Abstract
We previously characterized VE-statin/egfl7, a protein that is exclusively secreted by endothelial cells and modulates smooth muscle cell migration. Here, we show that VE-statin/egfl7 is the first known natural negative regulator of vascular elastogenesis. Transgenic mice, expressing VE-statin/egfl7 under the control of keratin-14 promoter, showed an accumulation of VE-statin/egfl7 in arterial walls where its presence correlated with an impaired organization of elastic fibres. In vitro, fibroblasts cultured in the presence of VE-statin/egfl7 were unable to deposit elastic fibres due to a deficient conversion of soluble tropoelastin into insoluble mature elastin. VE-statin/egfl7 interacts with the catalytic domain of lysyl oxidase (LOX) enzymes and, in endothelial cells, endogenous VE-statin/egfl7 colocalizes with LoxL2 and inhibits elastic fibre deposition. In contrast, mature elastic fibres are abundantly deposited by endothelial cells that are prevented from producing endogenous VE-statin/egfl7. We propose a model where VE-statin/egfl7 produced by endothelial cells binds to the catalytic domains of enzymes of the LOX family in the vascular wall, thereby preventing the crosslink of tropoelastin molecules into mature elastin polymers and regulating vascular elastogenesis.
Keywords: egfl7, elastogenesis, lysyl oxidases, VE-statin
Introduction
Elastic fibres are the most stable components of the extracellular matrix (ECM) that confer the essential resiliency to mechanical constraints of various organs, including skin, lungs and blood vessels (Kielty et al, 2002; Kielty, 2006). Elastic fibres are composed of an amorphous core of ‘insoluble' elastin assembled onto a microfibrillar scaffold made of several proteins, such as fibrillins, fibulins, emilins and MAGPs (Mithieux and Weiss, 2005). The formation of this heterogeneous ECM is a multistep process termed elastogenesis, the complexity of which is not yet entirely understood. To form fully functional elastic fibres, numerous cell types, such as fibroblasts, smooth muscle cells, endothelial cells and chondrocytes, secrete the soluble precursor tropoelastin, which is deposited on the pre-assembled microfibrillar scaffold (Hinek and Rabinovitch, 1994; Kielty et al, 2002; Mithieux and Weiss, 2005; Kielty, 2006). Adjacent tropoelastin molecules spontaneously aggregate and are then crosslinked into insoluble elastin polymers by enzymes of the copper-dependent family of lysyl oxidases (LOXs) (Kielty et al, 2002; Mithieux and Weiss, 2005; Kielty, 2006).
To date, five LOX family members have been identified (Lox, LoxL1, LoxL2, LoxL3 and LoxL4). They differ in substrate specificity and are able to crosslink several other ECM components in addition to elastin, such as collagens (Lucero and Kagan, 2006). Gene inactivation studies in mice confirmed the critical role of several LOXs in the final steps of elastin polymerization. Lox-deficient mice die after birth from structural alterations of their arterial walls eventually leading to aortic rupture (Maki et al, 2002; Hornstra et al, 2003). It is noteworthy that disruption of the lox gene only partially inhibits elastin and, to an even lesser extent, collagen crosslinks, suggesting that other LOXs participate in these processes. In contrast, LoxL1-deficient mice are viable but exhibit general elastic tissue defects resulting from the specific reduction in the levels of elastin crosslinks, whereas collagen crosslink content remains unchanged (Liu et al, 2004). Thus, LoxL1 appears to be specifically involved in elastogenesis, whereas Lox may crosslink other substrates as well (Maki et al, 2002; Hornstra et al, 2003; Liu et al, 2004). The remaining members of the Lox family (LoxL2, LoxL3 and LoxL4) share homology with Lox and LoxL1 in their carboxyl-terminal end, which corresponds to the copper-binding site, the catalytic domain and the carbonyl cofactor-binding site (Jourdan-Le Saux et al, 1999; Asuncion et al, 2001; Maki and Kivirikko, 2001; Maki et al, 2001). The precise functions and catalytic activities on tropoelastin and on collagen substrates of these LOXs are not yet understood. LoxL2 and LoxL3 were, however, found to interact and cooperate with the Snail transcription factor and to downregulate E-cadherin expression (Peinado et al, 2005).
We previously identified VE-statin/egfl7 as a novel ∼30 kDa protein that is exclusively expressed and secreted by vascular endothelial cells (Soncin et al, 2003). The protein is composed of an N-terminal signal peptide, an EMI domain, two centrally positioned EGF-like domains, including a calcium-binding one, and a leucine and valine-rich carboxy-terminal region (Soncin et al, 2003; Parker et al, 2004). During mouse embryogenesis, high levels of VE-statin/egfl7 transcripts are detected in endothelial progenitors and in endothelial cells of all blood vessels, whereas in adults VE-statin/egfl7 expression is reduced and is detectable only in a subset of vessels (Soncin et al, 2003; Fitch et al, 2004; Parker et al, 2004; Campagnolo et al, 2005). In adults, VE-statin/egfl7 expression resumes during re-endothelialization after arterial injury (Campagnolo et al, 2005) and coincides with extensive angiogenesis of human solid tumours (Parker et al, 2004). VE-statin/egfl7 expression is also detected in endothelial cells of human atherosclerotic plaques (Campagnolo et al, 2005). We previously demonstrated that VE-statin/egfl7 represses PDGF-BB-induced smooth muscle cell migration in vitro (Soncin et al, 2003), whereas its role in endothelial cell migration is controversial (Parker et al, 2004; Campagnolo et al, 2005). VE-statin/egfl7 is also able to promote endothelial cell adhesion in vitro; however, its cell adhesion properties are weaker than that of fibronectin or collagen (Parker et al, 2004). So far, VE-statin/egfl7 has been implicated in the process of perivascular cell recruitment (Soncin et al, 2003) and of vascular tubulogenesis in zebrafish model (Parker et al, 2004), but its functions are not fully elucidated. VE-statin/egfl7 knockout mice were recently generated. Half of the egfl7−/− embryos died between 14.5 and 15.5 days of gestation. In surviving egfl7−/− mice, blood vessels are tortuous and vascular development is delayed in egfl7−/− embryos or neonates. Retinal vasculature, which essentially develops after birth, is also defective in VE-statin/egfl7-deficient mice, although no obvious defects were found in smooth muscle and endothelial cell numbers or in the rates of endothelial cell proliferation and apoptosis. The egfl7−/− mouse phenotypes were attributed to a primary defect in the endothelium with a role for VE-statin/egfl7 in the spatial organization of endothelial cells in the angiogenic sprout (Schmidt et al, 2007).
In this study, we show that VE-statin/egfl7 is a negative regulator of elastic fibre maturation. This notion is based on in vivo data demonstrating that the forced expression of VE-statin/egfl7 impairs elastic fibre formation in transgenic mice and on in vitro experiments showing that the addition of exogenous VE-statin/egfl7 interferes with the crosslink of tropoelastin and correlates with a decrease in LOX activity. We further establish that VE-statin/egfl7 is capable of binding to each enzyme of the LOX family through the catalytic domain. siRNA-mediated silencing of VE-statin/egfl7 in endothelial cells stimulates LOX activity, tropoelastin expression and elastic fibre assembly.
