Summary
The five somatostatin receptor subtypes, named sst1 to sst5, activate both distinct and common signaling pathways and exhibit different patterns of receptor regulation. Until recently it was believed that once a particular somatostatin receptor was activated by an agonist, all the downstream signaling and regulatory effects characteristic of that receptor subtype in that cellular environment would be triggered. Thus, differences in the actions of somatostatin analogs between tissues were attributed to variability in the nature and concentration of the sst receptor subtypes and effectors expressed in different targets. However, agonists have recently been shown to exhibit functional selectivity at individual sst receptors such that they can elicit a subset of that receptor’s potential effects, a property known as biased agonism. This review will summarize the evidence for functionally selective somatostatin receptor agonists and discuss the implications and promise of these new findings.
Keywords: Somatostatin receptors, receptor signaling, receptor internalization, receptor desensitization, somatostatin analogs, biased agonism, stimulus trafficking
INTRODUCTION
The somatostatin receptor family is encoded by five genes, named sst1 – sst5. The mRNA produced from the sst2 gene is alternatively spliced in rodents and thus produces two protein products, sst2A and sst2B, that differ in their carboxy-termini. In contrast, the mRNAs for the other sst genes are not spliced. Thus, six different somatostatin receptor proteins are known and have been extensively characterized. Other articles in this compendium discuss recent developments in understanding the signaling and regulation of the individual sst receptor subtypes either alone or in the presence of heterologous G protein coupled receptors (GPCRs). These reviews demonstrate that the nature of the receptor isotypes present in a particular cellular environment is a critical determinant of the tissue response to somatostatin. However, recent studies have shown that the nature of the agonist is also critically important for determining the biological consequences of receptor activation. In this review I will discuss the evidence showing that agonists can exhibit functional selectivity at somatostatin receptors and direct a receptor to activate only select components of its biological repertoire.
Somatostatin receptor signaling and regulation involve multiple interacting partners
The binding of hormone to somatostatin receptors initiates a complex set of signaling events triggered by the interaction of the activated receptors with a large number of different protein partners (for recent reviews see (Csaba and Dournaud, 2001; Lahlou et al., 2004; Olias et al., 2004; Schonbrunn, 2004), reviews in this compendium). As members of the seven-transmembrane domain receptor family, it is not surprising that much of somatostatin receptor signaling involves activation of heterotrimeric G proteins. All somatostatin receptor subtypes inhibit adenylyl cyclase via the pertussis toxin sensitive G protein family, Gi/Go. Further, some somatostatin receptors activate K channels and/or inhibit Ca channels via Gi/Go proteins in endocrine or neuronal target cells leading to an inhibitory effect on secretion. In fact, recent evidence indicates that pertussis toxin sensitive G proteins also mediate the stimulation of tyrosine phosphatases by somatostatin receptors thereby producing some of the cytostatic actions of somatostatin (Arena et al., 2007) as well as the stimulation of phospholipase C activity leading to smooth muscle contraction (Murthy et al., 1996). In addition to activating the Gi/Go family of G proteins, somatostatin receptors can act via pertussis toxin independent mechanisms. In some cases these mechanisms involve activation of pertussis toxin insensitive G proteins, such as G12 or G14 (Hou et al., 1994; Komatsuzaki et al., 2001; Lin et al., 1996; Liu and Wong, 2005) whereas in other instances a direct interaction between sst receptors and phosphatases or kinases has been proposed (Bousquet et al., 2006; Ferjoux et al., 2003; Lopez et al., 1996). The list of molecules that bind to activated somatostatin receptors to initiate intracellular signaling is still undoubtedly incomplete and will continue to expand with additional studies. However, it is clear that the spectrum of interactions that occur between the activated receptor and its effectors determines the subsequent intracellular signaling events and, ultimately, the biological consequences of somatostatin receptor activation.
