Abstract
The PC cell line is a highly tumorigenic, insulin-independent, teratoma-derived cell line isolated from the nontumorigenic, insulin-dependent 1246 cell line. Studies of the PC cell growth properties have led to the purification of an 88-kDa secreted glycoprotein called PC cell-derived growth factor (PCDGF), which has been shown to stimulate the growth of PC cells as well as 3T3 fibroblasts. Sequencing of PCDGF cDNA demonstrated its identity to the precursor of a family of 6-kDa double-cysteine-rich polypeptides called epithelins or granulins (epithelin/granulin precursor). Since PCDGF was isolated from highly tumorigenic cells, its level of expression was examined in PC cells as well as in nontumorigenic and moderately tumorigenic cells from which PC cells were derived. Northern blot and Western blot analyses indicate that the levels of PCDGF mRNA and protein were very low in the nontumorigenic cells and increased in tumorigenic cell lines in a positive correlation with their tumorigenic properties. Experiments were performed to determine whether the autocrine production of PCDGF was involved in the tumorigenicity of PC cells. For this purpose, we examined the in vivo growth properties in syngeneic C3H mice of PC cells where PCDGF expression had been inhibited by transfection of antisense PCDGF cDNA. The results show that inhibition of PCDGF expression resulted in a dramatic inhibition of tumorigenicity of the transfected cells when compared with empty-vector control cells. These data demonstrate the importance in tumor formation of overexpression of the novel growth factor PCDGF.
The C3H mouse teratoma-derived cell line 1246 is an adipogenic cell line requiring insulin to proliferate and differentiate in defined medium (1, 2). Insulin-independent variant cell lines were isolated from 1246 cells maintained in insulin-free medium. One cell line called 1246-3A was analyzed in detail and shown to have lost the ability to differentiate and to have become moderately tumorigenic when 106 cells were injected into syngeneic host C3H mice (3). It was shown that the 1246-3A cells synthesized and secreted several factors that affected their proliferation and differentiation and the factors were biochemically characterized and identified (4–6). To establish a cell culture model of increased tumorigenicity, an in vitro–in vivo shuttle technique (7) was applied to isolate from the 1246-3A cells a highly tumorigenic cell line called PC (5). These highly tumorigenic PC cells gave rise to tumors when 104 cells were injected s.c. into syngeneic C3H mice (5). These three cell lines ranging from differentiating and nontumorigenic cells to differentiation-deficient, highly tumorigenic cells represented a unique model system to analyze the cellular and biochemical changes associated with the acquisition of tumorigenic properties. Comparative studies of the growth properties of the cell lines indicated that PC cells had lost the ability to respond to the growth factors required by 1246 and 1246-3A cells and instead became dependent on their own conditioned medium for proliferation (8). The factor responsible for this growth stimulation, PC cell-derived growth factor (PCDGF), was purified to homogeneity and sequenced (9). PCDGF was an 88-kDa glycoprotein consisting of a 68-kDa core polypeptide and a 20-kDa carbohydrate moiety (8), shown by amino acid and cDNA sequencing to be identical to the epithelin/granulin precursor (9, 10). Epithelin and granulins are 6-kDa double-cysteine-rich polypeptides originally purified from rat kidney (11) and from human granulocyte extracts (12). Although no function was attributed to granulins during their purification and characterization, epithelins were shown to be dual growth effectors for epithelial cells (10, 11). Cloning and sequencing of epithelin and granulin cDNAs showed that the 6-kDa polypeptides were encoded by a common precursor cDNA (9, 10). This epithelin/granulin precursor corresponded to a 63-kDa polypeptide contained 7.5 repeats of the 6-kDa epithelin or granulin polypeptides and several putative glycosylation sites (9, 10). As PC was a highly tumorigenic cell line that synthesized and secreted PCDGF, it was important to investigate the possible role of PCDGF on the growth of the teratoma cells. For this purpose, two experimental approaches were taken. One was to determine whether PCDGF was expressed preferentially in the highly tumorigenic cells. This was done by comparing the levels of expression of PCDGF mRNA and protein in the tumorigenic PC cell derivatives with the ones found in the nontumorigenic 1246 and moderately tumorigenic 1246-3A parent cells. The second approach was to examine the role of PCDGF expression on the tumorigenic properties of the PC cells by preventing the factor from being synthesized and examining the resulting changes in the growth properties of the cells. This was achieved by transfecting PC cells with an antisense PCDGF cDNA construct that blocked the growth factor expression. This approach has been used widely to examine the effect of inhibiting the expression of growth factors or their receptors on the growth properties of producer cells (13–19). Experiments presented here examined the in vitro and in vivo growth properties of PC cells in which PCDGF expression had been inhibited by transfection with antisense PCDGF cDNA.