Therefore, VE-statin/egfl7 secreted from endothelial cells is a regulator of vascular elastogenesis acting through a direct interaction with LOX catalytic domain leading to the specific inhibition of the crosslink activity of these enzymes.
Results
Ectopic expression of VE-statin/egfl7 by keratinocytes induces loose skin and vascular defects
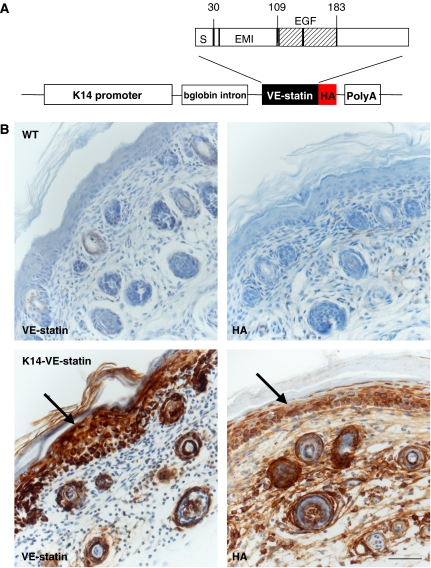
To investigate the putative effects of VE-statin/egfl7 on blood vessels in vivo, we generated transgenic mice that express a HA-tagged VE-statin/egfl7 protein in their epidermis using the human keratin-14 promoter (Figure 1A), a well-suited model previously used to address the angiogenic potential of secreted factors such as VEGF-A and angiopoietin-1 (Suri et al, 1998; Thurston et al, 1999). Two K14-VE-statin/egfl7 transgenic founders were obtained that expressed VE-statin/egfl7 (Supplementary Figure 1A) and transmitted the K14-VE-statin/egfl7 transgene. Immunostaining of skin sections from P5 transgenic mice with anti-HA and with anti-VE-statin/egfl7 antibodies confirmed VE-statin/egfl7 expression in keratinocytes (Figure 1B).
Figure 1.
Generation of K14-VE-statin/egfl7 transgenic mice. (A) Graphic representation of the K14-VE-statin/egfl7 transgene: S, signal peptide; EMI, EMI domain; EGF, EGF-like domains; HA, haemaglutinin influenza tag epitope. (B) Immunostaining for VE-statin/egfl7 of skin section of wild type (WT, top panels) or P5 transgenic (bottom panels). Details of the tail skin immunostained with anti-VE-statin/egfl7 (left panels) or anti-HA antibodies (right panels). Arrows indicate VE-statin/egfl7-expressing keratinocytes (scale bar, 50 μm).
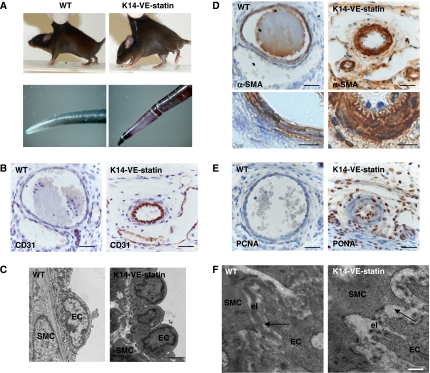
K14-VE-statin/egfl7 transgenic mice exhibit a loose and non-resilient skin (Figure 2A, upper panels), but they do not display any canonical macroscopic signs of angiogenic phenotype and neither do they reveal any increase in the number of CD31-positive dermal capillaries and venules (data not shown). Instead, by P5, K14-VE-statin/egfl7 transgenic mice develop necrotic tail tips, suggestive of regional ischaemia (Figure 2A, lower panels). Detailed histological and ultrastructural analyses revealed that transgenic tail arteries have a markedly reduced diameter when compared with wild-type control littermates (Figure 2B). These vessels have protruding CD31-positive endothelial cells and overlapping layers of smooth muscle- alpha-actin-positive mural cells (Figure 2B–D), suggesting stenosis. Additional immunohistochemistry revealed that the endothelial and smooth muscle cells of these vessels express proliferation-associated antigen, PCNA (Figure 2E). Electron microscopy showed that though junctions between arterial cells are unaltered in P5 K14-VE-statin/egfl7 pups (not shown), the internal elastic lamina is thicker in transgenic than in wild-type animals (599±33 versus 324±12.5 nm for VE-statin/egfl7 transgenic and wild-type control littermate arteries, respectively; P<0.0001). Moreover, a fragmented elastic fibre is observed in transgenic mice, whereas the internal elastic fibre is continuously deposited between endothelial and smooth muscle cells in control mice (Figure 2F). Finally, dermal elastic fibres are altered in transgenic compared with control mice as revealed by Van Gieson staining and elastin immunodetection, an observation that may explain their loose skin (Supplementary Figure 2). This initial phenotype analysis suggests that VE-statin/egfl7 expression affects elastogenesis in mice.
Figure 2.
Phenotype analysis of K14-VE-statin/egfl7 transgenic mice. (A) Upper panels: loose skin of K14-VE-statin/egfl7 transgenic mice compared with WT mice. Lower panels: overall phenotype of the transgenic pups at P5. Transgenic pups develop a flaky skin (not shown) and abnormal, blackened tails. (B) Main tail artery immunostained with anti-CD31 antibody to detect endothelial cells in WT or transgenic mice (scale bar, 30 μm). (C) Ultrastructural analysis of main tail artery in WT and transgenic mice. EC, endothelial cell; SMC, smooth muscle cell (scale bar, 2 μm). (D) Upper panels: smooth muscle cell alpha-actin immunostaining of main tail arteries of WT and transgenic mice, labelling SMC (scale bar, 30 μm). Lower panels: higher magnification of the arterial wall (scale bar, 10 μm). (E) WT or transgenic tail sections immunostained with anti-PCNA antibody to detect proliferating cells. (F) Ultrastructural analysis of the internal elastic laminae of WT and transgenic pups. Elastin lamella (el) appears continuous in WT mice, whereas it is disrupted in transgenic mice, EC, endothelial cell; SMC, smooth muscle cell (scale bar, 500 nm).
