays to the whole thorax with other organs shielded. Non-invasive measurements of breathing rate and arterial oxygen saturation, and invasive evaluations of bronchoalveolar lavage fluid, (for total protein, Clara cell secretory protein), vascular reactivity and histology were conducted for at least 6 time points up to 52 wks after irradiation.
Results
Irradiation with 10 Gy resulted in increased breathing rate, a reduction in oxygen saturation, an increase in bronchoalveolar lavage fluid protein and attenuation of vascular reactivity between 4–12 wks after irradiation. These changes were not observed with the lower dose of 5 Gy. Histological examination revealed perivascular edema at 4–8 wks after exposure to both doses, and mild fibrosis beyond 20 wks after 10 Gy.
Conclusions
Single-dose exposure of rat thorax to 10 but not 5 Gy X-irradiation resulted in a decrease in oxygen uptake and vasoreactivity and an increase in respiratory rate, which paralleled early pulmonary vascular pathology. Vascular edema resolved and was replaced by mild fibrosis beyond 20 wks after exposure, while lung function recovered.
Keywords: Radiation injury, non invasive assays, lung injury markers, vascular reactivity
Introduction
In the event of a radiological terrorist attack, accurate dosimetry and management of organ-specific damage will be vital (Coleman 2003). Having suitable markers for organ-level injury will not only aid in identifying individuals most likely to benefit from pharmacologic intervention, but will also play a critical role in optimizing any diagnostic or therapeutic modalities that are being developed or are available.
Pulmonary injuries caused by therapeutic irradiation have been well recognized in rodents and humans. Two phases of functional injury to the lungs have been described in humans: an acute phase of radiation pneumonitis occurring 4–30 wks after exposure and a later phase of fibrosis appearing 6–12 months after irradiation (Coggle et al. 1986). Inflammation is the predominant early histological and physiological finding. In rodents, radiation induces the synthesis of a variety of cytokines that lead to cellular infiltration and fibroblast stimulation, resulting in enhanced collagen synthesis and ultimately in fibrosis in the lungs (Franko & Sharplin 1994). During the acute phase, rodents exhibit changes in the production of prostacyclins (Ts’ao et al. 1983), cytokines (Van der Meeren et al. 2001), and adhesion molecules (Hallahan & Virudachalam 1999), as well as changes in hypervascular permeability (Maisin 1970). This is followed by an inflammatory response at 1–4 wks after exposure, with inflammatory cell recruitment in the lungs (Rosiello et al. 1993), cytokine production (Johnston et al. 1996), and activation of the coagulation system (Huang et al. 2001). Pneumonitis is followed by a phase of chronic inflammation and fibrosis that develops months to years after irradiation. In this phase, vascular damage and collagen deposition become apparent (Fajardo 1982).
While responses to high dose irradiation have been extensively studied, low to moderate radiation doses are less well characterized (Coleman 2003). Lehnert et al (1991) have demonstrated hyperpermeability following 15 Gy that was not observed in rats exposed to lower dose. Single doses of 11–13.5 Gy have been shown to result in a dose dependant decrease in relative blood flow in the irradiated lungs from 3–5 wks after exposure, with return to near normal flow by 5 wks (Peterson et al. 1992).
The present study is a first step towards developing a rat model for the radiation-induced lung injury that might occur after a radiological attack. The January 2008 Animal Models Workshop sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID)-Center of Medical Countermeasures against Radiation (CMCR) program confirmed that there is an urgent need to develop relevant rodent models for lung injury caused by whole thorax irradiation. We have characterized effects of a single dose of 5 or 10 Gy to the whole thorax of rats. Using this model, we intend to define spatial and temporal derangements in lung structure and function which might serve as markers of radiation-induced injury. We employ both invasive and non-invasive assays for this purpose: histology, oxygen saturation, breathing rate, bronchoalveolar lavage fluid (BALF) protein and cell count, changes in Clara Cell secretory protein (CC10), and vascular reactivity. Molteni et al (2000) reported that it is likely that angiotensin II (Ang II) is involved in the development of radiation-induced lung injury, since angiotensin converting enzyme (ACE) inhibitors and an Ang II type 1 receptor blocker were effective in mitigating radiation-induced pneumonitis and the development of lung fibrosis. We therefore studied vascular responses to Ang II, as a measure of vascular reactivity.
Materials And Methods
Animals
The study was approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin. Specific-pathogen-free female rats from the MCW colony of WAG/Rij MCW rats were used. The rats were housed in a moderate security barrier and were allowed sterilized food and water ad libitum.
Animal Injury Model
Un-anesthetized female rats were used at 9–10 wks of age and a weight of about 140 grams. Rats were treated using whole thorax irradiation with a single dose of 5 or 10 Gy, and one group was maintained as un-irradiated controls housed under identical conditions as the irradiated rats. Rats were examined for changes in structure and function at 6 time points- 3 days, 2 wks, 4 wks, 8 wks, 20 wks and 52 wks after irradiation.
For irradiation, the rat was acclimated and confined in a plexiglas jig that prevented the animal from rotating or from moving more than about 5 mm. A Pantak HF320 orthovoltage system (Therapax, Danbury, CT, USA) was used, with a 300 kVp beam, a half value layer of 1.4 mm Cu, and a midline dose-rate of 1.615 Gy/min. The absolute dose was measured using a calibrated Farmer-type ionization chamber. The radiation dose was delivered by two equally-weighted lateral beams in order to improve the dose uniformity. An 8 × 7.5 cm2 collimator was used to define a radiation field that encompassed the whole thorax; the whole lung is in the field, plus the heart and a small amount of the liver.
Histology
Basic histological examination was employed as a gold standard against which all other non-invasive endpoints were compared. Lung sections (tips of left lower lobe) from a minimum of 7 rats for each treatment and time point were taken from irradiated and control rats sacrificed following intraperitoneal injection of 50 mg/kg of pentobarbital. The remaining lungs were used for other assays such as vascular reactivity studies (described below). For histopathology, lung tissue samples were fixed in zinc formalin for 24 to 48 hr then processed using a Sakura Tissue Tek VIP5 processor (Sakura Finetek, Torrance, CA, USA). Processing was performed using 70%, 80%, 95% and 100% ethanols, xylene and paraffin. Following processing, samples were oriented (embedded) in paraffin and sectioned at 4 μm using a microtome. Sections were stained with hematoxylin and eosin (H & E) for identification. The samples were then scored in a blinded manner according to a semiquantitative scale (Canzian et al. 2007).
To ensure that the lower region of the lung that was used for histopathology was representative of that of the rest of the organ, areas spanning both the left and right lungs of rats exposed to 10 Gy, 8 wks after irradiation were scored. The average of the sum of the acute and chronic scores were 2.25, 2.75 and 3.25 for the upper, middle section and lower sections respectively. This suggested that the injury to the lower region of the lungs that we used were no less severe than that observed in the rest of the organ.