MATERIALS AND METHODS
Cell Culture.
Stock cultures of PC and 1246-3A cells were maintained in defined media as described previously (6). 1246 stock cells were cultivated in DME/F12 nutrient medium (1:1 mixture) (GIBCO/BRL) supplemented with 10% fetal bovine serum (FBS) (Gibco). For comparative studies, the three cell lines were cultivated in DME/F12 medium supplemented with 2% FBS.
Northern Blot Analysis of PCDGF mRNA Expression.
Total cellular RNA was isolated by RNAzol method (Cinna/Biotecx Laboratories, Friendswood, TX). Fifteen micrograms of total RNA was separated by electrophoresis on a denaturing 1.2% agarose gel containing 0.22 M formaldehyde in 1× MOPS (20). RNA samples were blotted onto nitrocellulose membrane (MSSI, Westboro, MA) by overnight capillary transfer in 10× SSC, and then hybridized at 42°C overnight in hybridization solution [50% formamide/5× SSPE/1% SDS/5× Denhardt’s solution/1 μg/ml poly(A)/100 μg/ml denatured salmon sperm DNA] with approximately 106 cpm/ml of randomly primed 32P-labeled mouse PCDGF cDNA probe to measure PCDGF mRNA expression. Filters were washed and exposed to x-ray film (Kodak X-Omat AR) for autoradiography. Ribosomal protein L32 mRNA (RPL32) was detected as an internal standard for normalizing RNA loading (21).
Immunoprecipitation and Western Blot Analysis of PCDGF Protein.
Since PCDGF is a secreted protein, its expression was measured in cell lysates and conditioned media collected in the presence of a protease inhibitor mixture of 200 μM phenylmethylsulfonyl fluoride (PMSF)/1 μM leupeptin/0.5 μM aprotinin/1 mM EDTA (all obtained from Sigma). Cells were lysed in PBS containing 1% Triton X-100 followed by sonication and centrifugation. For comparative studies of PCDGF expression, the samples used for immunoprecipitation and Western blot analysis were normalized to equivalent cell number (see figure legends), determined by counting cells from duplicate sets of dishes. Immunoprecipitation of PCDGF was carried out by incubating samples for 4 hr with 5 μg of affinity-purified anti-PCDGF IgG conjugated to agarose beads followed by centrifugation at 10,000 × g for 10 min. Immune complexes were resuspended in Laemmli sample buffer (22), boiled for 5 min, and separated by electrophoresis on a 10% polyacrylamide gel in the presence of SDS. Proteins were electrophoretically transferred to nitrocellulose membrane (Schleicher & Schuell). The membranes were blocked with 5% nonfat milk overnight at 4°C and then incubated for 1 hr at room temperature with 10 ng/ml of anti-PCDGF IgG conjugated to horseradish peroxidase, in the presence of 1% BSA. Immunoreactivity was visualized by the enhanced chemiluminescence detection system (Amersham).
Construction and Transfection of Antisense PCDGF cDNA Expression Vector in PC Cells.
PC cells were transfected with a PCDGF antisense cDNA fragment cloned into the expression vector pCMV4 (23) as described below. A 228-bp PCDGF cDNA fragment that included the start codon region was obtained by digesting full-length PCDGF cDNA with SmaI and XbaI enzymes. This fragment was cloned in the antisense orientation into XbaI and SmaI site of pCMV4 and is referred to hereafter as pAS-PCDGF. Transfection of PC cells with pAS-PCDGF by the calcium phosphate method (20) was performed when the cells reached 80% confluence in DME medium supplemented with 10% FBS. A calcium phosphate precipitate added to the cells contained 20 μg of pAS-PCDGF plasmid, 2 μg of pRSVneo plasmid carrying the neomycin-resistant gene as a selectable marker, and 20 μg of pSK plasmid as carrier. After 7 hr, the cells were shocked with 10% DMSO for 2–3 min, washed twice, and fed with DME/F12 medium supplemented with 10% FBS. One day after transfection, cells were subcultured at a 1:3 ratio and cultivated in serum-supplemented medium in the presence of 400 μg/ml of Geneticin (G-418 Sulfate, GIBCO/BRL). This medium first was changed 2 days later and then every 3–4 days thereafter. After 10–14 days, colonies of G418-resistant