VE-statin/egfl7 naturally accumulates in vascular walls and colocalizes with elastic fibres
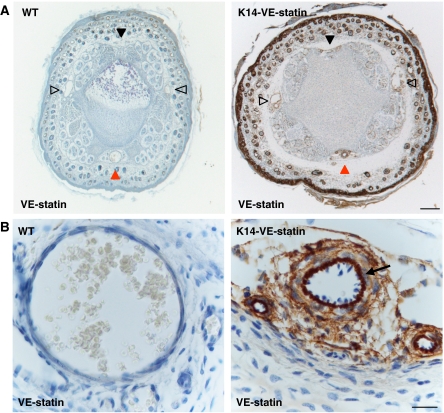
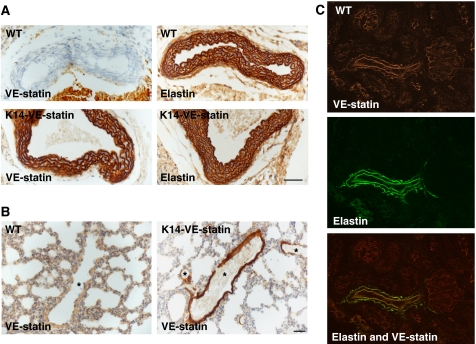
Although VE-statin/egfl7 is produced by keratinocytes in K14 transgenics, a peculiar accumulation of the protein is observed in the dermis (Supplementary Figure 2), around the hypodermal vascular bundles (Figure 3A and Supplementary Figure 1B) and within the wall of the main ventral artery of the tail (Figure 3B, arrow). Moreover, VE-statin/egfl7 secreted from transgenic keratinocytes accumulates in the aortic walls and within the smaller arteries of the lungs (Figure 4A and B). Additional immunohistochemical analyses further indicated that VE-statin/egfl7 decorates the elastic fibres, as evidenced with a parallel immunostaining using an anti-elastin antibody (Figure 4A, lower panels).
Figure 3.
Distribution of VE-statin/egfl7 in transgenic and WT animals. (A) Tail section of WT and transgenic P5 pups immunostained for VE-statin/egfl7. Solid black arrowhead: dorsal vascular bundle; open black arrowheads: lateral vascular bundles; red arrowhead: ventral vascular bundle (scale bar, 200 μm). (B) In the ventral vascular bundle, VE-statin/egfl7 accumulates between endothelial and smooth muscle cell layers (arrow) of the main artery (scale bar, 20 μm).
Figure 4.
VE-statin/egfl7 and elastin co-distribution in transgenic and wild-type mice. (A) Aorta sections of P5 WT and transgenic littermates immunostained for VE-statin/egfl7 (left panels) or for elastin (right panels) (scale bar, 50 μm). (B) Lung sections of P5 WT and transgenic littermates immunostained with VE-statin/egfl7 antibody, *lung arteries (scale bar, 30 μm). (C) Immunostaining for VE-statin/egfl7 (upper panel) or elastin (middle panel) and merged images (lower panel) in WT kidneys of P15 mice showing a similar pattern of distribution of the proteins in artery.
To determine whether VE-statin/egfl7 and elastic fibre co-distribution occurred naturally in non-transgenic animals, we compared the localization of VE-statin/egfl7 and of elastin in wild-type mouse embryonic and postnatal tissues. On E15.5 embryo sections, VE-statin/egfl7 and elastin colocalize in a subset of blood vessels (not shown). In a 15-day-old mouse kidney, VE-statin/egfl7 distribution fully overlaps that of elastin in arteries (Figure 4C), indicating a genuine natural co-distribution of VE-statin/egfl7 and components of elastic fibres during normal embryonic and postnatal development.
VE-statin/egfl7 interferes with the final assembly of elastic fibres
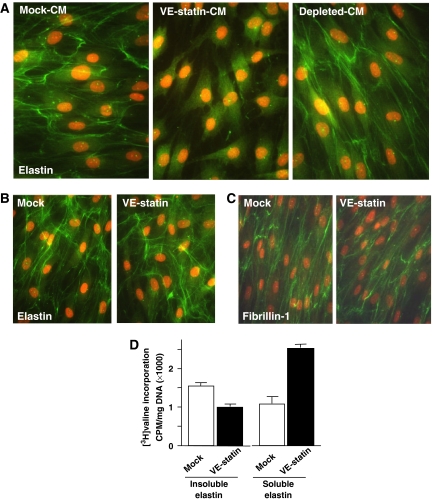
As VE-statin/egfl7 accumulation in the internal elastic lamina correlates with a structural alteration of the elastic fibres in transgenic mice, we tested whether VE-statin/egfl7 would directly affect the normal deposition of this ECM in vitro. Indeed, fibroblasts derived from human skin, which normally produce an abundant network of elastic fibres, fail to deposit elastic fibres when cultured in VE-statin-containing conditioned medium (CM; Figure 5A). On the other hand, fibroblasts maintained in VE-statin/egfl7-depleted CM produce a normal elastic fibre network, clearly indicating that VE-statin/egfl7 is a negative regulator of the net deposition of elastic fibres by these cells. Further, the addition of VE-statin/egfl7-containing medium to 7-day-old confluent cultures that had already deposited a normal elastic fibre network did not cause any destruction of these pre-established fibres during the next 3 days of culture (Figure 5B). These results indicate that VE-statin interferes with the initial elastogenesis process but does not induce elastolysis of previously deposited fibres. This notion was further validated by results showing that VE-statin/egfl7 did not affect elastin gene transcription during the next 3 days (Supplementary Figure 3) or the normal deposition of the microfibrillar scaffold, as revealed by immunodetection of fibrillin-1 (Figure 5C). Rather, dermal fibroblasts maintained in the presence of VE-statin/egfl7 accumulate more soluble elastin (as immunoprecipitable tropoelastin) than their counterparts maintained in mock medium (Figure 5D). As this phenomenon coexists with a significant reduction in the final deposition of insoluble (crosslinked) elastin, it suggests that VE-statin/egfl7 interferes with the elastin crosslink and the final steps of elastic fibre maturation.
Figure 5.
Defects in elastic fibre deposition by fibroblasts treated with VE-statin/egfl7. (A) Confluent fibroblasts were treated with mock (mock-CM), VE-statin/egfl7-conditioned-medium (VE-statin-CM) or VE-statin/egfl7 immunodepleted- (depleted CM) conditioned medium for 7 days after which elastic fibre deposition was assessed by immunofluorescence staining of elastin (green). Nuclei were stained with red propidium iodide. (B) Fibroblasts were cultured in normal medium for 7 days to allow the deposition of an elastic fibre network and then cultured for 3 days in the presence of mock- or VE-statin/egfl7-conditioned medium. Elastin deposition and nuclei were detected as in (A). (C) Microfibril organization is unaffected in fibroblasts cultured in the presence of VE-statin/egfl7 when compared with control, as evidenced by fibrillin 1 immunostaining. (D) Quantitative analysis of soluble and insoluble elastin in fibroblasts cultured with mock- or VE-statin/egfl7-conditioned medium as analysed by radioactive counting of NaOH-insoluble elastin and immunoprecipitable tropoelastin metabolically labelled with [3H]valine.