In addition to the multiplicity of partners involved in somatostatin receptor signaling, a different set of agonist triggered protein interactions lead to somatostatin receptor desensitization and internalization. Somatostatin binding is known to be rapidly followed by phosphorylation of sst1, sst2A and sst3 receptors by G protein coupled receptor kinases or GRKs (Elberg et al., 2002; Hipkin et al., 1997; Liu and Schonbrunn, 2001; Roth et al., 1997). The GRK family consists of six serine-threonine kinases that specifically bind to and phosphorylate agonist activated GPCRs (Moore et al., 2007; Reiter and Lefkowitz, 2006). Receptor phosphorylation results in the recruitment of cytoplasmic proteins called arrestins in a receptor subtype specific manner ((Brasselet et al., 2002; Kreuzer et al., 2001; Liu et al., 2005; Tulipano et al., 2004); Liu et al submitted for publication). Generally, the binding of arrestins to an activated, phosphorylated GPCR blocks further interaction between the receptors and G proteins and thus results in the desensitization of G-protein mediated signaling. In addition, arrestins play a key role in directing GPCRs to clathrin coated vesicles and to the endocytic macinery. The consequent receptor internalization removes GPCRs from the cell surface so that they are no longer available for agonist stimulation - a second mechanism for desensitization. Most interestingly, arrestins have recently been shown to also play an important role in G-protein independent GPCR signaling by recruiting cytosolic molecules to the receptor-arrestin complex (Dewire et al., 2007). Although the precise role of receptor phosphorylation and arrestin binding in the desensitization, trafficking and signaling of different somatostatin receptor subtypes has not been fully elucidated, these highly regulated and specific protein-protein interactions are recognized to play a critical role somatostatin receptor function.
This brief overview just begins to indicate the number of proteins that specifically recognize somatostatin receptors after, but not before, agonist binding as well as the resulting complexity involved in somatostatin receptor signaling and regulation. Thus, for somatostatin receptors, as for GPCRs in general, it has become critical to understand the nature of receptor activation.
Ligand binding and functional selectivity in G protein coupled receptors
Early models of receptor activation proposed that the binding of an agonist shifted an equilibrium from “the inactive”, basal conformation of a receptor to “the active” conformation by stabilizing the latter (Fig 1, Model 1) (Kenakin, 2004; Perez and Karnik, 2005). Agonists were proposed to differ both in their affinity for the receptor and in their ability to shift the equilibrium from the inactive to the active receptor state. However, receptors in “the active” conformation were able to couple to the entire spectrum of cytoplasmic effector proteins targeted by that receptor and therefore to produce all the biological consequences of those signaling interactions. Therefore, this two-state model for receptor activation predicted that, in a given cellular environment, the relative potencies of agonists for inducing any two biological effects from a single receptor would be the same (Kenakin, 2004; Perez and Karnik, 2005). This is because only the amount of activated receptor formed will vary with agonist not its structure.
Figure 1. Models for receptor activation.
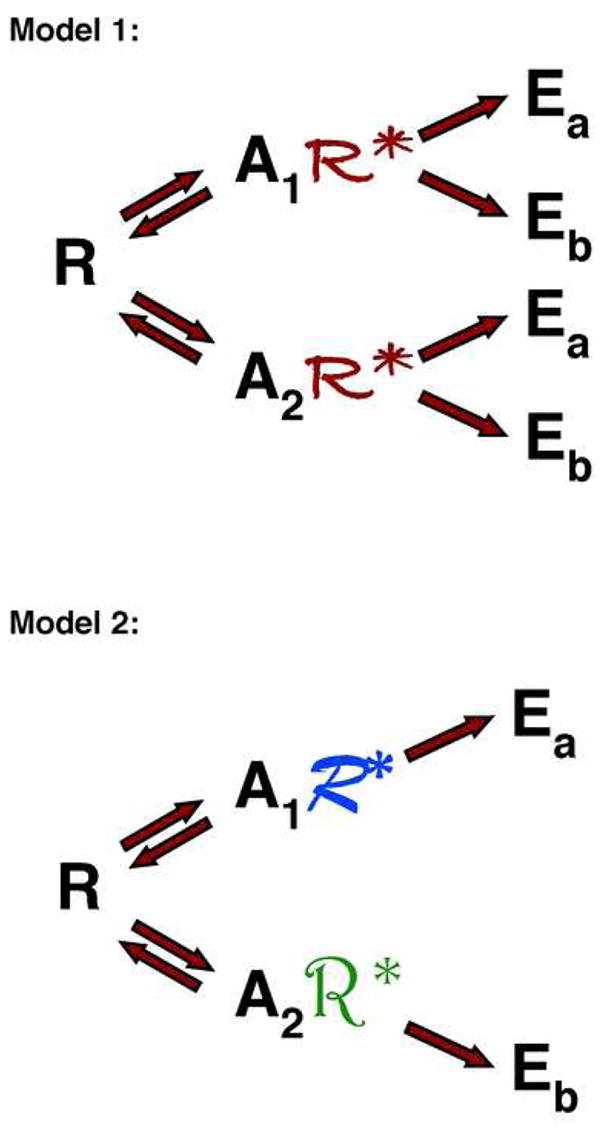
Model 1 shows two agonists (A1 and A2) that stabilize the same active receptor conformation (R*). The agonists differ in their binding affinity for the receptor and the effectiveness with which they can stabilize the active receptor conformation. However, since the conformation of the activated receptor (R*) is independent of the agonist bound, all agonists will stimulate the same spectrum of effectors (Ea and Eb) and produce the same spectrum of biological responses.