cells were picked with cloning rings and cultivated in DME/F12 medium supplemented with 10% FBS and 400 μg/ml G418. Control PC cells were transfected with pCMV4 and pRSVneo plasmid DNAs and isolated as described above. The presence of the transfected DNA (pAS-PCDGF or pCMV4) was determined by PCR analysis of genomic DNA isolated from G418-selected transfectants. For empty-vector control cells, PCR analysis was performed by using the primer pair of SP647, 5′-CCTACTTGGCAGTACATCTACGTA-3′, and AP912, 5′-CTGACGGTTCACTAAACGAGCTC-3′, corresponding to the cytomegalovirus (CMV) promoter region. The sense primer SP647 (described above) and antisense primer SP7 5′-CGAGAATTCAGGCAGACCATGTGGGTC-3′ located in the start codon region of PCDGF cDNA were used to test for the presence of antisense PCDGF cDNA in the genomic DNA of antisense transfectants. The transfectants were lysed in buffer A (100 mM KCl/10 mM Tris⋅HCl, pH 8.3/0.45% Tween 20/0.45% Nonidet P-40) and 120 μg/ml proteinase K (Boehringer Mannheim). Then, they were incubated at 60°C for 1 hr, followed by boiling for 15 min. DNA of each clone (50–100 ng) was used as template for PCRs. DNA from nontransfected cells was used as negative control. Plasmid DNA was used as a positive control. PCR was performed in a 20-μl reaction mixture containing 10 mM Tris⋅HCl, pH 9.0/50 mM KCl/1.5 mM MgCl2/0.1% Triton X-100/0.2 mM dNTP/0.5 units Taq DNA polymerase (Promega)/20 ng of each primer/50 ng genomic DNA template. The reaction tubes were heated to 95°C for 3 min and then subjected to 40 cycles of 95°C for 1 min, 55°C for 2 min, and 72°C for 3 min with a 10-min 72°C extension in a thermocycler (MJ Research, Cambridge, MA). Ten microliters of PCR product was analyzed on a 1% agarose gel and stained with ethidium bromide.
Measurement of Cell Proliferation.
Proliferation of pAS-PCDGF and control transfected PC cells was examined. Cells were plated in 12-well plates (Corning) at a density of 3 × 104 cells per well in 2 ml of either 2F defined medium consisting of DME/F-12 medium supplemented with 2 μg/ml human fibronectin (Upstate Biotechnology, Lake Placid, NY) and 10 μg/ml human transferrin (Sigma) or DME-F12 medium supplemented with 2% FBS. On day 5, cells were washed with PBS and enumerated with a Coulter counter after trypsinization of cells from duplicate wells.
Tumorigenicity Study.
Six-week-old female C3H mice from Taconic Farms (Germantown, NY) were injected s.c. with 106 pAS-PCDGF or control transfected PC cells. The appearance and size of tumors were examined daily. The mice were sacrificed 40 days after injection to measure tumor weight.
All experiments described here were repeated at least twice.
RESULTS
Comparison of PCDGF mRNA Expression in 1246, 1246-3A, and PC Cells.
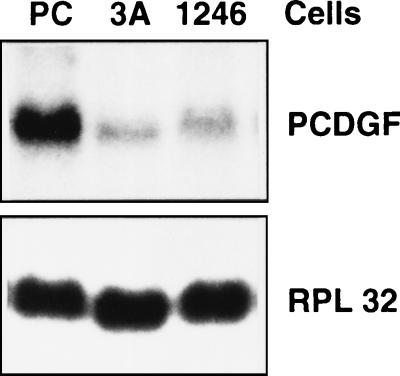
The cell lines 1246 (nontumorigenic), 1246-3A (moderately tumorigenic), and PC (highly tumorigenic) were cultivated in DME/F12 medium supplemented with 2% FBS. When the cells were 80% confluent, total RNA was extracted and PCDGF mRNA expression was measured by Northern blot analysis using a radiolabeled PCDGF cDNA probe. RPL32 mRNA expression was measured as an internal control for equal RNA loading. As shown in Fig. 1, the level of expression of PCDGF was very low in the nontumorigenic 1246 cells and in the moderately tumorigenic 1246-3A cells. In contrast, PCDGF mRNA expression increased by at least 20-fold in the highly tumorigenic PC cells. The size of the PCDGF mRNA transcript expressed by the cells was 2.2 kb.
Figure 1.
Comparison of PCDGF mRNA expression in 1246, 1246-3A, and PC cells. Cells were cultured in DME/F12 medium supplemented with 2% FBS until 80% confluent. Medium was changed 24 hr before RNA was collected. Fifteen micrograms of total RNA was analyzed by Northern blot with 32P-labeled PCDGF cDNA probe to measure PCDGF mRNA expression. Ribosomal protein RPL32 mRNA expression was used as internal standard for RNA loading.
PCDGF Protein Expression in the Three Cell Lines.