VE-statin/egfl7 inhibits LOX enzymes and interacts with their catalytic domain
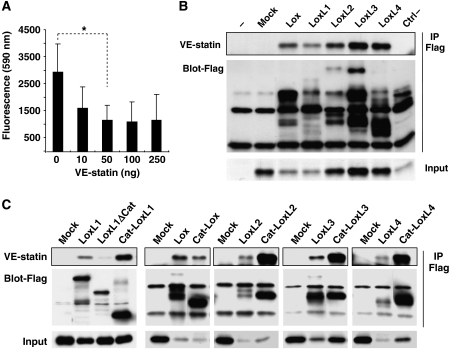
As tropoelastin crosslink is catalysed by LOX enzymes, we tested whether VE-statin/egfl7 could directly inhibit LOX activity, a property that would explain the above observations. MEF cells were treated with increasing amounts of recombinant VE-statin/egfl7 before measuring LOX activity. In the presence of 50 ng/ml of VE-statin/egfl7, LOX activity is reduced to 30% of untreated control, indicating that VE-statin/egfl7 is a direct inhibitor of LOX catalytic activity (Figure 6A).
Figure 6.
VE-statin/egfl7 inhibits lysyl oxidase activity and interacts with lysyl oxidase catalytic domains. (A) Lysyl oxidase activity was measured in MEF confluent cells after treatment with increasing amounts of recombinant VE-statin/egfl7. Each experiment was performed in quadruplicate. Results are expressed as mean±s.d. *Statistically significant (P<0.05). (B) Fibroblasts were co-transfected with expression vectors for HA-tagged VE-statin/egfl7 and for Flag-tagged LOXs before immunoprecipitation for Flag peptide (IP Flag) and immunoblot with anti-HA antibody (framed). VE-statin/egfl7 co-immunoprecipitates with every lysyl oxidase (framed). Mock and ctrl−, non-relevant flagged proteins do not interact with VE-statin/egfl7. Input, ∼4% of cell extracts. (C) Co-immunoprecipitation of VE-statin/egfl7 (framed) with LoxL1 (LoxL1ΔCat) lacking its catalytic domain or with the isolated Lox (Cat-Lox), LoxL1 (Cat-LoxL1), LoxL2 (Cat-LoxL2), LoxL3 (Cat-LoxL3) and LoxL4 (Cat-LoxL4) catalytic domains. VE-statin/egfl7 binds to Cat-Lox, Cat-LoxL1, Cat-LoxL2, Cat-LoxL3, Cat-LoxL4 but not to LoxL1ΔCat.
In an attempt to understand the molecular mechanisms by which VE-statin/egfl7 interferes with LOX activity, we first tested whether VE-statin/egfl7 could directly interact with LOX family members by co-immunoprecipitation assays. VE-statin/egfl7 interacts with all LOX enzymes but not with control protein (Figure 6B). Deletion of the catalytic domain of LoxL1 (LoxL1ΔCat; Figure 6C) prevented co-precipitation of this enzyme with VE-statin/egfl7. This suggests that the LOX catalytic domain, shared by the enzymes of the LOX family, mediates interaction between VE-statin/egfl7 and LOX enzymes. Accordingly, isolated Lox, LoxL1, LoxL2, LoxL3 and LoxL4 catalytic domains (Lox-Cat, LoxL1-Cat, LoxL2-Cat, LoxL3-Cat and LoxL4-Cat; Figure 6C) interacted with VE-statin/egfl7, confirming that VE-statin/egfl7 specifically associates with the LOX catalytic domains.
Secretion of endogenous VE-statin/egfl7 by endothelial cells correlates with their inability to deposit elastic fibres in vitro
As most of the above experiments were performed with overexpressed VE-statin/egfl7 or with recombinant protein, we next checked whether the endogenous, naturally produced VE-statin/egfl7 would also exert an effect as a natural inhibitor of elastogenesis in endothelial cells. We first examined primary endothelial HUVEC cells for their ability to synthesize VE-statin/egfl7 and to deposit elastic fibres. VE-statin/egfl7 is produced by HUVEC cells as it was detected in cell extracts by western blot (Figure 7A). Interestingly, VE-statin/egfl7 levels are higher in the detergent-resistant fraction later solubilized with urea than in the soluble fractions (Figure 7A, − and ctrl siRNA lanes). Immunofluorescence experiments performed on HUVEC cells cultured for 7 days after confluence revealed that VE-statin/egfl7 is found in the extracellular compartment where it organizes as a fibrillar meshwork (Figure 7B). However, HUVEC cells do not deposit any elastin-containing fibre (Figure 7C), whereas control dermal human primary fibroblasts from neonates cultured under similar conditions (which do not express VE-statin/egfl7 as expected) organize elastic fibres (Figure 7B and C). RT–PCR analysis of elastic fibre components showed that HUVEC cells express both tropoelastin and LOXs (mainly LoxL2 and Lox; Figure 7D), indicating that these cells should be able to convert tropoelastin into crosslinked elastin. In addition, we also examined the distribution of both VE-statin/egfl7 and LoxL2 on confluent HUVEC cell cultures by immunofluorescence and found that VE-statin/egfl7 and LoxL2 colocalize (Figure 7E). This colocalization occurred in the ECM as revealed by VE-statin/egfl7 and LoxL2 immunofluorescences performed on actin-stained HUVEC cells or directly on HUVEC cell matrix after cell removal (Supplementary Figure 4).
Figure 7.
Endogenous VE-statin/egfl7 secreted by HUVEC endothelial cells colocalizes with LoxL2. (A) Endogenous VE-statin/egfl7 is detected mainly in insoluble extracts from HUVEC cells (soluble compared with urea extractions) as evidenced by western blot. Anti-VE-statin/egfl7 antibody specificity is established by complete signal loss in cell extracts from HUVEC cells transfected with siRNA targeting VE-statin/egfl7 messenger. ns, nonspecific signal. (B) Endogenous VE-statin/egfl7 is detected by immunofluorescence in HUVEC cells cultured to high density but not in normal human dermal fibroblasts from neonates (NHDF). (C) Mature elastic fibres are undetectable in HUVEC cells but are actively deposited by NHDF in similar culture conditions. (D) RT–PCR analysis of endogenous VE-statin/egfl7, elastin and lysyl oxidases mRNA levels in HUVEC and NHDF cells cultured at confluence. (E) Colocalization of endogenous VE-statin/egfl7 (top) and LoxL2 (middle) in HUVEC cells by immunofluorescence. Bottom: merged image of VE-statin/egfl7 and LoxL2.
We thus hypothesized that endothelial cells have all the necessary components to deposit mature elastic fibre but that endogenous VE-statin/egfl7 secreted by these cells naturally inhibits this process.