Pulse Oximetry
Oxygen saturation was measured in un-anesthetized rats using a veterinary pulse oximeter (Nonin, 8600V series, Plymouth, MN, USA), the probe of which was placed over the rat tail artery (Decker et al. 1989, Mirsattari et al. 2004). During the procedure, the rat was held in a plexiglas jig housed in a glass box, the interior of which was heated to a steady temperature of 30 °C. In order to enhance the sensitivity of the test, oxygen saturation was also measured after the rats were exposed to a hypoxic gas mixture of 12% oxygen and a balance of nitrogen for 10 min. Rats were used for this non-invasive assay after exposure to 10 Gy (n=6–15/group) or 5 Gy (n=4–6/group), which were compared with respective age-matched controls. These rats were studied serially at all time points. The rats studied with pulse oximetry were also examined for changes in breathing rate before being sacrificed for invasive protocols such as vasoreactivity or levels of lung injury markers as described below.
Breathing rate assay
Respiratory frequency is a well validated marker of radiation induced lung damage in rodents (Van Eerde et al. 2001, Novakova-Jiresova et al. 2005). In our experiments rats immobilized in a plexiglas jig were placed in a transparent, airtight box. The box was connected to a differential pressure transducer which in turn was connected to a data acquisition device (Dataq-DI 158U, Dataq Instruments Inc, Akron, OH, USA). The frequency of pressure changes inside the box was recorded and analyzed. Recordings were continuously captured for a maximum of 10 min per rat after two training sessions which desensitized the rats to the jig as well as the airtight box to minimize stress. The mean breathing rate in each rat was then calculated from a minimum of four steady regions of the recording lasting greater than 15 s. If the measurement required more than 5 min to obtain, the rat was released from the box and rested to prevent anxiety as well as drop of oxygen inside the box. A mean breathing rate (breaths per min) of a dose group was calculated from the means of individual rats at each time point.
Estimation of lung injury markers in lung lavage fluid
Rats (n=3–8) from each cohort were anesthetized intraperitoneally with pentobarbital (50 mg/kg), and a tracheostomy was performed. An 18 G cannula with a flexible tip was inserted into the trachea and the lungs were lavaged with a total volume of 5 ml of phosphate-buffered saline. The BALF was centrifuged (1000 g for 10 min at 4 ºC) and the resultant cell free supernatant was analyzed for determination of levels of markers of lung injury. The cell pellet was resuspended in 75 μl of phosphate buffered saline (PBS) and aliquots of the cell suspension were used to determine the cell number and viability (trypan blue exclusion) using a haematocytometer.
Total protein in the BALF was estimated using the Coomassie Brilliant Blue method with a Bio-Rad Protein Assay Kit (Cat. # 161–0435, Bio-Rad, Hercules, CA, USA). CC10 in BALF was assayed by an enzyme linked immunosorbent assay (ELISA). BALF was diluted 1:10 with coating buffer (8.4 g/L NaHCO3, 3.56 g/L NaCO3, pH 9.5). This solution (100 μl) was incubated overnight at 4 ºC in 96 well ELISA plates. Following incubation, the wells were washed 3 times with 200 μl/well of wash buffer (0.05% Tween 20 in TBS). The wells were then coated with 200 μl/well of blocking buffer (Superblock T20 PBS, Pierce, Rockford, IL, USA), and the plate was incubated at room temperature for 1 hr. After washing, the wells were coated with the primary antibody (rabbit polyclonal IgG against CC10, 1:500, CC10 FL-96, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the plate was incubated at room temperature for 1 hr. The wells were then washed 3 times as previously described. Horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:20,000, 100 μl per well, Cat. # 170–6515, Bio Rad, Hercules, CA, USA) was then added. Following incubation at room temperature for 1 hr, the wells were washed 5 times and 100 μl of the HRP substrate solution (equal amounts of substrate A and B, BD Opt E1A, BD Biosciences, San Jose, CA, USA) at room temperature was added to each well. The reaction was allowed to proceed for 15 min after which it was stopped with 2N H2SO4. The plates were read at 450 nm within 30 min of stopping the reaction.
Wet/Dry weight
The upper and middle lobes of the right lung from samples used for vascular reactivity studies were harvested, gently wiped and weighed immediately. The same samples were dried at 60 °C for 24 hr. After drying, they were weighed again to calculate the ratio of wet to dry weight of lung tissue. Tissue from rats that underwent lung lavage were not used for this assay.
Vascular reactivity studies
These studies were carried out in a manner previously published (Zhu et al. 1998). Briefly, the chests were opened for removal of the heart and lungs en bloc in anesthetized rats. Tissue (from n=4–11, most groups were represented by 8 rats) was harvested for: (i) measurement of wet and dry weight; (ii) histological examination; and (iii) microdissection of pulmonary arteries. Pulmonary arteries (1–1.5 mm in diameter) were cut into rings, mounted on a force transducer placed in an organ bath having pH-adjusted, oxygenated Ringer’s solution (mM: NaCl 118, KCl 4.7, MgSO4 0.6, KH2PO4 1.18, CaCl2 2.5, glucose 10 and NaHCO3 27, pH 7.4) at 37 °C. Rings were initially loaded with 0.3 g tension by incremental application over 30 min and then equilibrated for an additional 30–40 min before the studies were started. A dose dependent constriction to Ang II (10−10 M to 10− 7 M, Sigma A-9525, St. Louis, MO, USA) was examined in isolated pulmonary arteries at different times post-irradiation. Pulmonary artery rings were washed repeatedly with Ringer’s solution until tone recovered to basal level and then KCl (80 mM) was applied to detect the non-receptor mediated response of the rings. Tension data were relayed from the pressure transducers to a signal amplifier (600 series eight-channel amplifier, Gould Electronics, Chandler, AZ, USA). Data were acquired and analyzed with CODAS software (DataQ Instruments Inc, Akron, OH, USA). On an average, 4 rings were taken from each rat and data from 4–11 rats were combined for each result.
Statistical Analyses
Data are calculated as mean ± sem (standard error of the means). Potential differences among the experimental groups (irradiated rats versus their un-irradiated, age-matched siblings) were assessed by using the Student’s unpaired t-test for 2 groups and ANOVA for repeated measures with post hoc comparison by Tukey’s for comparison of more than two groups or changes from baseline over more than two times. Values were considered significantly different when p≤0.05. Depending on the difference between means of the groups being compared, some assays required lower sample sizes to achieve the same power of analysis.
Results
Histology
Histological examinations were performed on lungs that were also used to dissect pulmonary artery rings, and therefore were obtained from un-inflated organs. Representative stained sections are shown in Figures 1a–1f. Considerable acute perivascular edema was noted at 4 wks following exposure to 10 Gy (marked by arrow in Figure 1e). Other significant findings included chronic changes such as vessels wall hyalinization, interstitial thickening and mild fibrosis. These changes were scored in a blinded manner using a semiquantitative scale of 0–4, with 4 being the most severe (Canzian et al. 2007). These scores were averaged and tabulated to reflect injury at different time points (Table I). Interstitial thickening was not scored, as this end point was often ambiguous in un-inflated tissue. However, the pathologist described that at 20 and 52 wks after irradiation 50% of the rats irradiated with a dose of 10 Gy exhibited interstitial thickening and fibrosis, which were less obvious in rats exposed to 5 Gy after 20 wks. Acute and chronic histological abnormalities were highest at 4–8 wks after exposure to radiation and appeared to recover by 20 wks, with the injuries being more pronounced at the 10 Gy dose than at 5 Gy.
Figure 1.