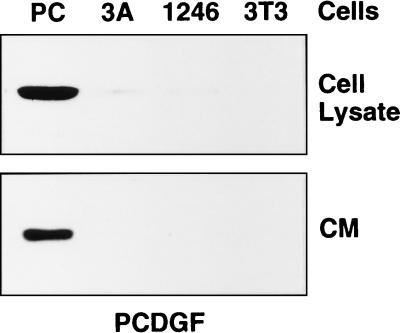
Next we examined the expression of PCDGF protein in the three cell lines. Since PCDGF is a secreted protein (8), we examined its level not only in cell lysates but also in the culture medium of the three cell lines by a combination of immunoprecipitation and Western blot analysis by using an anti-PCDGF antibody. As shown in Fig. 2, the level of expression of PCDGF in cell lysates and conditioned media of the highly tumorigenic PC cells was much higher than in 1246 and 1246-3A cells, similar to the differences observed for PCDGF mRNA expression (Fig. 1). PCDGF protein expression was very low in the 1246-3A cell lysate and culture medium and undetectable in 1246 cells. The data presented in Figs. 1 and 2 suggest that the level of PCDGF expression in the three cell lines correlated with the degree of tumorigenicity of the cells. Based on these results, it was hypothesized that PCDGF overexpression in PC cells was directly associated with their tumorigenic properties. To examine this hypothesis, experiments were carried out to isolate PC cells in which PCDGF expression had been inhibited by stable transfection with PCDGF antisense cDNA and to compare the tumorigenic properties of these antisense transfected cells with empty-vector transfected PC cells.
Figure 2.
PCDGF protein expression in 1246, 1246-3A, and PC cells. Cells were cultivated as described in the legend of Fig. 1. Cell lysates and conditioned media were collected and normalized by cell number. Samples corresponding to 18 × 105 cells (cell lysates) and 3 × 105 cells (conditioned media) were used to measure PCDGF expression by immunoprecipitation and Western blot by using an anti-PCDGF antibody as described under Materials and Methods.
Isolation of Antisense PCDGF Transfected PC Cells.
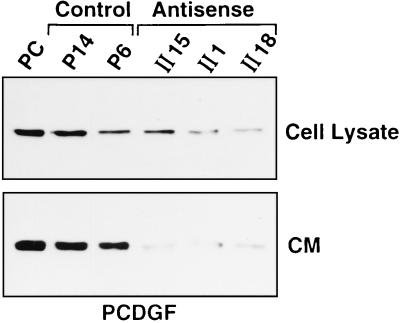
PC cells were cotransfected with the pAS-PCDGF plasmid (Fig. 3) and with the pRSVneo plasmid as described under Material and Methods. Control PC cells were cotransfected with empty pCMV4 and pRSVneo plasmids. In both cases, transfected cells were selected for growth in medium containing G418. Colonies were assayed for the presence of the transfected plasmids by PCR analysis of genomic DNA (Fig. 4). Antisense pAS-PCDGF and empty-vector control transfected cells were tested further to determine the level of PCDGF protein expression by Western blot analysis (Fig. 5). All antisense clones tested exhibited a significantly reduced level of PCDGF expression both in cell lysates and in conditioned media when compared with empty-vector controls (P6 and P14) and with wild-type PC cells. Three antisense clones (AS-II1, AS-II15, and AS-II18) and two empty-vector control clones (P6 and P14) were chosen and studied further. Inhibition of PCDGF protein expression was observed in the three antisense transfected clones to varying degrees (Fig. 5). The level of PCDGF protein secreted in the conditioned media of empty-vector control cells (P14 and P6) was similar to that secreted by untransfected PC cells.
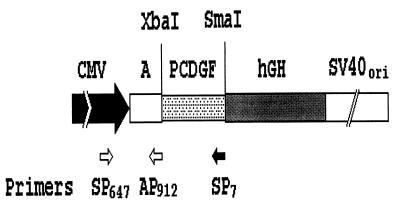
Figure 3.
Construction of antisense PCDGF cDNA. A 228-bp PCDGF cDNA fragment (−45 to +183 bp) including the start codon region was ligated in the antisense orientation into the XbaI-SmaI site of pCMV4 expression vector. Immediate early promoter region (CMV, stippled block), a DNA copy of a segment of the alfalfa mosaic virus 4 RNA that contains a translational enhancer (A), transcription termination and polyadenylation signals from the human growth hormone gene (hGH, gray block), and the simian virus 40 (SV40) origin of DNA replication and early region enhancer sequences (SV40ori, white block) are shown. Locations of primers used for PCR analysis of transfectants are indicated.