Endogenous VE-statin/egfl7 is directly responsible for the endothelial cell inability to deposit mature elastin
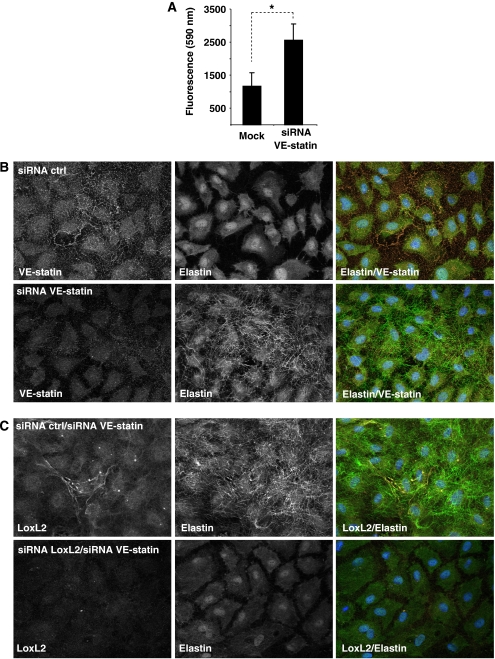
As we observed that exogenous VE-statin/egfl7 represses LOX activity (Figure 6A) and that exogenous and, most probably, endogenous VE-statin/egfl7 interact with LOXs (Figures 6B and 7E), we hypothesized that endogenous VE-statin/egfl7 may bind to LoxL2 in HUVEC cells and inhibit its LOX enzymatic activity, thus preventing elastin deposition. We thus measured LOX activity in HUVEC cells transfected with control- or with VE-statin/egfl7-siRNA. VE-statin/egfl7 silencing induced a more than two-fold increase in LOX activity when compared with control cells (Figure 8A), confirming that endogenous VE-statin/egfl7 expression directly prevents LOX activity. Further, we found that endogenous VE-statin/egfl7 is responsible for the natural poor elastogenic activity of HUVEC cells, as silencing VE-statin/egfl7 induced a massive deposition of mature elastic fibres by these cells, whereas control-silenced cells did not (Figure 8B). As LoxL2 is the most prominently expressed LOX in HUVEC cells (Figure 7D), we analysed the effect of LoxL2 inhibition on elastic fibre deposition in VE-statin/egfl7-silenced HUVEC cells. Whereas the sole silencing of VE-statin/egfl7(siRNA-control/siRNA-VE-statin/egfl7)enhanced elastogenesis as observed above, LoxL2 silencing completely abolished elastic fibre formation induced by VE-statin/egfl7 silencing (Figure 8C), indicating that LoxL2 activity accounts for most of the tropoelastin conversion into crosslinked elastin in HUVEC cells.
Figure 8.
Endogenous VE-statin/egfl7 inhibits elastic fibre deposition by repressing lysyl oxidase activity in HUVEC cells. (A) Measure of lysyl oxidase enzymatic activity in control- (mock) or VE-statin/egfl7-siRNA (siRNA VE-statin)-transfected HUVEC cells. Each experiment was performed in triplicate. Results are expressed as mean±s.d. *P<0.05. (B) VE-statin/egfl7 is immunodetected (top left panel), whereas elastic fibres are not observed (top central panel) in siRNA control (siRNA ctrl)-transfected HUVEC cells. After VE-statin/egfl7 siRNA transfection, VE-statin/egfl7 is no longer detected (bottom left panel), whereas mature elastic fibres are abundantly deposited by HUVEC cells (bottom central panel). Right panel: merged images of the same field. (C) After co-transfection of control siRNA and VE-statin/egfl7 siRNA (siRNA ctrl/si RNA VE-statin), LoxL2 and mature elastin are detected by immunofluorescence in HUVEC cells. Co-transfection of LoxL2-siRNA and VE-statin/egfl7-siRNAs (siRNA LoxL2/siRNA VE-statin) in HUVEC cells prevents elastic fibre formation.
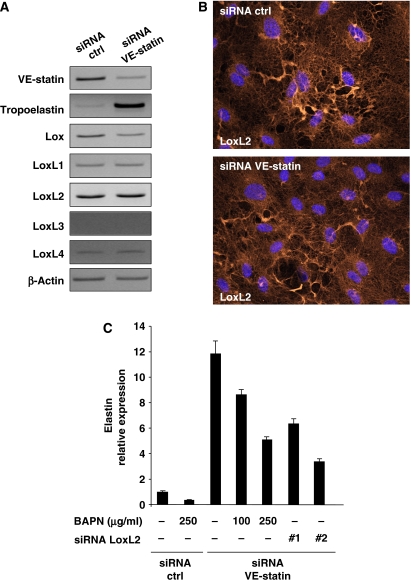
RT–PCR analysis revealed that mRNA levels of LOXs were not significantly modified by VE-statin/egfl7 silencing in HUVEC cells. Moreover, we did not find dramatic changes either of LoxL2 protein levels or of LoxL2 protein organization pattern in VE-statin/egfl7 siRNA-transfected HUVEC cells compared with controls (Figure 9B).
Figure 9.
VE-statin/egfl7 indirectly represses tropoelastin transcription. (A) RT–PCR analysis of tropoelastin and LOX transcripts in HUVEC cells transfected with control- (siRNA ctrl) or VE-statin/egfl7- (siRNA VE-statin) siRNA. (B) Immunolocalization of LoxL2 in HUVEC cells transfected with control- (siRNA ctrl) or VE-statin/egfl7- (siRNA VE-statin) siRNA. (C) Quantitative RT–PCR analysis of tropoelastin expression in HUVEC cells transfected with control- (siRNA ctrl) or VE-statin/egfl7-siRNA (siRNA VE-statin) and treated with BAPN or in HUVEC cells transfected with both VE-statin/egfl7-siRNA and two distinct LoxL2- siRNA (siRNA LoxL2 nos. 1 and 2). Reactions were performed in triplicate. Results are expressed as mean of relative quantity±s.d.
Altogether, our results show that endogenous VE-statin/egfl7 naturally represses elastogenesis in HUVEC cells, a process that is mostly under the control of LoxL2.
VE-statin/egfl7 is indirectly responsible for tropoelastin transcription control
Interestingly, tropoelastin transcripts were markedly upregulated in VE-statin-silenced HUVEC cells (Figure 9A), suggesting that VE-statin/egfl7 may repress tropoelastin gene expression either directly or through indirect mechanisms such as LOX activity inhibition. To discriminate between these two possibilities, we examined first the effects of beta-aminopropionitrile (BAPN), a potent inhibitor of LOX activity, on elastin messenger expression induced by VE-statin/egfl7 silencing in HUVEC cells. As expected from the previously reported stimulatory effect of LOX activity on elastin gene transcription (Oleggini et al, 2007), quantitative RT–PCR analysis revealed that increasing amounts of BAPN significantly repressed elastin expression induced by VE-statin/egfl7 silencing (Figure 9C). Similarly, the inhibition of LoxL2 expression, using two distinct siRNA (nos. 1 and 2; Figure 9C), in VE-statin/egfl7 silencing HUVEC cells also downregulated elastin expression when compared with control cells, indicating that elastin expression in HUVEC cells is dependent on LoxL2 lysyl oxidase activity. Quantitative RT–PCRs confirmed the efficient knockdown of VE-statin/egfl7 and of LoxL2 (Supplementary Figure 5) in cells transfected with either VE-statin/egfl7/ctrl- or VE-statin/egfl7/LoxL2-siRNAs.
Altogether, these results suggest that VE-statin/egfl7 indirectly controls tropoelastin transcription through the regulation of LoxL2 activity.