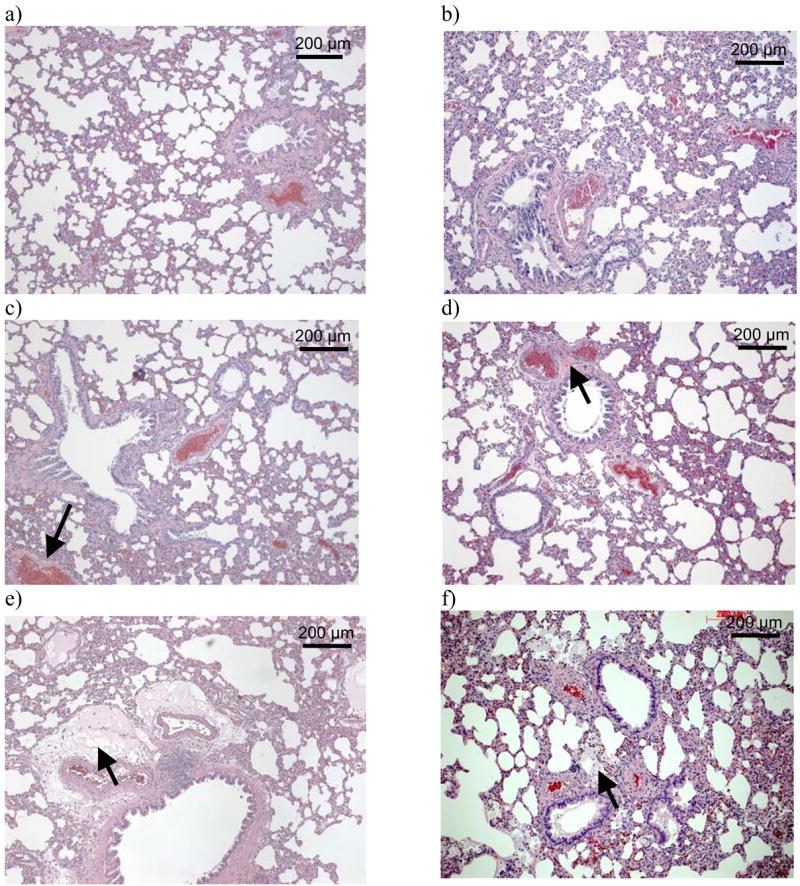
Hematoxylin-eosin-stained lung sections from a minimum of 7 rats were examined at each time point. Representative images for the major findings are shown as follows: a) control (0 Gy) at 3 days after irradiation; b) 0 Gy at 52 wks after irradiation; c) 5 Gy at 8 wks after irradiation, vessel wall hyalinization marked with arrow; d) 5 Gy at 20 wks after irradiation, chronic vessel changes marked with arrow; e) 10 Gy at 4 wks after irradiation, perivascular edema marked with arrow; f) 10 Gy at 52 wks after irradiation, mononuclear cells marked with arrow.
Table I.
Histological Scoring. The results of blinded scoring using a semiquantitative scale. Acute and chronic changes in the vascular compartment and the airways were scored as follows- 0: absence of histological changes; 1: parenchymal alterations in 1 to 25% of the tissue examined; 2: parenchymal alterations in 26 to 50% of the tissue examined; 3: parenchymal alterations in 51 to 75% of the tissue examined; 4: parenchymal alterations in 76% to 100% of the tissue examined. The 1st and 2nd columns show the time after irradiation and the specific changes and compartments scored respectively. The figures represent mean scores ± sem for each compartment (n=7–10).
| Time PI | Compartment - Characteristic | 0 Gy Mean±sem | 5 Gy Mean±sem | 10 Gy Mean±sem |
|---|---|---|---|---|
| 3 days | Acute Vascular changes - Perivascular edema | 0.67±0.17 | 0.5±0.26 | 1.3±0.36 |
| 2 wks | Acute Vascular changes - Perivascular edema | 0.55±0.22 | 0.3±0.16 | 1±0.32 |
| 4 wks | Acute Vascular changes - Perivascular edema | 0.63±0.32 | 0.88±0.29 | 1.63±0.41 |
| 8 wks | Acute Vascular changes - Perivascular edema | 0.4±0.22 | 1.38±0.32 | 0.4±0.26 |
| Chronic vascular changes- Vessel wall hyalinization, perivascular fibrosis | 1.1±0.27 | 1.38±0.32 | 1.2±0.36 | |
| 20 wks | Acute Vascular changes - Perivascular edema | 0 | 0 | 0 |
| Chronic vascular changes- Vessel wall hyalinization, perivascular fibrosis | 0.6±0.22 | 1±0.43 | 1.13±0.22 | |
| 52 wks | Acute Vascular changes - Perivascular edema | 0.22±0.14 | - | 0.88±0.35 |
| Chronic vascular changes- Vessel wall hyalinization, perivascular fibrosis | ±0.33 | - | 1.25±0.36 | |
| Chronic Airway changes- mononuclear cells | 0.78±0.36 | - | 0.88±0.35 |
Breathing Rate
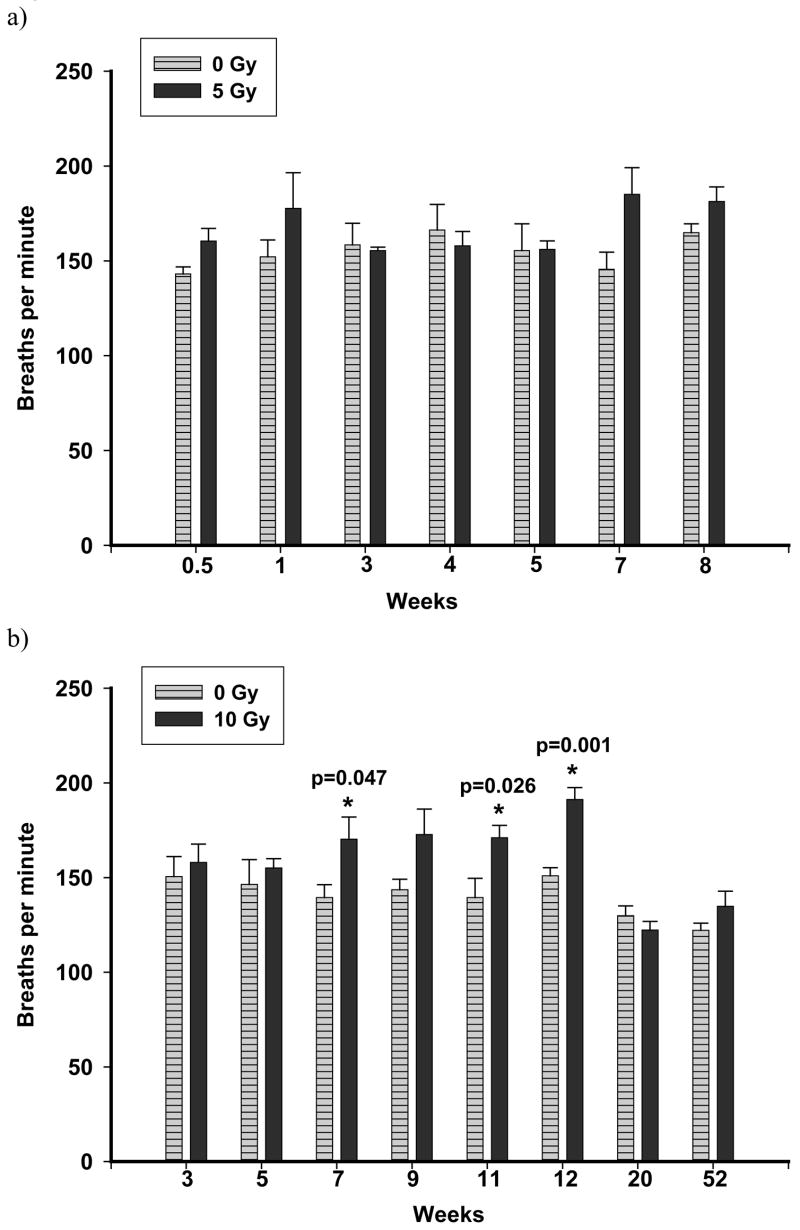
Respiratory frequency although elevated, was not significantly different in rats irradiated with a dose of 5 Gy compared to controls up to 8 wks after irradiation (Figure 2a). However, respiratory rates were generally higher in rats irradiated with a dose of 10 Gy compared to controls and were significantly elevated between 7 to 12 wks (Figure 2b).