Model 2 shows two agonists (A1 and A2) that each stabilize a different active receptor conformation (denoted by R* in different fonts). Agonists differ both in their binding affinity for the receptor and the conformation of the activated receptor that they stabilize. Each receptor conformation will activate a characteristic subset of effectors (e.g. Ea or Eb) from the spectrum of possible effectors for the receptor and therefore each agonist can induce a characteristic set of biological responses that can differ from those induced by other agonists acting at the same receptor.
The two-state model for receptor activation has dominated much of the literature on somatostatin receptors. In most instances analog activities have been deduced from only one or two measurements such as inhibition of hormone secretion or modulation of second messenger production. Such representative measurements were then used to indicate the overall potency and efficacy of the analogs under investigation.
Subsequent studies in the signal transduction field have shown that a two-state model cannot explain the effect of many agonists to induce only some of the possible responses following receptor activation. The concept of selective receptor modulators is probably most widely recognized in the nuclear receptor field where it has had an enormous impact on steroid receptor targeted therapeutics (Jordan, 2007). However, recent studies have demonstrated that the general concept of selective receptor modulators also applies to GPCRs (for recent reviews see (Galandrin et al., 2007; Kenakin, 2004; Neubig, 2007; Perez and Karnik, 2005; Urban et al., 2007; Violin and Lefkowitz, 2007)) and this evolution in our understanding again promises to have important consequences for drug development (Mailman, 2007). New terms have been coined to describe the idea that agonists can selectively activate different signaling pathways and responses via a single GPCR. These terms include “functionally selective agonism”, “protean agonism”, “biased agonism” and “ligand directed trafficking of receptor signals” among others. Such selective signaling is explained by a model in which agonists not only exhibit different affinities for a receptor, but they also stabilize different active receptor conformations (Fig 1, Model 2). These different activated receptor structures determine the affinity of the receptor for the spectrum of cytoplasmic proteins which mediate its signaling effects, such as G proteins, kinases, and phosphatases, as well as its regulation, such as GRKs or arrestins. Thus, this model predicts that in any given cell type, the relative potencies or efficacies of two agonists for various biological effects may differ even though their actions are triggered by a single receptor subtype.
Functionally selective agonists at somatostatin receptors
The first systematic studies to characterize the effects of a large number of somatostatin analogs on multiple signaling end points were carried out by Hoyer’s group (Siehler and Hoyer, 1999a; Siehler and Hoyer, 1999b; Siehler and Hoyer, 1999c; Siehler et al., 2005). These investigators compared the potencies and efficacies of somatostatin analogs in CCL39 cells stexpressing one of the sst receptor subtypes on three parameters: (1) stimulation of GTPγS binding in membrane preparations, (2) inhibition of forskolin stimulated cyclic AMP production in intact cells, and (3) stimulation of phosphoinositide accumulation also in intact cells. Cyclic AMP production was a readout for receptor inhibition of adenylyl cyclase via pertussis toxin sensitive G proteins whereas inositol phosphate production resulted from phospholipase C stimulation via both PTX sensitive and insensitive pathways.