A proposed mechanism of VE-statin/egfl7 inhibitory effect on elastogenesis
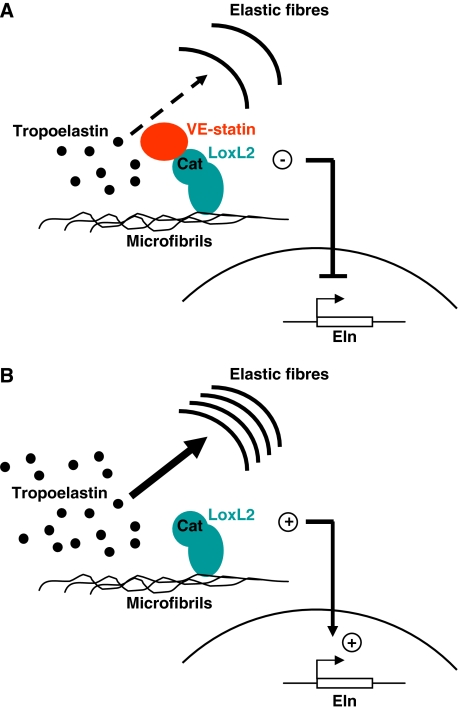
To illustrate the functions of VE-statin/egfl7 in the regulation of vascular elastogenesis, we propose the following model (Figure 10): when secreted by endothelial cells, VE-statin/egfl7 is deposited onto the vascular ECM and interacts with the catalytic domain of the LOXs (i.e. the LoxL2 for HUVEC cells), which are previously docked onto the microfibrillar scaffold (i.e. fibulins; Liu et al, 2004). This association directly prevents the conversion of soluble tropoelastin into mature elastic fibres by LOXs. As a consequence of LOX activity inhibition by VE-statin/egfl7, elastin gene transcription is repressed (Figure 10A). In the absence of VE-statin/egfl7, LOXs are highly active and efficiently convert soluble tropoelastin into elastic fibres (Figure 10B), which promotes tropoelastin transcript synthesis.
Figure 10.
Proposed model for VE-statin/egfl7 function during elastogenesis. (A) VE-statin/egfl7 interacts with LoxL2 catalytic domain preventing conversion of tropoelastin into mature insoluble elastin. The inhibition of lysyl oxidase activity subsequently represses tropoelastin transcription. (B) The absence of VE-statin/egfl7 relieves lysyl oxidase activity, which allows abundant mature elastic fibres to organize. The sudden increase of lysyl oxidase activity induces tropoelastin transcription by a molecular mechanism that still needs to be elucidated.
Discussion
This study was the first attempt to create transgenic animals using our recently found VE-statin/egfl7 gene. On the basis of ectopic expression of VE-statin/egfl7 in transgenic animals and in vitro experiments using exogenous and endogenous VE-statin/egfl7, it demonstrates that VE-statin/egfl7 interferes with elastic fibre deposition in blood vessels.
Elastogenesis is a complex process involving a large number of proteins, whose mutual structural and functional interactions are still poorly understood. Among these proteins, LOXs have emerged as key factors of the elastogenic process over the past years (Maki et al, 2002; Hornstra et al, 2003; Liu et al, 2004). These enzymes catalyse the crosslink of monomeric tropoelastin into polymers of elastin ensuring elastic fibre stability and mechanical properties. Gene inactivation approaches have already revealed the need for some of these enzymes during elastogenesis (Maki et al, 2002; Hornstra et al, 2003; Liu et al, 2004). Yet little is known about the regulation of LOX activity. Bone morphogenetic protein family related proteins, Tolloid-like proteins and fibronectin regulate LOX activity by controlling the maturation of LOX proenzymes into active isoforms (Borel et al, 2001; Uzel et al, 2001; Fogelgren et al, 2005), but so far no direct regulation of active LOX proteins has been identified. In this study, we demonstrate that an endogenous control of elastogenesis naturally exists in endothelial cells, which involves the regulation of LOX activities through a direct interaction between the LOX catalytic domain and VE-statin/egfl7.
VE-statin/egfl7 transgenic mice exhibit elastic fibre defects that are reminiscent of those reported in mice deficient for Lox and for LoxL1, such as non-resilient skin and disrupted elastic lamellae in some arteries (Maki et al, 2002; Hornstra et al, 2003; Liu et al, 2004). This is consistent with the fact that VE-statin/egfl7 inhibits LOX activity through a direct interaction with the LOX catalytic domain of every LOX protein. Whether all LOX members are genuine targets of VE-statin/egfl7 in vivo remains to be defined. Lox and LoxL1 are clearly involved in vascular elastogenesis, but the requirement of the other LOXs in this process is still unknown. We found that LoxL2 is the most predominant LOX family member expressed in primary endothelial cells and that it is also the main actor of elastogenesis in the absence of VE-statin/egfl7 in these cells. It might also participate in this process in vivo. We did not notice obvious signs of lung emphysema or pelvic prolapse after parturition in K14-VE-statin transgenic animals, indicating that VE-statin/egfl7 overexpression only partially phenocopied Lox and LoxL1 deficiencies (Maki et al, 2002; Hornstra et al, 2003; Liu et al, 2004). Further, although skin elastogenesis defects are most likely attributable to diffusion of VE-statin/egfl7 into deeper dermal layers, VE-statin/egfl7 accumulation in arterial elastic lamellae indicates that a fraction of VE-statin/egfl7 very likely enters the blood circulation and is remotely distributed in various tissues. In these tissues, VE-statin/egfl7 is almost exclusively confined to blood vessels, mostly arteries, as transgenic lungs, for instance, did not show a significant accumulation of VE-statin/egfl7 around airways, whereas the protein was detected on elastic fibres of arteries in this organ. This suggests that circulating VE-statin/egfl7 is quickly trapped by vascular elastic fibres, a phenomenon that probably prevents its further diffusion and interaction with non-vascular elastic fibres in this organ and subsequent emphysema development.
In transgenic animals, organization defects of elastic lamellae were not readily seen in aortic walls on which VE-statin/egfl7 yet accumulated massively. Although we cannot exclude a discrete alteration of aorta that would require deeper ultrastructural analyses, this suggests that normal elastogenesis occurred in this tissue despite the presence of VE-statin/egfl7 (Figure 4A). This is probably because the onset of elastogenesis in large arteries precedes the vast production of VE-statin/egfl7 by keratinocytes that is initiated after the mid-gestation activation of the K14 promoter in the transgenic animals (Vassar et al, 1989). It is thus probable that the transgenic VE-statin/egfl7 secreted at later developmental stages had no effects because elastogenesis was already largely achieved in large arteries and because VE-statin/egfl7 does not alter the structure of already deposited elastic fibres (Figure 5B). An earlier expression of VE-statin/egfl7 using a different promoter would probably have had a more closely matched phenotype to that observed in Lox-deficient mice, resulting in embryonic lethality.