Figure 2.
The breathing rate of rats was measured non-invasively using an airtight plethysmograph. Breaths per min following 5 Gy (panel a) and 10 Gy (panel b) were compared to controls. Values are mean breaths per min ± sem; n=4–6 (5 Gy), n=6–15 (10 Gy). No increase in breathing rate was observed following exposure to 5 Gy up to 8 wks. A significant elevation (p≤0.05 vs Ctrl) in breathing rate was noted at 7, 11 and 12 wks following irradiation with 10 Gy.
Oxygen saturation
There were no differences in resting oxygen saturation in rats exposed to a dose of 5 Gy relative to control (data not shown). We observed a small, but statistically-significant, lowering in oxygen saturation in the rats irradiated with a dose of 10 Gy compared to controls between 3–8 wks after irradiation (control 97 ± 0.2, n=11, 10 Gy 95.8 017 ± 0.37, n=7; p=0.028).
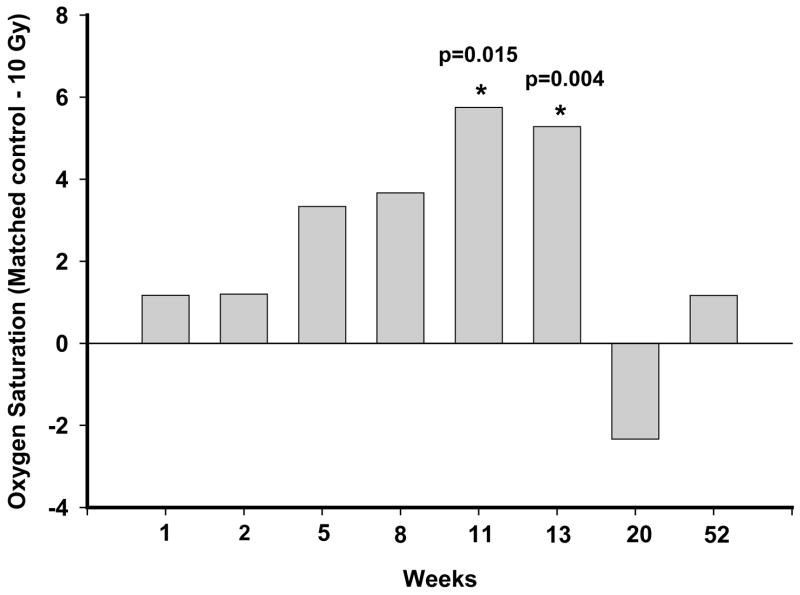
There was also no difference in oxygen saturation following exposure to hypoxia (breathing 12% oxygen in nitrogen for 10 min) in rats exposed to 5 Gy up to 8 wks after irradiation (data not shown). Rats irradiated with a dose of 10 Gy demonstrated a further decrease in oxygen saturation after exposure to hypoxia at 11 wks and 13 wks after exposure as shown in Figure 3. At 20 and 52 wks, the values were similar in the irradiated and un-irradiated groups.
Figure 3.
Oxygen saturation was measured non-invasively using a veterinary pulse oximeter 10 min after exposure to a hypoxic gas mixture of 12% oxygen in nitrogen. Rats were compared to controls at each time point. Data is shown for exposure to 10 Gy. The difference of mean oxygen saturation values between the age-matched controls and irradiated rats is presented for 10 Gy only. The mean ± sem values recorded were: wk1–10 Gy: 83.0 ±1.6, 0 Gy: 84.2 ± 0.7; wk2–10 Gy: 82.8 ± 1.0, 0 Gy: 84.0 ± 1.0; wk5–10 Gy: 80.3 ± 1.3, 0 Gy: 83.7 ± 1.4; wk8–10 Gy: 81.3 ± 1.5, 0 Gy: 85.0 ± 1.8; wk11–10 Gy: 79.5 ± 1.2, 0 Gy: 85.3 ± 1.5; wk13–10 Gy: 80.8 ± 0.9, 0 Gy: 86.1 ± 1.6; wk20–10 Gy: 85.7 ± 2.4, 0 Gy: 83.3 ± 1.2; wk52–10 Gy: 85.2 ± 1.4, 0 Gy: 86.3 ± 1.3; n=6–15. A significant reduction (p≤0.05 vs Ctrl) in oxygen saturation was observed in rats exposed to a dose of 10 Gy at 11 and 13 wks after irradiation.
Lung Injury Markers
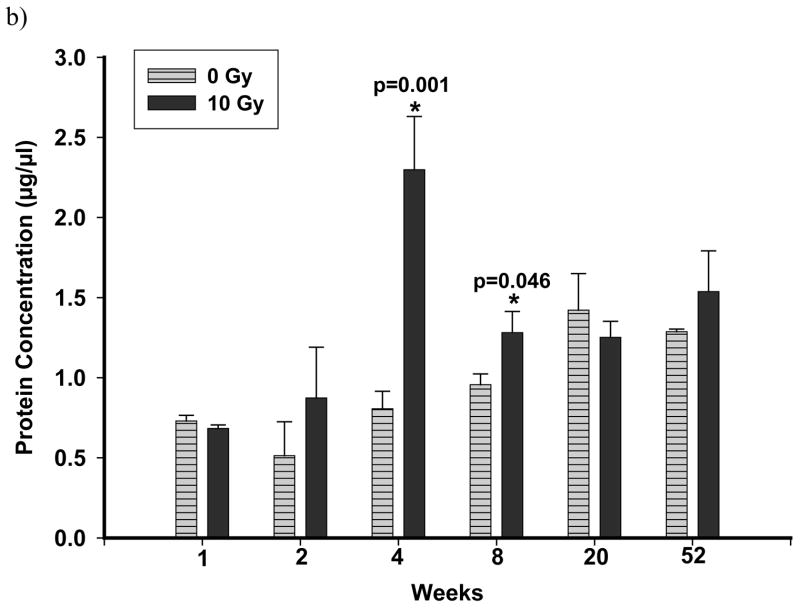
BALF protein was significantly elevated at 4 and 8 wks after exposure to 10 Gy, but not at 20 or 52 wks (Figure 4b) while no effects were seen after 5 Gy (Figure 4a). A significant reduction in CC10 levels in the BALF of rats irradiated with 10 Gy was observed at 4 wks after exposure (control 4.8 ± 0.5 μg/ml, 10 Gy 1.6 ± 0.31 μg/ml, n=6 each; p<0.001), but not at any other times. No difference was seen after an exposure of 5 Gy (control 4.30 ± 0.6 μg/ml, 5 Gy 2.2 ± 0.7μg/ml, n=3–7, 4 wks after irradiation). While cell counts in BALF were elevated at 4 and 8 wks after irradiation, no significant differences were observed between the control and irradiated (10 Gy) rats (data not shown).