In general, agonist-induced GTPγS binding, phosphoinositide turnover, and inhibition of adenylyl cyclase showed major differences both in the rank order of potencies and in the relative efficacies of different analogs. However, although suggestive, the lack of correlation observed in these studies did not directly demonstrate preferential coupling of individual somatostatin receptors to particular signaling pathways largely because the assays used did not segregate the signaling events triggered by sst receptor activation. Thus, agonist stimulation of GTPγS binding reflects receptor activation of all receptor-interacting G proteins, including the pertussis toxin sensitive G proteins that couple somatostatin receptors to adenylyl cyclase and the pertussis toxin insensitive G proteins that are at least partially responsible for coupling receptors to phospholipase C. Further, since cyclic AMP measurements were made after 15 min of agonist stimulation whereas phosphoinositide production was determined after 50 min of stimulation, the effect of receptor desensitization will differ in the two assays. We now know that desensitization can occur after few minutes of agonist stimulation for several somatostatin receptor subtypes (Beaumont et al., 1998; Elberg et al., 2002; Engstrom et al., 2006; Hipkin et al., 1997; Holliday et al., 2007; Liu et al., 2000). Thus, desensitization will influence second messenger formation in assays conducted in intact cells and the impact of desensitization on such measurements will vary with the time of incubation. Nonetheless, the observed differences in the activities of somatostatin agonists to regulate diverse second messenger systems via the same receptor did provide suggestive evidence for the existence of multiple agonist-specific receptor conformations preferentially coupled to particular intracellular signaling events (Siehler and Hoyer, 1999b; Siehler and Hoyer, 1999c).
To further test whether functional selectivity occurs with somatostatin receptors we examined the effects of 11 somatostatin analogs on two distinct receptor-activated pathways (Liu et al., 2005). Our aim was to minimize cross-over effects in the end-points used to measure receptor interaction with different effector molecules. Using CHO cells expressing only sst2A receptors, we measured the potencies and efficacies of a series of analogs to inhibit cyclic AMP production and to stimulate receptor internalization. Receptor internalization is unaffected by pertussis toxin pretreatment and depends on arrestin binding to the receptor (Liu et al manuscript submitted). In contrast, inhibition of cyclic AMP production is abolished by pertussis toxin and is therefore mediated entirely by receptor activation of Gi/Go proteins. Finally, to minimize the effects of receptor desensitization, cyclic AMP measurements were made after only 10 min of agonist stimulation.
All but one of the analogs tested behaved as full agonist for both inhibition of cyclic AMP production and stimulation of receptor endocytosis (Liu et al., 2005). However, their relative potencies for the two activities varied about 10-fold. Interestingly, the analogs tested fell into two groups. The native peptides SS14, SS28 and cortistatin were all close to equipotent at inhibiting cyclic AMP production and at stimulating receptor endocytosis. In contrast, synthetic, short somatostatin analogs, such as [Tyr3]octreotide and vapreotide were 5 to 10 times more potent at signal generation than receptor internalization (Liu et al., 2005). These differences in potency ratios demonstrate that agonists do not stabilize a common active state of the sst2A receptor that then causes both cyclase inhibition and receptor internalization (Liu et al., 2005). Rather, agonists must stabilize different receptor conformations with varying abilities to activate downstream signaling pathways.
Surprisingly, the peptidomimetic L-779,976 was unique in that it was a partial agonist for stimulating receptor internalization even though it was more potent than the native peptides for both cyclic AMP inhibition and receptor endocytosis. Consistent with the lower efficacy of L-779,976 for receptor internalization, the sst2A receptor-arrestin complex formed upon agonist binding was far less stable when the receptor was occupied by L-779,976 than when it was occupied by SS14 (Liu et al., 2005). These results show a direct, agonist-specific effect on the interaction of the sst2A receptor with one of its effector proteins. Therefore, they provide strong support for the conclusion that the conformation of the activated receptor depends on the agonist bound and, further, that agonists determine the nature of the interactions between the receptor and down-stream effectors.
Two subsequent studies indicated that agonist specific receptor conformations also exist with other sst receptor subtypes. Cescato et al showed that several of the most potent synthetic sst5 agonists, such as KE108, and L-817,818, were unable to produce internalization of this receptor subtype even though the native peptides, SS14 and SS28 were effective (Cescato et al., 2006). The striking dissociation between the high potency and efficacy of these analogs to inhibit cyclic AMP production (Reubi et al., 2002; Rohrer et al., 1998) and their inability to stimulate receptor internalization (Cescato et al., 2006) demonstrates functional selectivity of agonists at the sst5 receptor.