VE-statin/egfl7 localization pattern in transgenic mice demonstrates a strong tropism of the protein for elastic fibres. Either VE-statin/egfl7 has specific LOX partners in elastogenic sites or other proteins of the elastic fibre are required to more or less specifically dock VE-statin/egfl7 onto elastic fibres. Such anchoring proteins are already known to have essential functions in elastogenesis. Liu et al (2004) showed that LoxL1 interacts with the microfibrillar protein fibulin 5, restricting the position of the LOX onto the nascent elastic fibres. Regardless of whether fibulins or other proteins of the microfibrillar scaffold are required for VE-statin/egfl7, exclusive interaction with elastic fibres will therefore be tested. Further, in wild-type animals, VE-statin/egfl7 gene expression is restricted to endothelial cells (Soncin et al, 2003; Parker et al, 2004) and we noticed here that endogenous VE-statin/egfl7 protein accumulates mainly in vascular beds. Such a restricted pattern of gene expression and protein accumulation strongly suggests that VE-statin/egfl7 inhibits LOX activity and elastogenesis mainly or strictly in vascular beds.
Although both our in vivo and in vitro experiments provide the proof of the principle for our proposed model, a crucial question remains: what is the role of the VE-statin/egfl7 during normal development? In other words, what would be the physiological consequences of the loss of such an elastogenesis regulator? Very recently, Schmidt et al (2007) reported the generation of VE-statin/egfl7-deficient mice. As early as E13.5, a significant number of VE-statin/egfl7−/− were oedematous and about half the VE-statin/egfl7−/− embryos died around E14.5–E15.5, whereas the remaining VE-statin/egfl7−/− animals developed normally to adulthood and were fertile. Vascular development defects were reported in numerous tissues of both embryos and neonates and were mostly characterized by a delayed development of blood vessels and a tortuous pattern. These problems were attributed to endothelial cell migration defects. Although elastic fibre organization of blood vessels was not investigated in these embryos, migration of various cell types has been shown to be controlled by LOXs (Li et al, 2000; Payne et al, 2005; Laczko et al, 2007; Polgar et al, 2007). Although little is known about the LOX activity requirement during endothelial cell migration, one might hypothesize, however, that LOX activity modulation would also influence endothelial cell migratory properties. Moreover, collagen crosslink is ensured by LOXs. Although we did not find collagen organization defects in VE-statin/egfl7-treated fibroblast cultures (not shown), we cannot exclude that VE-statin/egfl7 may also regulate the LOX-dependent organization of collagen ECM whose modification would obviously affect cell migration capacities. VE-statin/egfl7 would then have a central role in the global regulation of LOX activities.
Materials and methods
Generation of transgenic mice
Mouse VE-statin/egfl7 HA-tagged construct was amplified by PCR using the oligonucleotides listed in Supplementary Table 1 and cloned into the BamH1 site of pG3Z.K14 vector containing the K14 promoter cassette (Turksen et al, 1992). The sequence-verified, purified transgene was injected into fertilized oocytes of B6D2 hybrid mouse. Founders were genotyped by PCR and western blotting before breeding with C57Bl6/J mice.
Antibodies, immunoblotting and immunodetection
Antibodies raised against recombinant murine VE-statin/egfl7 (Caetano et al, 2006) were obtained from Antibody by Design. Antibody against human VE-statin/egfl7 was from R&D; antibodies against human LoxL2 were from Santa Cruz or R&D; antibodies against HA, elastin, fibrillin1, CD31 and αSMA were from Babco, Abcam, Elastin Product Company, BD Pharmingen and Sigma, respectively. Immunoblotting was performed as previously described (Soncin et al, 2003). Immunodetection was carried out using a Ventana Discovery automat for HA, VE-statin/egfl7 and elastin detection on paraffin sections. Briefly, slides were deparaffinized and treated with protease 1 (Ventana) for 8 min before incubating with antibodies at 37°C for 30 min. Slides were then incubated with corresponding biotinylated secondary antibodies before staining with DAB using DaBMap kit (Ventana). Immunofluorescence for elastin and fibrillin-1 on fibroblasts were performed as previously described (Hinek et al, 2000) Immunofluorescence analysis for elastin, VE-statin and LoxL2 was performed on HUVEC cells after methanol fixation by incubating cells with primary antibodies diluted in PBS/0.5% BSA before incubation with corresponding secondary antibodies labelled with Alexa Fluor 594 or Alexa Fluor 488 in the same dilution buffer.
LOX activity measurement
MEF cells were treated with or without mouse recombinant VE-statin (Caetano et al, 2006) the day before measuring LOX activity. HUVEC cells were transfected with control- or VE-statin/egfl7-siRNA as described hereafter and cultured 4 days before measuring LOX activity.
LOX activity was measured using the Amplex Red Monoamine Oxidase Assay Kit (Invitrogen), using either benzylamine or cadaverine as substrate, in urea 1.2 M, Na borate 50 mM pH 8.2. Reactions were carried out at 37°C and readings were performed using a Fluostar Optima (BMG Labtech) spectrofluorimeter. LOX activity was calculated as the difference between total activity and activity in BAPN (500 μM)-containing wells of the corresponding sample. Statistical analyses were performed using Student's t-test using the GraphPad Prism software.
Cell cultures and elastin deposition assay
NIH3T3 cells were transiently transfected with pcDNA3-VE-statin/egfl7-HA or pcDNA3 as control, and conditioned media were produced following the protocol described (Soncin et al, 2003). Skin fibroblasts derived from biopsies of healthy human skin (Hinek et al, 2005) were plated at 20 000 cells/cm2 density and cultured in 2% FBS supplemented DMEM until confluence to be then treated for 7 days with different conditioned media before immunodetection. To analyse the VE-statin/egfl7 effect on already deposited elastic fibres, confluent fibroblast cultures were initially maintained in media with 2% FBS for 7 days to stimulate elastic fibre deposit and then maintained with VE-statin/egfl7-conditioned media for the next 3 days before immunostaining.
NHDF and HUVEC cells (Lonza) were routinely cultured following the manufacturer's instructions. siRNA (Dharmacon) were transfected using Primefect siRNA (Lonza) on HUVEC cells plated at 250 00 cells/cm2.
Northern blot and RT–PCR analysis
Confluent fibroblast cultures were maintained for 24 h with conditioned media. Total RNA was extracted using TRI-reagent (Sigma), and steady-state levels of elastin mRNA were analysed by northern blot as described previously (Urban et al, 2002).
Confluent HUVEC cells were cultured for 7 days and total RNA was extracted using Trizol (Invitrogen) following the manufacturer's instructions. Semiquantitative RT–PCR was performed as previously described (Lelievre et al, 2000) using primers mentioned in Supplementary Table 2. Quantitative RT–PCR analysis was carried out using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and human egfl7, elastin and LoxL2 Taqman Gene Expression assay systems following the manufacturer's instructions for use with the Stepone real-time PCR system (Applied Biosystem). Relative quantification was calculated using the ΔΔCt method, where ΔCt is (target Ct−actin Ct), and ΔΔCt is (ΔCt sample−ΔCt untreated control). Relative quantity is 2−ΔΔCt.