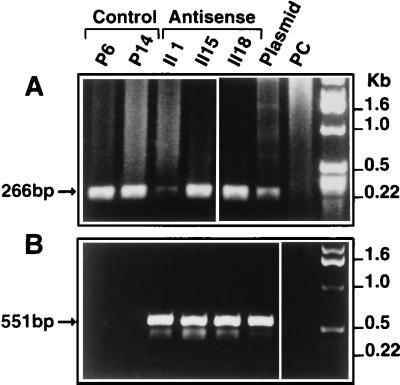
Figure 4.
PCR analysis of antisense and control transfected clones. PCR analysis of DNA from the selected clones was performed with primer pairs as described in Materials and Methods. (A) SP647 (sense primer) and the AP912 (antisense primer), both located in the CMV promoter (Fig. 3), were used to amplify a 266-bp fragment from cells transfected with the CMV promoter expression vector (antisense and empty vector transfected cells). (B) Sense primer SP647 (described above) and antisense primer SP7, located in the start codon region of PCDGF cDNA (Fig. 3), were used to amplify a 551-bp DNA fragment in the pAS-PCDGF transfectants only. No amplified band was obtained with DNA from control transfected cells. pAS-PCDGF plasmid DNA and genomic DNA from nontransfected PC cells were used as positive and negative control template for PCR, respectively.
Figure 5.
PCDGF protein expression in antisense and control transfected clones. PC cells and antisense and control transfectants were cultivated in DME/F12 medium supplemented with 2% FBS. The medium was replaced with fresh medium 24 hr before the samples were collected. Cell lysates and conditioned media were normalized by cell numbers of 18 × 105 and 3 × 105 cells, respectively, and were immunoprecipitated with anti-PCDGF IgG and analyzed by Western blot as described in Materials and Methods.
In Vitro Growth Properties of Antisense and Control Transfected Cells.
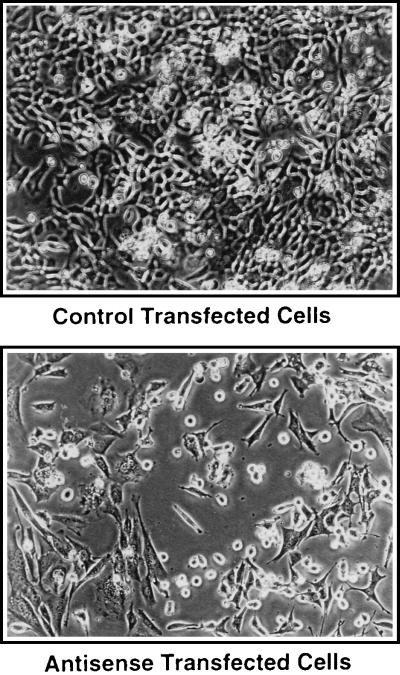
The morphology of antisense and control transfected cells was examined. Phase-contrast micrographs indicated that the pAS-PCDGF transfected cells did not spread as well as the control cells and they maintained a rounded morphology when compared with control transfected PC cells (Fig. 6).
Figure 6.
Morphology of antisense and control transfectants in monolayer culture. Phase-contrast micrographs of antisense and control transfectant cells. (×100.)
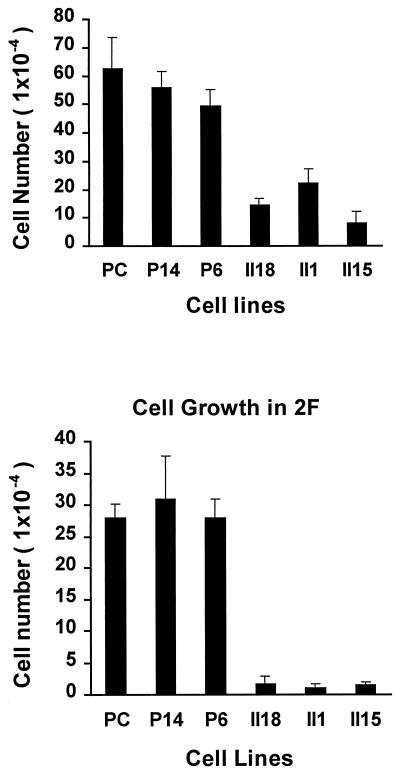
Growth of both types of transfected cells was measured in serum-supplemented medium and in serum-free defined medium (Fig. 7). pAS-PCDGF transfected cells cultivated either in defined medium (Fig. 7 Lower, 2F medium) or in serum-containing medium (Fig. 7 Upper) had a reduced proliferative capacity when compared with control transfected cells and untransfected PC cells cultivated in similar culture conditions. After 5 days in culture, the number of pAS-PCDGF transfected cells was reduced by 90% in defined medium and by 50% in serum-supplemented medium compared with control cells cultivated in the same conditions. Moreover, after 5 days, the number of cells in defined medium was 10% of those in serum-containing medium. In contrast, empty-vector control cells had the same proliferation capacity as nontransfected PC cells, and they displayed only a 50% reduction in cell number after 5 days in defined medium when compared with serum-containing medium.