Figure 4.
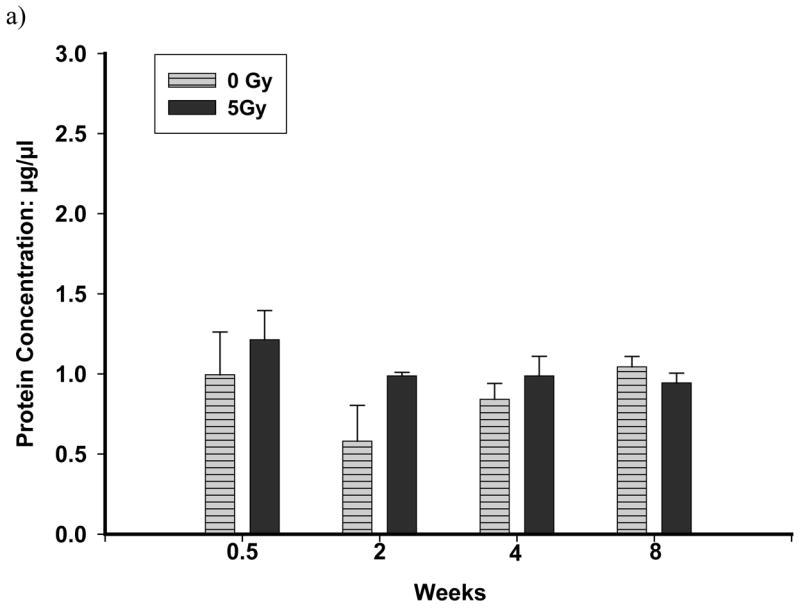
Total protein levels in the BALF of rats exposed to a dose of 5 Gy (panel a) and 10 Gy (panel b) compared to controls. Each data point represents mean ± sem; n=3–8. Following exposure to 5 Gy, no significant increase in total proteins was observed up to 8 wks after irradiation. Total Proteins were significantly elevated (p≤0.05 vs Ctrl) at 4 wks and at 8 wks after irradiation in rats exposed to a dose of 10 Gy compared to un-irradiated control rats.
Wet/Dry weight
The ratio of wet to dry weight of lung tissue was not significantly different in control and radiated rats with doses of 5 Gy up to 20 wks after irradiation (4 wks: control 4.7 ± 0.1, 5 Gy 4.7 ± 0.05, n=8 each; 8 wks: control 4.8 ± 0.07, 5 Gy 4.8 ± 0.1, n=8 each). However, with a dose of 10 Gy, there was a significant increase in wet:dry weight of lung tissue at 4 and 8 wks after exposure (4 wks: control 4.5 ± 0.1, 10 Gy 5.1 ± 0.1, n=9 each, p<0.01; 8 wks: control 4.6 ± 0.04, 10 Gy 4.8 ± 0.03, n=8 each, p<0.05).
Vascular reactivity studies
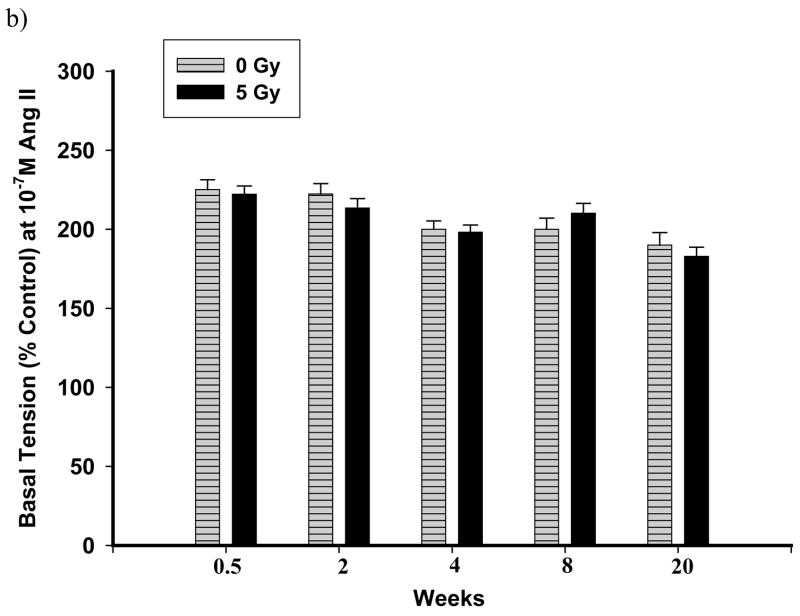
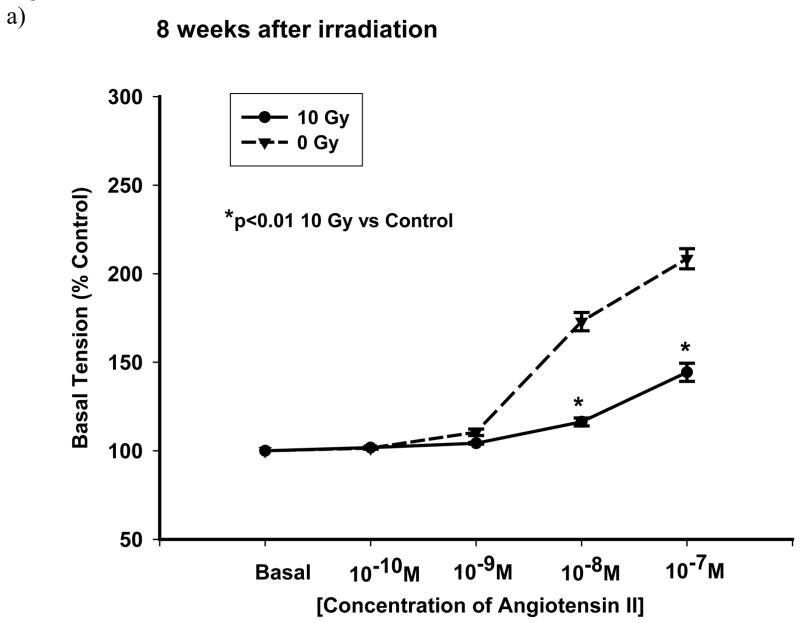
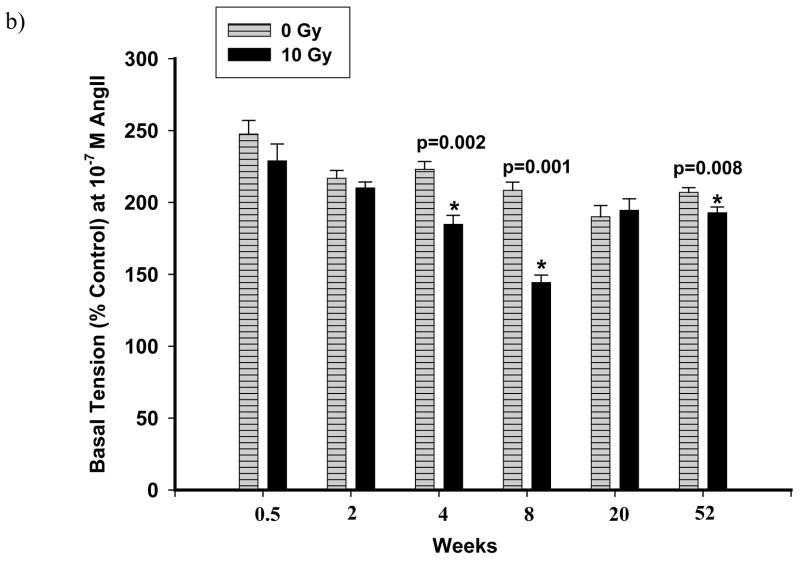
Dissected pulmonary arteries were examined for constriction to Ang II. The non-receptor mediated response of pulmonary arteries to KCl (80 mM) was also studied. In control vascular rings, the pulmonary arteries constricted to Ang II (10− 10 M–10− 7 M) in a concentration-dependent manner (dashed lines in Figure 5a and Figure 6a). Figure 5a is a representative graph demonstrating reactivities after exposure to 5 Gy. There were no significant changes as compared to un-irradiated rats up to 20 wks after exposure (Figure 5b). However, with the dose of 10 Gy, vasoreactivity was considerably altered at different time points summarized in Figure 6b, which shows the response to 10− 7 M Ang II at the different time points following exposure to 10 Gy. Three days after exposure, there was a mild but insignificant attenuation in reactivity to Ang II. Two weeks after irradiation, there was no change in reactivity to Ang II. At 4 wks following irradiation, there was a significant loss of constriction to Ang II (10− 8 M and 10− 7 M) which was even more marked after 8 wks after exposure (Figure 6a). Interestingly, these changes in reactivity of pulmonary arteries were not observed after 20 wks after irradiation. However, the vasoreactivity of pulmonary arteries from radiated rats were again diminished over that of controls after 52 wks after irradiation (Figure 6b).