Using cytosensor microphysiometry, two groups have reported differential agonist activity at sst4 receptors (Engstrom et al., 2006; Smalley et al., 1998). Both groups measured analog stimulation of the extracellular acidification rate (EAR), which results from activation of the Na+/H+ antiporter via pertussis toxin sensitive G proteins. They measured the increase in EAR during a 10 to 50 sec agonist stimulation of sst4 expressing CHO cells as well as the desensitization of this response to multiple peptide challenges. Strikingly, in both studies the ability of agonists to produce an EAR response was unrelated to their ability to induce desensitization. The results support the conclusion that analogs cause differential activation of receptor-effector pathways and the formation of multiple activated conformations of sst4. Moreover, these studies provide the first demonstration that somatostatin receptor desensitization can be separated from receptor signaling, an observation with important therapeutic implications.
In summary, it is now clear that signaling by, and regulation of, sst 2, sst4 and sst5 receptors are sensitive to the nature of the activating agonist. It is likely that additional examples of functional agonist selectivity will be forthcoming at these as well as other somatostatin receptor subtypes, as such examples are now streaming in for the entire GPCR family.
The identification of biased agonists at sst receptors means that we can no longer assume that all agonists are functionally equivalent and produce the same spectrum of effects at individual sst receptors. Moreover, we may have to re-examine some past studies in which the effects of specific and nonselective agonists are compared. Previously, the effects of one analog which was able to simultaneously active multiple receptors were often compared with the effects of another analog that was specific for a single somatostatin receptor isotype. Differences in the activities of two such analogs were interpreted as resulting from an interaction, either direct or indirect, between the receptor subtypes activated by the nonselective agonist. However, the new results summarized here indicate that some of the previous observations comparing specific and broad spectrum agonists may have resulted from differential effects of the two compounds at target receptors rather than from receptor interactions. The use of receptor specific antagonists together with agonist that simultaneously target multiple receptors will undoubtedly help to sort out the potentially complex interactions that may occur between somatostatin receptor subtypes as well as between somatostatin receptors and heterologous GPCRs.
The promise and challenge of functionally selective somatostatin analogs
Biased agonism has now been demonstrated for several somatostatin receptor subtypes. However, the number of functionally selective somatostatin receptor agonists is still small and their discriminatory effects on receptor action have been limited to a few end points -namely receptor signaling via pertussis toxin sensitive G proteins and receptor regulation (internalization or desensitization) by mechanisms which are likely to involve arrestin recruitment. Further, the structural basis for the observed differences in receptor activity are unknown and we are nowhere near the point that we can identify specific ligand-receptor interactions that can direct the receptor to the activation of particular effectors. Despite these gaps in our understanding, it is clear that the potential for selective therapeutic effects at somatostatin receptors is much broader than previously recognized, as drugs may not only target particular somatostatin receptor subtypes but may also induce differential activities at specific receptors. Thus, for somatostatin receptors, as for GPCRs in general, the possibility of new drugs with different pharmacological profiles at specifically targeted receptor subtypes holds great promise.