Assays of metabolically labelled soluble and insoluble elastins
Quadruplicate confluent cultures of dermal fibroblasts incubated for 72 h in the presence or absence of conditioned media were exposed to 20 μCi [3H]valine. The contents of radioactive NaOH-insoluble elastin and immunoprecipitable soluble tropoelastin were assessed separately in each culture as previously described (Hinek et al, 2000). Final results reflecting the amounts of metabolically labelled, insoluble and soluble elastin were expressed as CPM per microgram of DNA. DNA amounts were determined after purification with DNeasy Tissue System (Qiagen).
Electron microscopy ultrastructural analysis
Ultrastructural analyses were performed following previously described methods (Mattot et al, 2002). Elastin staining was done by tannic acid treatment of glutaraldehyde-osmium fixed tissues (Simionescu and Simionescu, 1976).
Co-immunoprecipitations
Co-immunoprecipitation experiments were performed as previously described (Zanetti et al, 2004) with minor modifications. Briefly, NIH3T3 cells (106 cells in 100 mm Ø dish) were transfected with 5 μg of either pcDNA3 (Invitrogen) control vector or pcDNA3-VE-statin/egfl7HA and 5 μg of pIRES-hrGFP-1a vector (Stratagene) or FLAG LOXs in pIRES-hrGFP-1a or a respective mutated version (amplified using primers described in Supplementary Table 1). Cells were lysed in 1 ml of 50 mM Tris pH 8.0, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 0.5 mM CaCl2 and 0.5 mM MgCl2 and 1X EDTA-free Complete protease inhibitor cocktail (Roche). Complexes were immunoprecipitated using anti-FLAG M2 affinity Gel (Sigma), and used for HA immunoblotting analysis as described above. Flagged protein expression was assessed with monoclonal anti-Flag M2 antibody in re-probing experiments. Whole-cell extracts (input) saved for HA-tagged protein expression were tested by HA immunoblotting.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr Elaine Fuchs for the pG3Z.K14 vector, Dr Cécile Goujet (SEAT, UPS44-CNRS, Villejuif) for help with transgenic mouse generation and Dr Pascal Sommer for a critical reading of the manuscript. We thank Nathalie Spruyt for technical help in transgenic mouse genotyping. We thank the Microscopy Facility of the Institut Pasteur de Lille Campus. This study was supported by ‘Ligue Nationale contre le Cancer' and ‘Association pour la Recherche sur le Cancer (ARC)'. EL was supported by ‘ARC' and ‘Ligue Nationale contre le Cancer'. FS is Directeur de Recherche INSERM.
References
- Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K (2001) A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol 20: 487–491 [DOI] [PubMed] [Google Scholar]
- Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B (2001) Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem 276: 48944–48949 [DOI] [PubMed] [Google Scholar]
- Caetano B, Drobecq H, Soncin F (2006) Expression and purification of recombinant vascular endothelial-statin. Protein Expr Purif 46: 136–142 [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H (2005) EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol 167: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H (2004) Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn 230: 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B, Polgar N, Szauter KM, Ujfaludi Z, Laczko R, Fong KS, Csiszar K (2005) Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem 280: 24690–24697 [DOI] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M (1994) 67-kD elastin-binding protein is a protective ‘companion' of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol 126: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R (2000) Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet 66: 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Wang Y, Liu K, Mitts TF, Jimenez F (2005) Proteolytic digest derived from bovine Ligamentum Nuchae stimulates deposition of new elastin-enriched matrix in cultures and transplants of human dermal fibroblasts. J Dermatol Sci 39: 155–166 [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD (2003) Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278: 14387–14393 [DOI] [PubMed] [Google Scholar]
- Jourdan-Le Saux C, Tronecker H, Bogic L, Bryant-Greenwood GD, Boyd CD, Csiszar K (1999) The LOXL2 gene encodes a new lysyl oxidase-like protein and is expressed at high levels in reproductive tissues. J Biol Chem 274: 12939–12944 [DOI] [PubMed] [Google Scholar]
- Kielty CM (2006) Elastic fibres in health and disease. Expert Rev Mol Med 8: 1–23 [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA (2002) Elastic fibres. J Cell Sci 115: 2817–2828 [DOI] [PubMed] [Google Scholar]
- Laczko R, Szauter KM, Jansen MK, Hollosi P, Muranyi M, Molnar J, Fong KS, Hinek A, Csiszar K (2007) Active lysyl oxidase (LOX) correlates with focal adhesion kinase (FAK)/paxillin activation and migration in invasive astrocytes. Neuropathol Appl Neurobiol 33: 631–643 [DOI] [PubMed] [Google Scholar]
- Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F (2000) ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene 19: 2438–2446 [DOI] [PubMed] [Google Scholar]
- Li W, Liu G, Chou IN, Kagan HM (2000) Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem 78: 550–557 [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182 [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM (2006) Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Kivirikko KI (2001) Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem J 355: 381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R (2002) Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509 [DOI] [PubMed] [Google Scholar]
- Maki JM, Tikkanen H, Kivirikko KI (2001) Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol 20: 493–496 [DOI] [PubMed] [Google Scholar]
- Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, Carmeliet P (2002) Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol 13: 1548–1560 [DOI] [PubMed] [Google Scholar]
- Mithieux SM, Weiss AS (2005) Elastin. Adv Protein Chem 70: 437–461 [DOI] [PubMed] [Google Scholar]
- Oleggini R, Gastaldo N, Di Donato A (2007) Regulation of elastin promoter by lysyl oxidase and growth factors: cross control of lysyl oxidase on TGF-beta1 effects. Matrix Biol 26: 494–505 [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin S, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DYR, De Sauvage FJ, Ye W (2004) The endothelial-cell-derived secreted factor egfl7 regulates vascular tube formation. Nature 428: 754–758 [DOI] [PubMed] [Google Scholar]
- Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SF, Csiszar K, Hendrix MJ, Kirschmann DA (2005) Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res 65: 11429–11436 [DOI] [PubMed] [Google Scholar]
- Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24: 3446–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar N, Fogelgren B, Shipley JM, Csiszar K (2007) Lysyl oxidase interacts with hormone placental lactogen and synergistically promotes breast epithelial cell proliferation and migration. J Biol Chem 282: 3262–3272 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Paes K, De Maziere A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W (2007) EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 134: 2913–2923 [DOI] [PubMed] [Google Scholar]
- Simionescu N, Simionescu M (1976) Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol 70: 608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncin F, Mattot V, Lionneton F, Spruyt V, Lepretre F, Begue A, Stehelin D (2003) VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J 22: 5700–5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD (1998) Increased vascularization in mice overexpressing angiopoietin-1. Science 282: 468–471 [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM (1999) Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514 [DOI] [PubMed] [Google Scholar]
- Turksen K, Kupper T, Degenstein L, Williams I, Fuchs E (1992) Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci USA 89: 5068–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A (2002) Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams–Beuren syndrome. Am J Hum Genet 71: 30–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC (2001) Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem 276: 22537–22543 [DOI] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E (1989) Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci USA 86: 1563–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan GM (2004) EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol Cell Biol 24: 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information