Figure 7.
In vitro growth properties of antisense and control transfected clones. PC cells, antisense cDNA transfected cells, and empty vector control transfected cells were plated either in DME/F-12 medium supplemented with 2 μg/ml human fibronectin and 10 μg/ml human transferrin (2F medium, Lower) or with 2% FBS added 12 hr after plating (Upper). At day 5, cells from duplicate wells were trypsinized and counted with a Coulter counter. The experiment was repeated twice. Each bar represents the mean number of cells (±SD) calculated from both experiments.
Tumorigenic Properties of Antisense and Control PC Cells.
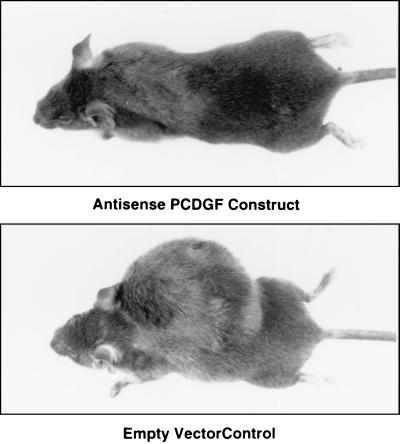
The tumorigenic properties of the three pAS-PCDGF transfected clones and two empty-vector control clones were examined by s.c. injection of 106 cells per mouse into 6-week-old syngeneic C3H female mice. Tumor formation was followed by daily monitoring of the mice, and tumor growth was determined by measuring the dimensions of the tumors. Fig. 8 shows mice injected with control cells and pAS-PCDGF transfected cells 40 days after injection. The mice were sacrificed and the tumors were collected and weighed. As shown in Table 1, all of the mice injected with either one of the control transfected PC cell lines (P14 and P6) developed tumors rapidly. Tumors were visible as early as 15 days after injection for both the control transfectants and nontransfected PC cells. These results indicated that the growth properties of the control cells had not been affected significantly by the transfection and selection processes. In contrast, all three clones that had been transfected with pAS-PCDGF showed a marked inhibition of tumor growth. For AS-II1 and AS-II18, inhibition was complete because after 40 days, none of the mice injected with either of these clones had tumors. For AS-II15, two of the five mice injected developed tumors, but the inhibition of tumor growth was still significant as AS-II15 tumors were 5–10% of the weight of the control transfected PC cell tumors. Even 60 days after injection in C3H mice, AS-II1 and AS-II18 did not form palpable tumors (data not shown).
Figure 8.
Comparison of mice injected with antisense and control transfected cells. C3H female mice were photographed 40 days after s.c. injection of 106 cells from antisense (Upper) or control PC transfectants (Lower).
Table 1.
In vivo tumorigenicity of PC cells transfected with antisense PCDGF cDNA and with empty vector
| Cells injected | Day of appearance ±SD | Mice with tumors | Weight, g, ±SD |
|---|---|---|---|
| AS-II1 | — | 0/5 | — |
| AS-II18 | — | 0/5 | — |
| AS-II15 | 36 ± 14 | 2/5 | 0.35 ± 0.05 |
| P14 | 15 ± 3 | 5/5 | 5.4 ± 2.0 |
| P6 | 15 ± 9 | 5/5 | 3.6 ± 1.9 |
| PC | 15 ± 4 | 5/5 | 6.4 ± 2.6 |
C3H female mice were injected s.c. with 106 cells from PCDGF antisense cDNA transfectants (AS-II1, AS-II18, and AS-II15), control transfectants (P14, P6), or nontransfected PC cells. Average day of tumor appearance, number of mice (of five) with tumors at 40 days, and tumor weight in g ± SD are provided.
