Abstract
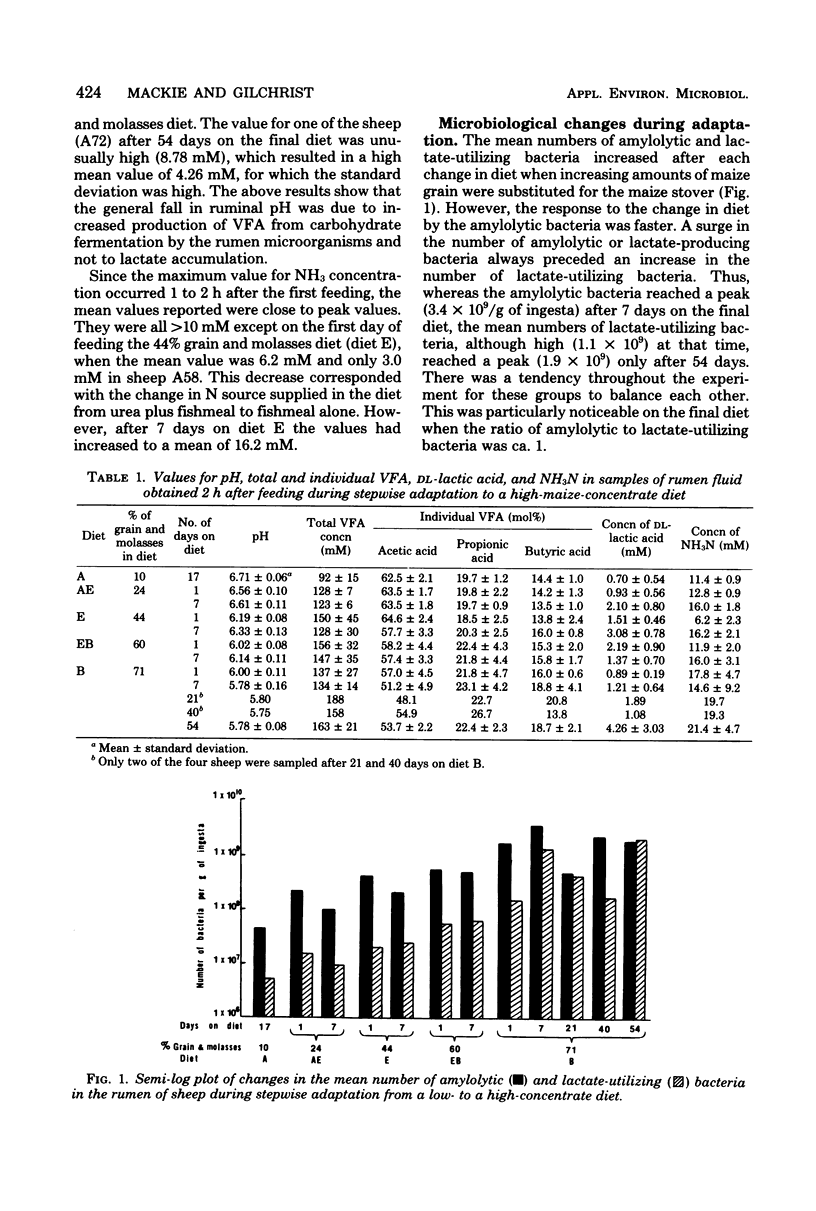
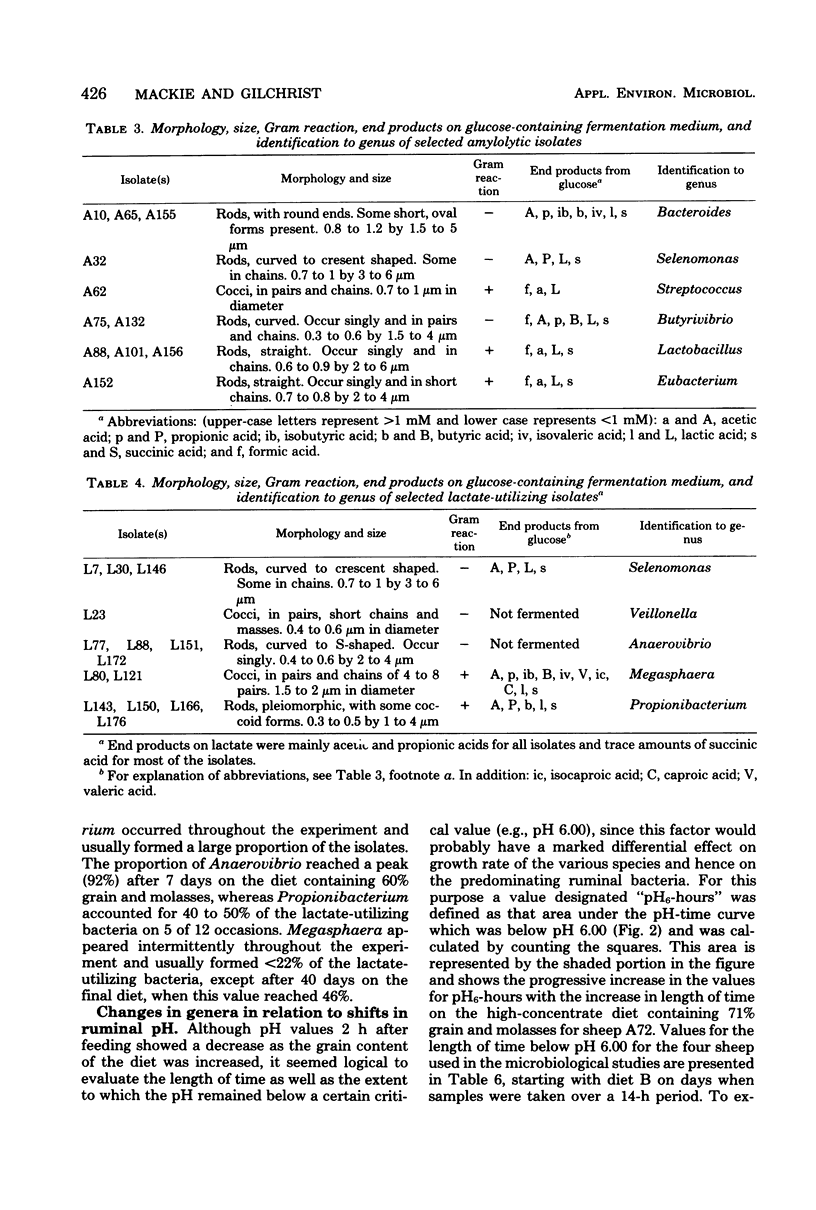
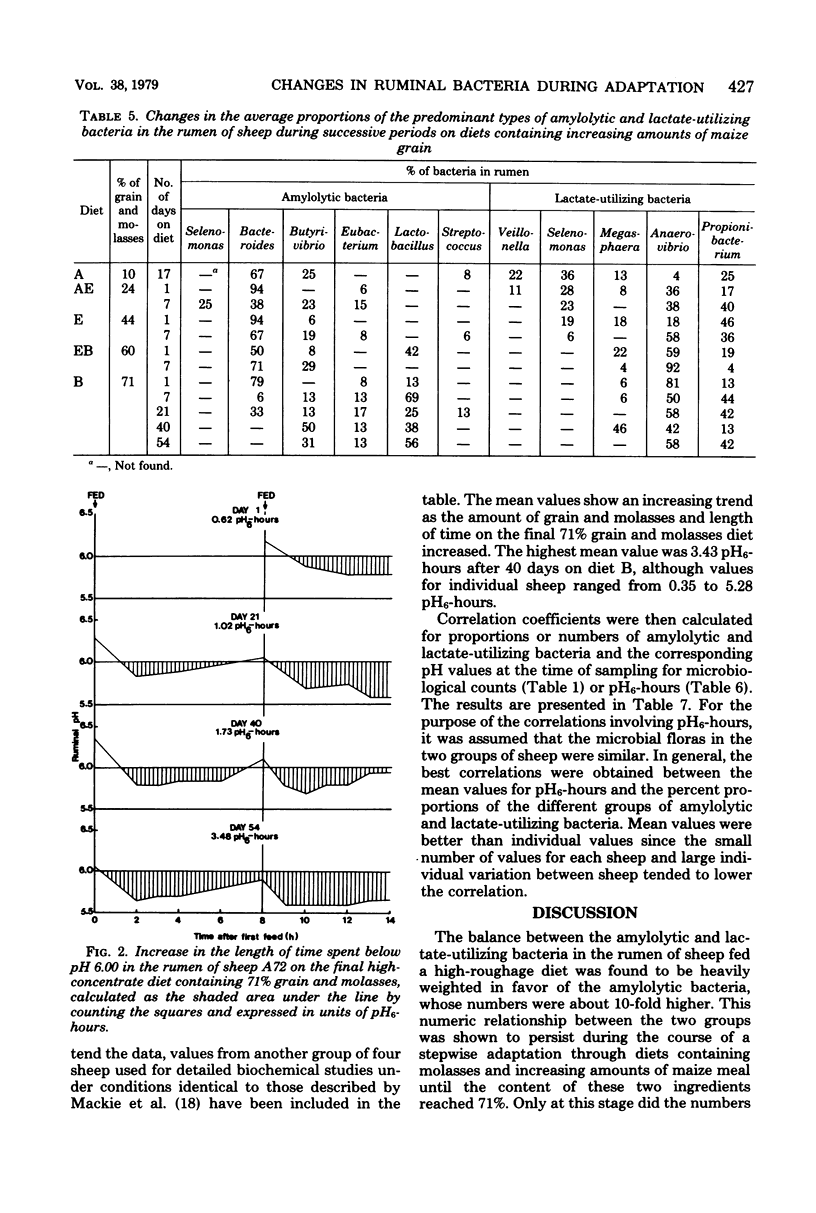
Changes in the numbers and types of lactate-producing and lactate-utilizing bacteria in the rumen of sheep were followed during stepwise adaptation from a low- to a high-concentrate diet. The mean numbers of bacteria increased after each change in diet when increasing amounts of maize grain were substituted for maize stover. A surge in number of amylolytic bacteria always preceded an increase in lactate-utilizing bacteria, and with the final diet containing 71% grain and molasses the two groups tended to balance each other, which resulted in low lactic acid accumulation. The lactate utilizers thus played a key role in controlling the fermentation. Orderly shifts occurred among the predominating amylolytic and lactate-utilizing bacteria in response to the gradual decrease in ruminal pH as the amount of maize meal in the diet increased. Among the lactate utilizers, the succession began with acid-sensitive Veillonella and Selenomonas, which were superseded by more acid-tolerant Anaerovibrio and Propionibacterium. Among the amylolytic bacteria, Bacteroides was superseded by more acid-tolerant Lactobacillus and Eubacterium. The ecological succession of predominating genera was shown to be correlated significantly with ruminal pH and, more specifically, with the length of time as well as the extent to which the pH remained below a certain critical undefined value in the rumen, arbitrarily set at pH 6.00.