Figure 5.
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 5 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; n=32 rings from 8 rats (panel a), n=20–34 rings from 5–9 rats (panel b). No significant differences were observed at 8 wks after irradiation (panel a). The response of pulmonary artery rings to 10−7 M Ang II at the different times after irradiation found no significant differences between the two groups (panel b).
Figure 6.
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 10 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; n=27 rings from 7 rats (panel a), n=16–40 rings from 4–11 rats (panel b). There was a fall in reactivity in irradiated vessels at 8 wks after irradiation (panel a). The response of the rings to 10−7 M Ang II at different time points following exposure showed significant attenuation to vasoreactivity at 4, 8 and 52 wks after irradiation (* p<0.01 vs Ctrl, panel b).
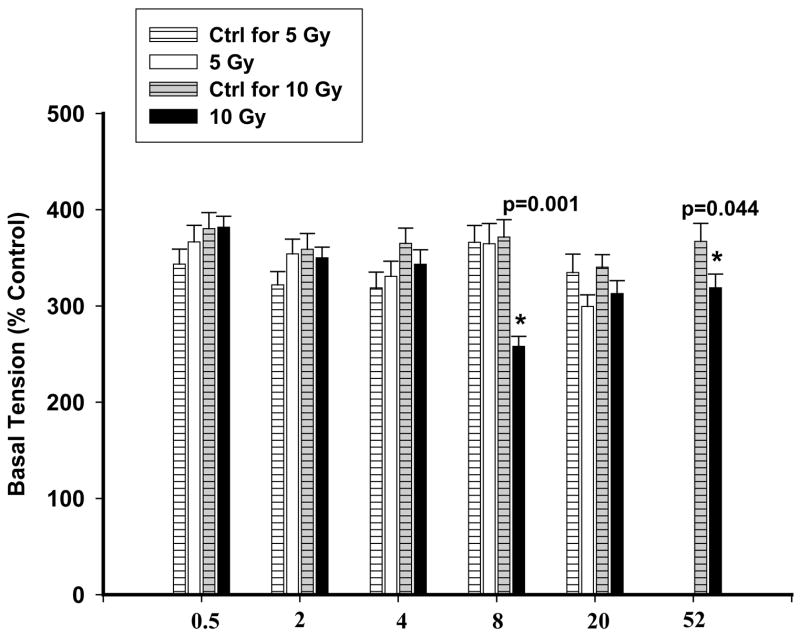
The vessels from rats irradiated with a dose of 10 Gy and examined after 8 wks and 52 wks demonstrated an attenuated constriction with KCl (80 mM), which was similar to the trend observed with Ang II. The response to KCl at the other time points was the same as that seen with control rings and is summarized in Figure 7.
Figure 7.
The effect of KCl on rat PA rings at different doses and time points. The blank and black columns represent 5 Gy and 10 Gy respectively. No significant differences were observed between 5 Gy and control rings (striped columns). Significant attenuation to KCl was noted at 8 wks and 52 wks following exposure to 10 Gy compared to controls (grey-striped columns). Each data is mean ± sem and expressed as % of baseline contraction (* p<0.05 vs Ctrl); n=16–40 rings from 4–11 rats.
Discussion
Most of the research to date on normal tissue radiation effects and radioprotectors, has been investigated using high-dose and/or fractionated radiation as models of cancer radiotherapy. In the context of a radiological accident/attack, survival is possible with exposure to whole body doses at or below 10 Gy (Coleman et al. 2003). We have developed a rat model of radiation-induced lung injury employing a single dose of 5 or 10 Gy. To study effects due to exposure to the lungs, and not secondary effects attributable to injury to other organs including kidneys, GI tract, brain etc., we irradiated the whole thorax only and not the total body. Clearly these studies will need to be extended to radiological terrorism models that would include other types of irradiation (e.g., internal emitters) and both larger and more nonuniform fields. The Chernobyl accident in 1986 resulted in large amounts of radioactive materials being released into the environment. On site personnel and emergency workers were exposed to doses ranging from 2–20 Gy with some early fatalities. Among the general population exposed to the Chernobyl radioactive fallout, however, the radiation doses were relatively low, and acute radiation syndrome and associated fatalities did not occur (The Chernobyl forum 2003–2005). At longer times after exposure in the low-moderate dose range, there is the potential for the expression of injury in other tissues such as the kidney and lungs. Our model simulates the lung injury that might be sustained in such a scenario.
The results with our model demonstrate, using six independent assays, that a dose of 10 Gy induces transient pulmonary dysfunction around 8–13 wks after irradiation. We detected, in a non-invasive manner, increased breathing rate and decreased oxygen saturation under resting conditions and after hypoxic stress. Examination of the BALF (invasively collected from rat lungs) demonstrated an increase in total protein with decrease in the lung specific marker CC10. Vascular reactivity, measured by constriction of pulmonary arteries ex vivo, to the peptide hormone Ang II, was blunted after exposure to 10 Gy. We also observed the most significant histological derangements around 8 wks as compared to 5 other time points. These results correlated structural damage with the functional deteriorations. Interestingly, all parameters of injury, including most histological changes significantly recovered by 20 wks after irradiation. We also examined rats exposed to 5 Gy and observed no remarkable alterations in pulmonary function up to 8 wks. However, this time point may be relatively early for definitive changes following exposure to 5 Gy, which may either have a longer latency, less severity of injury or more significant recovery, thereby masking detection. We did not test rats beyond 20 wks as we had already observed recovery from the more serious injuries in the 10 Gy cohort by this time.