DISCUSSION
PCDGF is a growth factor belonging to a family of double-cysteine-rich polypeptides that includes the 6-kDa cysteine-rich polypeptides epithelins and granulins (8–10). PCDGF is secreted as an 88-kDa glycoprotein by the highly tumorigenic PC cell line. PC cells are insulin-independent teratoma cells isolated for their high tumorigenic properties in syngeneic C3H mice (3). Histological analysis of the tumors generated by s.c. injection of PC cells in C3H mice indicate that PC cells form malignant fibrous histiocytomas (unpublished data). Sequencing of full-length PCDGF cDNA isolated from PC cell cDNA library indicated that PCDGF was identical to mouse epithelin/granulin precursor (10–11) and that PCDGF expressed by the highly tumorigenic cells did not contain any mutations (unpublished data). Time course studies of PCDGF synthesis and secretion in PC cells have shown that PCDGF is secreted as early as 2 hr after synthesis (data not shown). This observation and the fact that PC cells were growth-stimulated by PCDGF (8) and presented PCDGF cell-surface binding sites (24) indicated that secreted PCDGF was an autocrine growth factor for the highly tumorigenic cells. Based on these results, it was hypothesized that PCDGF expression may be increased in tumorigenic cells when compared with their normal counterparts. Increased expression of several growth factors in many different tumor cells, such as IGF-I in glioblastoma (14) and transforming growth factor-α in human breast cancer cells (25), has been observed. The model system consisting of nontumorigenic 1246 cells (1) and moderately tumorigenic 1246-3A (2) and highly tumorigenic PC cells (5) provided an experimental system to investigate directly the role of PCDGF (epithelin/granulin precursor) in tumorigenic cell lines derived from the same nontumorigenic and hormone-responsive parent cell line. The analysis of PCDGF mRNA and protein levels in the three cell lines indicated that PCDGF expression was undetectable in the nontumorigenic 1246 cells and significantly increased in the highly tumorigenic cells in correlation with the degree of tumorigenicity of the teratoma cells.
To determine whether increased PCDGF expression in PC cells contributed to their high tumorigenic properties, we used the antisense cDNA transfection approach to block PCDGF expression in PC cells and examine their growth characteristics. Antagonizing mRNA with the help of “hybridization competitor” in inhibiting protein synthesis was first introduced by Zamecnik et al. (26) and Plesner et al. (27). Recent years have seen considerable progress in studying the role of antisense RNA as an inhibitor of oncogenic protein production (13). The development of stable transfected clones with antisense cDNA is advantageous in that it allows a continuous supply of antisense RNA to disrupt protein synthesis and it is well suited for in vivo tumorigenic assays. Our results demonstrate that decreasing PCDGF protein synthesis and secretion by expression of antisense PCDGF mRNA in the highly tumorigenic PC cells reduced their growth ability in vitro and inhibited their tumorigenicity in vivo. Phase-contrast microscopic analysis showed that the antisense transfected cells with inhibited PCDGF expression had a decreased ability to spread on the tissue culture substrate. Many cells were rounded and floated in the culture medium. In contrast, empty vector control transfectants expressing normal PCDGF levels were well spread and grew as well as untransfected PC cells. Localization of PCDGF in PC cells by immunofluorescence with anti-PCDGF antibody revealed PCDGF staining at sites of cell–cell contact (unpublished data). This evidence suggests that PCDGF may affect cell growth by a complex mechanism including autocrine stimulation of PC cell growth and contribution in the regulation of adhesion and communication between cells.
In vivo studies indicated that inhibiting PCDGF expression in the teratoma cells resulted in an inhibition of tumor growth. Comparison of tumorigenicity (Table 1) and Western blot analysis showing the level of residual PCDGF expression in the pAS-PCDGF transfected cells (Fig. 5) suggests that the degree of inhibition of tumorigenicity correlates with the degree of inhibition of PCDGF expression in the cells. The two cell lines (AS-II1 and AS-II18) with the lowest level of PCDGF production and secretion were not tumorigenic since none of the injected mice developed tumors even after 60 days while tumors appeared in 15 days in mice injected with control cells. In contrast, AS-II15 cells, in which inhibition of PCDGF expression by antisense PCDGF cDNA transfection was not as efficient, maintained some degree of tumorigenicity (although a reduced one) since two of five mice developed smaller tumors, which first appeared after 36 days. Teratocarcinoma stem cells are known to originate from a breakdown in the normal regulatory processes controlling proliferation and differentiation rather than from mutations in genes responsible for the normal control of cell behavior (28). The abnormal behavior of such embryonic stem cells can be reversed if they are placed in a normal environment. Little is known about the molecular mechanism of the malignant transformation of teratoma. The fact that malignant teratoma arise from relatively undifferentiated cells implies the existence of a relationship between tumorigenesis and differentiation (29). The three cell lines, 1246, 1246-3A, and PC, with increasing tumorigenic properties were derived from a C3H mouse teratoma and provide a good model system to study the molecular mechanisms involved in the loss of differentiation properties and the acquisition of tumorigenic properties. The studies with this model system described here demonstrate that the epithelin/granulin precursor also known as PCDGF is an essential autocrine modulator resulting in the autonomous growth of undifferentiated, highly tumorigenic PC cells in vitro and in vivo. The data presented here also demonstrate that overexpression of epithelin/granulin precursor, PCDGF, may play an important function in teratocarcinogenesis and in tumorigenesis.