Acknowledgments
I thank members of my laboratory, Qisheng Liu, Dian Dewi and Sunny Liu, for their contributions to the research covered in this review as well as our collaborators on these projects, Jean-Claude Reubi, Renzo Cescato, and Jean Rivier. Our studies in this area were supported by a research grants from the National Institute of Arthritis, Diabetes, Digestive, and Kidney Disease (DK-32234).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Arena S, Pattarozzi A, Massa A, Esteve JP, Iuliano R, Fusco A, Susini C, Florio T. An intracellular multi-effector complex mediates somatostatin receptor 1 activation of phospho-tyrosine phosphatase{eta} Mol Endocrinol. 2007;21:229–46. doi: 10.1210/me.2006-0081. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Hepworth MB, Luty JS, Kelly E, Henderson G. Somatostatin receptor desensitization in NG108-15 cells. A consequence of receptor sequestration. J Biol Chem. 1998;273:33174–83. doi: 10.1074/jbc.273.50.33174. [DOI] [PubMed] [Google Scholar]
- Bousquet C, et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. 2006;25:3943–54. doi: 10.1038/sj.emboj.7601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasselet S, Guillen S, Vincent JP, Mazella J. beta-Arrestin is involved in the desensitization but not in the internalization of the somatostatin receptor 2A expressed in CHO cells. FEBS Lett. 2002;516:124–8. doi: 10.1016/s0014-5793(02)02517-6. [DOI] [PubMed] [Google Scholar]
- Cescato R, et al. Internalization of sst2, sst3 and sst5 receptors: Effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502–11. [PubMed] [Google Scholar]
- Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides. 2001;35:1–23. doi: 10.1054/npep.2001.0848. [DOI] [PubMed] [Google Scholar]
- Dewire SM, Ahn S, Lefkowitz RJ, Shenoy SK. beta-Arrestins and Cell Signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Elberg G, Hipkin RW, Schonbrunn A. Homologous and heterologous regulation of somatostatin receptor 2. Mol Endocrinol. 2002;16:2502–14. doi: 10.1210/me.2002-0207. [DOI] [PubMed] [Google Scholar]
- Engstrom M, Savola JM, Wurster S. Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J Pharmacol Exp Ther. 2006;316:1262–8. doi: 10.1124/jpet.105.094128. [DOI] [PubMed] [Google Scholar]
- Ferjoux G, et al. Critical Role of Src and SHP-2 in sst2 Somatostatin Receptor-mediated Activation of SHP-1 and Inhibition of Cell Proliferation. Mol Biol Cell. 2003;14:3911–28. doi: 10.1091/mbc.E03-02-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–30. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Hipkin RW, Friedman J, Clark RB, Eppler CM, Schonbrunn A. Agonist-induced desensitization, internalization, and phosphorylation of the sst2a somatostatin receptor. Journal of Biological Chemistry. 1997;272:13869–13876. doi: 10.1074/jbc.272.21.13869. [DOI] [PubMed] [Google Scholar]
- Holliday ND, Tough IR, Cox HM. A functional comparison of recombinant and native somatostatin sst(2) receptor variants in epithelia. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Gilbert RL, Barber DL. Subtype-specific signaling mechanisms of somatostatin receptors SSTR1 and SSTR2. Journal of Biological Chemistry. 1994;269:10357–62. [PubMed] [Google Scholar]
- Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. 2007;99:350–6. doi: 10.1093/jnci/djk062. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–92. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki K, Terashita K, Kinane TB, Nishimoto I. Somatostatin type V receptor activates c-Jun N-terminal kinases via Galpha(12) family G proteins. Biochem Biophys Res Commun. 2001;289:1211–7. doi: 10.1006/bbrc.2001.6085. [DOI] [PubMed] [Google Scholar]
- Kreuzer OJ, Krisch B, Dery O, Bunnett NW, Meyerhof W. Agonist-Mediated Endocytosis of Rat Somatostatin Receptor Subtype 3 Involves beta-Arrestin and Clathrin Coated Vesicles. J Neuroendocrinol. 2001;13:279–287. doi: 10.1046/j.1365-2826.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- Lahlou H, Guillermet J, Hortala M, Vernejoul F, Pyronnet S, Bousquet C, Susini C. Molecular signaling of somatostatin receptors. Ann N Y Acad Sci. 2004;1014:121–31. doi: 10.1196/annals.1294.012. [DOI] [PubMed] [Google Scholar]
- Lin X, Voynoyasenetskaya TA, Hooley R, Lin CY, Orlowski J, Barber DL. G-alpha-12 differentially regulates Na+-H+ exchanger isoforms. Journal of Biological Chemistry. 1996;271:22604–22610. doi: 10.1074/jbc.271.37.22604. [DOI] [PubMed] [Google Scholar]