Histological analysis revealed vascular changes, mainly perivascular edema, notably at 4–8 wks following irradiation with 10 Gy and at 8 wks following 5 Gy. Minimal histological evidence of radiation damage was noted in the 5 Gy cohort up to 20 wks. Rats belonging to the 10 Gy cohort exhibited fibrosis at 20 wks and 52 wks after exposure to 10 Gy. There was also observable interstitial thickening in the 10 Gy group past 20 wks, which could not be accurately scored in un-inflated lungs. Molteni et al (2000) have reported vascular changes in histology, which included detachment of endothelial cells, subendothelial and perivascular edema, a few days after injury, in a rat model of hemithoracic irradiation with 20 or 30 Gy of γ-rays. This progressed to severe arteritis and interstitial collagen deposition at 3 months, and then on to severe pneumonitis and extensive pulmonary fibrosis at 6 months. We did not observe such effects with 5 or 10 Gy.
Breathing rate is a sensitive non-invasive measure of pulmonary dysfunction and with radiotherapy relevant doses of radiation, investigators have noted a dose-dependant elevation in respiratory frequency in rats (Novakova-Jiresova et al. 2005). We have recorded a drop in respiratory frequency beyond 20 wks after irradiation indicating recovery (confirmed by other functional assays) as well as a possible age related decline of this parameter in rats. Pulse oximetry is widely used in the clinical setting. Studies indicate that the physiological responses to hypoxic stress, including changes in arterial blood oxygenation in the rodent model, are similar to those seen in humans (Gonzalez et al. 1991). We have observed a small but significant decrease in oxygen saturation under resting conditions and following hypoxic stress in the rats irradiated with a dose of 10 Gy. It is likely that the sensitivity of our measures for pulse oximetry was lower as compared to corresponding measures in humans, owing to the difficulty in obtaining steady readings from un-anesthetized rats and due to the thickness of the integument of the tail. Our findings do however suggest that pulse oximetry might be a viable diagnostic and prognostic tool following radiation injury to the lungs. In clinical practice, oximetry combined with mild/moderate exercise to bring cardiopulmonary disorders or lack of reserve to light exercise (e.g. six min walk test) would likely have more sensitivity than resting oximetry, similar to what we observed after exposure of rats to hypoxia (Baughmann et al. 2007).
Clara cells are non-ciliated, non-mucous, secretory cells of the pulmonary airways, which are known to secrete a variety of proteins, including Clara cell 10 KDa protein/uteroglobin (Hermans et al. 1999, Parker & Townsley 2004). It has been speculated that CC10 acts to protect the lungs from oxidative damage although it’s exact physiological function remains to be clarified (Broeckaert et al. 2000). Several investigators have suggested that BALF and serum levels of this protein can serve as a marker of Clara cell damage and bronchoalveolar blood barrier permeability (Hermans et al. 1999, Parker & Townsley 2004). Arsalane and colleagues (2000) demonstrated that there is a marked decrease in the secretion and synthesis of CC10 during LPS-induced acute lung inflammation in rats. In a murine model of ventilator induced lung injury, plasma CC10 was found to increase with lung injury and correlated with peak inflation pressure and BALF albumin and total protein concentrations (Yoshikawa et al. 2003). CC10 levels following thoracic irradiation have not been studied. After 10 Gy of thoracic irradiation we observed a significant reduction of this protein in the BALF, while total protein levels were markedly elevated. It is worthwhile noting that the effects we observed were not present after a dose of 5 Gy. Serum levels of CC10 were very low in all the rats studied with no differences between groups (data not shown) indicating that BALF CC10 levels may be a better predictor of sub-lethal radiation lung injury in rats than blood levels.
The effects of irradiation on vascular artery tone and dynamics have been studied by several investigators (Perkett et al. 1986, Warfield et al. 1990, Bourlier et al. 1998, Eder et al. 2004). It has been reported that 20 Gy whole-body irradiation results in a decreased response of the abdominal aorta to U46619, a stable thromboxane A2 mimic (Warfield et al. 1990). A high dose of irradiation (60 Gy) increased serotonin induced contraction of the pulmonary artery, whereas lower (20 Gy) doses slightly decreased it compared with control (Eder et al. 2004). Kwock et al (1987) demonstrated that irradiation with 30 Gy of gamma irradiation resulted in a 70% decrease in pulmonary artery perfusion using technetium-99m microaggregated albumin in the irradiated lung by 2–3 weeks after irradiation in rats. In another study conducted in sheep, a substantial increase in pulmonary vascular resistance as well as pulmonary arterial pressure in the weeks following whole lung irradiation was observed (Perkett et al. 1986). Our findings indicate that irradiation with the lower dose (5 Gy) does not affect the vasoreactivity of pulmonary arteries to Ang II, ex vivo. However, we demonstrate that with the higher dose (10 Gy), there is a loss of constriction to Ang II at 4–8 wks followed by substantial recovery after 20 wks though we observed mild attenuation of vasoreactivity again after 52 wks after irradiation. It is possible that the attenuation in vasoreactivity may be more pronounced at longer time points after irradiation. The decrease in reactivity that we observed may occur as a result of changes in the expression of surface receptors to Ang II, or alterations in intracellular signaling pathways. Since the capacity for vasoconstriction is required for matching ventilation and perfusion, radiation induced vascular injury may be expected to lead to oxygen desaturation, particularly under conditions of stress.
In summary, we have shown that single exposure to a sub-lethal dose of irradiation (10 Gy) to the whole thorax of the rat results in histological alterations with functional consequences. While all these changes reversed, mild fibrosis appeared after 20 wks that did not substantially affect the functional assays after exposure to 10 Gy. It has been noted previously that the first phase of early injury to the lung generally subsides after several wks and is followed by chronic inflammation and fibrosis that develops months or years after irradiation (McDonald et al. 1995). We conclude that moderate doses of radiation only temporarily injure rat lungs, but unlike previous models utilizing higher doses of radiation, pulmonary function recovers even without intervention when rats are maintained in a moderate security barrier facility. Further studies are needed to determine if lung function is reduced after times beyond 1 year after irradiation and if this recovery is unique to the rat. It is also possible that recovery may not occur in rats receiving similar doses delivered to the whole body, where the kidneys and the entire bone marrow are exposed, or where there is combined injury such as infection.
Acknowledgments
This work was supported by NIAID cooperative agreement AI067734. We thank Lynn Gruman of the Histology Core for the sectioning and staining of lung tissues, and Marylou Mäder, Vladimir Semenenko and X. Allen Li in the Irradiation Core for conducting irradiation and dosimetry.