Acknowledgments
We thank Drs. Dominic Eisinger and Jun Hayashi for helpful discussion, Dr. J. Denry Sato for discussion and critically reading this manuscript, and Drs. Koichiro Tanaka, Xianmin Xia, and Jian Zhou and Mr. Steve Goodrich for technical suggestions. This work was supported by Grants NIH RO1 CA 55439 (GS) from the National Institutes of Health and DAMD 17-96-1-6072 (GS) from the U.S. Army Medical Research and Material Command.
ABBREVIATIONS
- FBS
fetal bovine serum
- PCDGF
PC cell-derived growth factor
- CMV
cytomegalovirus
References
- 1.Serrero G, Khoo J C. Anal Biochem. 1982;120:351–359. doi: 10.1016/0003-2697(82)90357-8. [DOI] [PubMed] [Google Scholar]
- 2.Serrero G. In Vitro Cell Dev Biol. 1985;21:537–540. doi: 10.1007/BF02620848. [DOI] [PubMed] [Google Scholar]
- 3.Yamada Y, Serrero G. Proc Natl Acad Sci USA. 1988;85:5936–5940. doi: 10.1073/pnas.85.16.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada Y, Serrero G. J Cell Physiol. 1989;140:254–263. doi: 10.1002/jcp.1041400210. [DOI] [PubMed] [Google Scholar]
- 5.Serrero G, Zhou J, Lepak N M. J Cell Physiol. 1991;149:503–511. doi: 10.1002/jcp.1041490321. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Serrero G. Growth Factors. 1993;9:123–131. doi: 10.3109/08977199309010827. [DOI] [PubMed] [Google Scholar]
- 7.Buonassisi V, Sato G H, Cohen A I. Proc Natl Acad Sci USA. 1962;48:1184–1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Gao G, Crabb J C, Serrero G. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 9.Bhandary V, Palfree R G E, Bateman A. Proc Natl Acad Sci USA. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plowman G D, Green J M, Neubauer M G, Buckley S D, McDonald V L, Todaro G J, Shoyab M. J Biol Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- 11.Shoyab M, McDonald V, Byles C, Todaro G J, Plowman G D. Proc Natl Acad Sci USA. 1990;87:7912–7916. doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Biochem Biophys Res Commun. 1990;173:1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 13.Gold L. J Biol Chem. 1995;270:13581–13584. doi: 10.1074/jbc.270.23.13581. [DOI] [PubMed] [Google Scholar]
- 14.Trojan J, Blossey B K, Johanson T R, Rudin S D, Tyrocinski M, Ilan J, Ilan J. Proc Natl Acad Sci USA. 1992;89:4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnicoff M, Li W, Basak S, Herlyn D, Baserga R, Rubin R. Cancer Immunol Immunother. 1996;42:64–68. doi: 10.1007/s002620050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C T, Wu S, Gabrilovich D, Chen H, Nadaf-Rahrov S, Ciernik I F, Carbone D P. Cancer Res. 1996;56:3038–3041. [PubMed] [Google Scholar]
- 17.Pass Hi, Mew D J, Carbone M, Matthews W A, Donongton J S, Baserga R, Walker C L, Resnicoff M, Steinberg S M. Cancer Res. 1996;56:4044–4048. [PubMed] [Google Scholar]
- 18.Burfeind P, Chernicky C L, Rininsland F, Ilan J, Ilan J. Proc Natl Acad Sci USA. 1996;93:7263–7268. doi: 10.1073/pnas.93.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maret A, Galy B, Arnaud E, Bayard F, Prats H. Cancer Res. 1995;55:5075–5079. [PubMed] [Google Scholar]
- 20.Ausubel F M, Brent R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current Protocols in Molecular Biology. New York: Greene & Wiley; 1994. [Google Scholar]
- 21.Bowman L H. Mol Cell Biol. 1987;7:4464–4471. doi: 10.1128/mcb.7.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K, Beguin F, Gujer-Kellenberger G. J Mol Biol. 1970;47:69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- 23.Andersson S, Davis D L, Dahlback H, Jornvalif H, Russell D W. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 24.Xia X, Serrero G. Biochem Biophys Res Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 25.Dickson R B, Lippman M E. Endocrine Rev. 1995;16:559–589. doi: 10.1210/edrv-16-5-559. [DOI] [PubMed] [Google Scholar]
- 26.Zamecnik P C, Goodchild J, Taguchi Y, Sarin P S. Proc Natl Acad Sci USA. 1986;83:4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plesner P, Goodchild J, Kalckar H M, Zamecnik P C. Proc Natl Acad Sci USA. 1987;84:1936–1939. doi: 10.1073/pnas.84.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce G B. Am J Pathol. 1974;77:103–118. [PMC free article] [PubMed] [Google Scholar]
- 29.Martin G R. Science. 1980;209:768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]