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Robinson I. M., Dougherty R. W., Bucklin J. A. Grain overload in cattle and sheep: changes in microbial populations in the cecum and rumen. Am J Vet Res. 1975 Feb;36(2):181–185. [PubMed] [Google Scholar]
- BALDWIN R. L., WOOD W. A., EMERY R. S. Conversion of lactate-C14 to propionate by the rumen microflora. J Bacteriol. 1962 Apr;83:907–913. doi: 10.1128/jb.83.4.907-913.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop R. H., Hammond P. B. D-lactic acidosis of ruminants. Ann N Y Acad Sci. 1965 Jul 31;119(3):1109–1132. doi: 10.1111/j.1749-6632.1965.tb47466.x. [DOI] [PubMed] [Google Scholar]
- Eadie J. M., Hyldgaard-Jensen J., Mann S. O., Reid R. S., Whitelaw F. G. Observations on the microbiology and biochemistry of the rumen in cattle given different quantities of a pelleted barley ration. Br J Nutr. 1970 Mar;24(1):157–177. doi: 10.1079/bjn19700018. [DOI] [PubMed] [Google Scholar]
- Elam C. J. Acidosis in feedlot cattle: practical observations. J Anim Sci. 1976 Oct;43(4):898–901. doi: 10.2527/jas1976.434898x. [DOI] [PubMed] [Google Scholar]