- Liu AM, Wong YH. Activation of nuclear factor {kappa}B by somatostatin type 2 receptor in pancreatic acinar AR42J cells involves G{alpha}14 and multiple signaling components: a mechanism requiring protein kinase C, calmodulin-dependent kinase II, ERK, and c-Src. J Biol Chem. 2005;280:34617–25. doi: 10.1074/jbc.M504264200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Cescato R, Dewi DA, Rivier J, Reubi JC, Schonbrunn A. Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol. 2005;68:90–101. doi: 10.1124/mol.105.011767. [DOI] [PubMed] [Google Scholar]
- Liu Q, Prejusa A, Schonbrunn A. Agonist induced phosphorylation of the sst1 somatostatin receptor: relationship to desensitization and internalization. Proceedings of the 82nd Meeting of the Endocrine Society; Toronto, Ontario. 2000. [Google Scholar]
- Liu Q, Schonbrunn A. Agonist-induced phosphorylation of somatostatin receptor subtype 1 (sst1). Relationship to desensitization and internalization. J Biol Chem. 2001;276:3709–17. doi: 10.1074/jbc.M008873200. [DOI] [PubMed] [Google Scholar]
- Lopez F, Esteve JP, Buscail L, Delesque N, Saintlaurent N, Vaysse N, Susini C. Molecular mechanisms of antiproliferative effect of somatostatin - involvement of a tyrosine phosphatase. Metabolism: Clinical & Experimental. 1996;45:14–16. doi: 10.1016/s0026-0495(96)90071-2. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–6. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of Receptor Trafficking by GRKs and Arrestins. Annu Rev Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor-mediated signaling in smooth muscle. Activation of phospholipase C-beta3 by Gbetagamma and inhibition of adenylyl cyclase by Galphai1 and Galphao. J Biol Chem. 1996;271:23458–63. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- Neubig RR. Missing links: mechanisms of protean agonism. Mol Pharmacol. 2007;71:1200–2. doi: 10.1124/mol.107.034926. [DOI] [PubMed] [Google Scholar]
- Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–91. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–61. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Eisenwiener KP, Rink H, Waser B, Macke HR. A new peptidic somatostatin agonist with high affinity to all five somatostatin receptors. Eur J Pharmacol. 2002;456:45–9. doi: 10.1016/s0014-2999(02)02651-1. [DOI] [PubMed] [Google Scholar]
- Rohrer SP, et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–40. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- Roth A, Kreienkamp HJ, Meyerhof W, Richter D. Phosphorylation of four amino acid residues in the carboxyl terminus of the rat somatostatin receptor subtype 3 is crucial for its desensitization and internalization. J Biol Chem. 1997;272:23769–74. doi: 10.1074/jbc.272.38.23769. [DOI] [PubMed] [Google Scholar]
- Schonbrunn A. Somatostatin Receptors. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry. Vol. 4. Elsevier; Oxford: 2004. [Google Scholar]
- Siehler S, Hoyer D. Characterisation of human recombinant somatostatin receptors. 2. Modulation of GTPgammaS binding. Naunyn Schmiedebergs Arch Pharmacol. 1999a;360:500–9. doi: 10.1007/s002109900142. [DOI] [PubMed] [Google Scholar]
- Siehler S, Hoyer D. Characterisation of human recombinant somatostatin receptors. 3. Modulation of adenylate cyclase activity. Naunyn Schmiedebergs Arch Pharmacol. 1999b;360:510–21. doi: 10.1007/s002109900143. [DOI] [PubMed] [Google Scholar]
- Siehler S, Hoyer D. Characterisation of human recombinant somatostatin receptors. 4. Modulation of phospholipase C activity. Naunyn Schmiedebergs Arch Pharmacol. 1999c;360:522–32. doi: 10.1007/s002109900144. [DOI] [PubMed] [Google Scholar]
- Siehler S, Nunn C, Zupanc GK, Hoyer D. Fish somatostatin sst3 receptor: comparison of radioligand and GTPgammaS binding, adenylate cyclase and phospholipase C activities reveals different agonist-dependent pharmacological signatures. Auton Autacoid Pharmacol. 2005;25:1–16. doi: 10.1111/j.1474-8673.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Feniuk W, Humphrey PP. Differential agonist activity of somatostatin and L-362855 at human recombinant sst4 receptors. Br J Pharmacol. 1998;125:833–41. doi: 10.1038/sj.bjp.0702133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Hollt V, Schulz S. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–82. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. beta-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]