References
- Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A. Clara cell specific protein (CC16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. American Journal of Respiratory and Critical Care Medicine. 2000;161:1624–1630. doi: 10.1164/ajrccm.161.5.9812157. [DOI] [PubMed] [Google Scholar]
- Baughmann RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–213. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- Bourlier V, Diserbo M, Joyeux M, Ribuot C, Multon E, Gourmelon P, Verdetti J. Early effects of acute gamma-radiation on vascular arterial tone. British Journal of Pharmacology. 1998;123:1168–1172. doi: 10.1038/sj.bjp.0701744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Annals of the New York Academy of Sciences. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- Canzian M, Soeiro A, Taga MF, Farhat C, Barbas CSV, Capelozzi VL. Semiquantitative assessment of surgical lung biopsy: predictive value and impact on survival of patients with diffuse pulmonary infiltrate. Clinics. 2007;62:23–30. doi: 10.1590/s1807-59322007000100005. [DOI] [PubMed] [Google Scholar]
- Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environmental Health Perspective. 1986;70:261–2003. doi: 10.1289/ehp.8670261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Stone HB, Tofilon PJ, Wong RSL. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: Report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiation Research. 2003;159:812–834. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- Kwock L, Davenport WC, Clark RL, Zarembra J, Lingle B, Chaney EL, Friedman M. The effects of ionizing radiation on the pulmonary vasculature of intact rats and isolated pulmonary endothelium. Radiation Research. 1987;111:276–291. [PubMed] [Google Scholar]
- Decker MJ, Conrad KP, Strohl KP. Noninvasive oximetry in the rat. Biomedical Instrumentation and Technology. 1989;23:222–228. [PubMed] [Google Scholar]
- Eder V, Gautier M, Boissière J, Girardin C, Rebocho M, Bonnet P. Gamma irradiation induces acetylcholine-evoked, endothelium-independent relaxation and activates K-channels of isolated pulmonary artery of rats. International Journal of Radiation Oncology, Biology, Physics. 2004;60:1530–1537. doi: 10.1016/j.ijrobp.2004.07.698. [DOI] [PubMed] [Google Scholar]
- Fajardo LF. Pathology of radiation injury. New York: Masson; 1982. [Google Scholar]
- Franko AJ, Sharplin J. Development of fibrosis after lung irradiation in relation to inflammation and lung function in a mouse strain prone to fibrosis. Radiation Research. 1994;140:347–355. [PubMed] [Google Scholar]
- Gonzalez NC, Sokari A, Clancy RC. Maximum oxygen uptake and arterial blood oxygenation during hypoxic exercise in rats. Journal of Applied Physiology. 1991;71:1041–1049. doi: 10.1152/jappl.1991.71.3.1041. [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiation Research. 1999;152:6–13. [PubMed] [Google Scholar]
- Hermans C, Knoops B, Wiedig M, Arsalane K, Toubeau G, Falmagne P, Bernard A. Clara cell protein as a marker of Clara cell damage and bronchoalveolar blood barrier permeability. European Respiratory Journal. 1999;13:1014–1021. doi: 10.1034/j.1399-3003.1999.13e14.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Ogushi F, Tani K, Ogawa H, Kawano T, Endo T, Izumi K, Sono N, Ueno J, Sone S. Thrombin promotes fibroblast proliferation during the early stages of experimental radiation pneumonitis. Radiation Research. 2001;156:45–52. doi: 10.1667/0033-7587(2001)156[0045:tpfpdt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1a, interleukin-1b and tumor necrosis factor a mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiation Research. 1996;145:762–767. [PubMed] [Google Scholar]
- Lehnert BE, Dethloff LA, Finkelstein JN, Van Der Kogel AJ. Temporal sequence in early alterations in rat lung following thoracic irradiation. International Journal of Radiation Biology. 1991;60:657–675. doi: 10.1080/09553009114552481. [DOI] [PubMed] [Google Scholar]
- Maisin JR. The ultrastructure of the lung of mice exposed to a supra-lethal dose of ionizing radiation on the thorax. Radiation Research. 1970;44:545–564. [PubMed] [Google Scholar]
- McDonald S, Rubin P, Phillips TL, Marks LB. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. International Journal of Radiation Oncology Biology Physics. 1995;31:1187–1203. doi: 10.1016/0360-3016(94)00429-O. [DOI] [PubMed] [Google Scholar]
- Mirsattari SM, Bihari F, Leung LS, Menon RS, Wang Z, Ives JR, Bartha R. Physiological monitoring of small animals during magnetic resonance imaging. Journal of Neuroscience Methods. 2004;144:207–213. doi: 10.1016/j.jneumeth.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, Wolfe LF, Brizio-Molteni L, Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. International Journal of Radiation Biology. 2000;76:523–532. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- Novakova-Jiresova A, van Luijk P, van Goor H, Kampinga HH, Coppes RP. Pulmonary Radiation Injury: Identification of risk factors associated with regional hypersensitivity. Cancer Research. 2005;65:3568–3576. doi: 10.1158/0008-5472.CAN-04-3466. [DOI] [PubMed] [Google Scholar]
- Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. American Journal of Physiology – Lung. 2004;286:231–246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- Perkett EA, Brigham KL, Meyrick B. Increased vasoreactivity and chronic pulmonary hypertension following thoracic irradiation in sheep. Journal of Applied Physiology. 1986;61:1875–1881. doi: 10.1152/jappl.1986.61.5.1875. [DOI] [PubMed] [Google Scholar]
- Peterson LM, Evans ML, Graham MM, Eary JF, Dahlen DD. Vascular Response to radiation injury in the rat lung. Radiation Research. 1992;129:139–148. [PubMed] [Google Scholar]
- Rosiello RA, Merrill WW, Rockwell S, Carter D, Cooper JA, Jr, Care S, Amento EP. Radiation pneumonitis. Bronchoalveolar lavage assessment and modulation by a recombinant cytokine. American Review of Respiratory Disease. 1993;148:1671–1676. doi: 10.1164/ajrccm/148.6_Pt_1.1671. [DOI] [PubMed] [Google Scholar]
- The Chernobyl Forum. 2003–2005. Chernobyl’s legacy: health, environmental and socioeconomic impacts and recommendations to the governments of Belarus, the Russian Federation and Ukraine. WHO, IAEA and UNDP.
- Ts’ao CH, Ward WF, Port CD. Radiation injury in rat lung. I. Prostacyclin (PGI2) production, arterial perfusion, and ultrastructure. Radiation Research. 1983;96:284–293. [PubMed] [Google Scholar]
- Van der Meeren A, Monti P, Lebaron-Jacobs L, Marquette C, Gourmelon P. Characterization of the acute inflammatory response after irradiation in mice and its regulation by interleukin 4. Radiation Research. 2001;155:858–865. doi: 10.1667/0033-7587(2001)155[0858:cotair]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Van Eerde MR, Kampinga HH, Szabo BG, Vujaskovic G. Comparison of three rat strains for development of radiation-induced lung injury after hemithoracic irradiation. Radiotherapy and Oncology. 2001;58:313–316. doi: 10.1016/s0167-8140(00)00301-7. [DOI] [PubMed] [Google Scholar]
- Warfield ME, Schneidkraut MJ, Ramwell PW, Kot PA. WR2721 ameliorates the radiation-induced depression in reactivity of rat abdominal aorta to U46619. Radiation Research. 1990;121:63–66. [PubMed] [Google Scholar]
- Yoshikawa S, Reynolds SD, Parker JC. Ventilator induced lung injury detected by plasma levels of Clara cell specific protein in mice. American Journal of Respiratory and Critical Care Medicine. 2003;167:A775. [Google Scholar]
- Zhu D, Effros RM, Harder D, Roman RJ, Jacobs ER. Tissue sources of cytochrome P4504A and 20-HETE syntheses in rabbit lungs. American Journal of Respiratory Cell and Molecular Biology. 1998;18:1–8. doi: 10.1165/ajrcmb.19.1.3145. [DOI] [PubMed] [Google Scholar]