- Giesecke D., Lawlor M. J., Walser-Kärst K. Comparative studies on the digestive physiology of sheep fed on semi-purified or roughage-concentrate diets. 2. Microbiological investigations. Br J Nutr. 1966;20(2):383–392. doi: 10.1079/bjn19660038. [DOI] [PubMed] [Google Scholar]
- Grubb J. A., Dehority B. A. Effects of an abrupt change in ration from all roughage to high concentrate upon rumen microbial numbers in sheep. Appl Microbiol. 1975 Sep;30(3):404–412. doi: 10.1128/am.30.3.404-412.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. L., Cooley J. H., Goetsch D. D., Das N. K. Lactic acid-utilizing bacteria in ruminal fluid of a steer adapted from hay feeding to a high-grain ration. Am J Vet Res. 1976 May;37(5):611–613. [PubMed] [Google Scholar]
- Latham M. J., Sharpe M. E., Sutton J. D. The microbial flora of the rumen of cows fed hay and high cereal rations and its relationship to the rumen fermentation. J Appl Bacteriol. 1971 Jun;34(2):425–434. doi: 10.1111/j.1365-2672.1971.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Storry J. E., Sharpe M. E. Effect of low-roughage diets on the microflora and lipid metabolism in the rumen. Appl Microbiol. 1972 Dec;24(6):871–877. doi: 10.1128/am.24.6.871-877.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie D. D. Production and utilization of lactic acid by the ruminant. A review. J Dairy Sci. 1967 Nov;50(11):1772–1786. doi: 10.3168/jds.S0022-0302(67)87715-4. [DOI] [PubMed] [Google Scholar]
- Mackie R. I., Heath S. Enumeration and isolation of lactate-utilizing bacteria from the rumen of sheep. Appl Environ Microbiol. 1979 Sep;38(3):416–421. doi: 10.1128/aem.38.3.416-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. O. Some effects on the rumen micro-organisms of overfeeding a high barley ration. J Appl Bacteriol. 1970 Jun;33(2):403–409. doi: 10.1111/j.1365-2672.1970.tb02213.x. [DOI] [PubMed] [Google Scholar]
- Ogimoto K., Giesecke D. Untersuchungen zur Genese und Biochemie der Pansenacidose. 2. Mikroorganismen und Umsetzung von Milchsäure-Isomeren. Zentralbl Veterinarmed A. 1974 Jul;21(7):532–538. [PubMed] [Google Scholar]
- Potter E. L., Dehority B. A. Effects of changes in feed level, starvation, and level of feed after starvation upon the concentration of rumen protozoa in the ovine. Appl Microbiol. 1973 Nov;26(5):692–698. doi: 10.1128/am.26.5.692-698.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Slyter L. L., Oltjen R. R., Kern D. L., Weaver J. M. Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr. 1968 Feb;94(2):185–192. doi: 10.1093/jn/94.2.185. [DOI] [PubMed] [Google Scholar]
- WARNER A. C. Some factors influencing the rumen microbial population. J Gen Microbiol. 1962 Apr;28:129–146. doi: 10.1099/00221287-28-1-129. [DOI] [PubMed] [Google Scholar]


