Abstract
Glucocorticoids are widely used anti-inflammatory and immunomodulatory agents, of which the action mechanism is mainly based on interference of hormone-activated glucocorticoid receptor (GR) with the activity of transcription factors, such as nuclear factor-κB (NF-κB). In addition to the well described interaction-based mutual repression mechanism between the GR and NF-κB, additional mechanisms are at play, which help to explain the efficacy of glucocorticoid-mediated gene repression. In this respect, we found that glucocorticoids counteract the recruitment of activated Mitogen- and Stress-activated protein Kinase-1 (MSK1) at inflammatory gene promoters resulting in the inhibition of NF-κB p65 transactivation and of concurrent histone H3 phosphorylation. Additionally, we observed that activated GR can trigger redistribution of nuclear MSK1 to the cytoplasm through a CRM1-dependent export mechanism, as a result of an interaction between liganded GR and activated MSK1. These findings unveil a novel aspect within the GR-mediated NF-κB-targeting anti-inflammatory mechanism.
Keywords: glucocorticoid, inflammation, MSK1, NF-κB, translocation
Introduction
Challenging the human body with microbial agents, such as lipopolysaccharide, or the self-produced inflammatory cytokine tumor necrosis factor (TNF), generates a local and systemic immune response. This is mediated by the expression of other inflammatory cytokines and chemokines, among which interleukin-6 (IL6), interleukin-8 (IL8) and RANTES, and of adhesion molecules (Barnes et al, 1998). It has been well documented that transcription of the above mentioned genes is regulated by either or both activator protein 1 and nuclear factor-κB (NF-κB) transcription factor families. In general, NF-κB is a dimer that can consist of different NF-κB family members. The NF-κB heterodimer comprising a p65 and a p50 subunit, of which the former harbors transactivation functions and the latter can increase inducible DNA-binding affinity of NF-κB (Ghosh and Karin, 2002), is the most commonly studied. In uninduced cells, NF-κB predominantly resides in the cytoplasm under the restraint of the inhibitory, NF-κB-binding protein (IκB). Upon stimulation, IκB is phosphorylated by the IκB kinase complex Ikk, ubiquitinylated and consequently degraded by the 26S proteasome (Ben-Neriah, 2002). The released, activated NF-κB transcription factor translocates into the nucleus, where it binds to specific promoter recognition sites of various pro-inflammatory genes to activate them. Besides the classical NF-κB activation pathway, additional regulatory mechanisms have been identified that fine tune nuclear NF-κB responses in a gene-specific way. As inflammatory gene expression is also co-determined by mitogen-activated protein kinase (MAPK) signaling (Vanden Berghe et al, 1998), we identified the downstream mitogen- and stress-activated protein kinase-1 (MSK1) as an essential NF-κB p65 S276 kinase (Vermeulen et al, 2003). Phosphorylation of this serine residue mediates CBP histone acetylase effects (Zhong et al, 1998) and thus ensures optimal expression of the IL6 gene. In addition, MSK1 may also selectively mark histone H3 S10 phosphorylation at inflammatory gene promoters, which helps to establish a transcription-prone chromatin environment at the IL6 gene promoter (Saccani et al, 2002; Soloaga et al, 2003; Vermeulen et al, 2003).
Following inflammation, immune homeostasis in the body is restored by stimulating the release of glucocorticoid (GC) hormones, which activate the glucocorticoid receptor (GR). Therefore, synthetic GCs are administered to combat inflammatory disorders. The GR, which belongs to the superfamily of nuclear receptors, contains an N-terminal domain, enclosing a transactivation function, a DNA-binding domain, comprising dimerization functions, and a C-terminal ligand-binding domain (LBD), that is also involved in transactivation. Upon activation by ligand, GR is freed from its cytoplasmic chaperones and translocates into the nucleus, where it gives rise to positive or negative transcriptional effects (De Bosscher et al, 2003). Constitutive shuttling between nucleus and cytoplasm has been reported for the non-activated as well as for the activated forms of GR (Haché et al, 1999). Whereas nuclear import of GR is mediated by the nuclear localization signals NL1 and NL2 (Savory et al, 1999), different mechanisms have been described to account for the nuclear export of liganded or unliganded GR (Savory et al, 1999; Liu and DeFranco, 2000; Holaska et al, 2002; Carrigan et al, 2007).
Previous reports explored GC and MAPK signaling crosstalk, affecting NF-κB-driven inflammatory gene promoters. Pro-inflammatory activated MAPKs can deactivate GR through phosphorylation and subsequent retranslocation to the cytoplasm (Irusen et al, 2002; Itoh et al, 2002; Szatmary et al, 2004; Wang et al, 2004). In turn, active GR can inactivate the MAPK signaling pathway, either directly or indirectly through the upregulation of the nuclear dual-specificity phosphatase DUSP1, known to dephosphorylate extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun N-terminal kinase JNK in different cell lines (Caelles et al, 1997; Kassel et al, 2001; Chen et al, 2002; Lasa et al, 2002). As our group observed no significant inhibition by GCs of p38 and ERK MAPK activation in L929sA mouse fibroblasts (De Bosscher et al, 2001) and A549 human epithelial cells (IME Beck, unpublished data), we further explored whether in these cells the downstream kinase MSK1 might be a target of GCs and thus could contribute to transrepression of NF-κB-driven inflammatory genes. Concisely, in this study, we focus on the effects of GCs on the spatiotemporal dynamics of MSK1, resulting in downmodulation of NF-κB-driven gene expression.
Results
GCs and H89 interfere with the phosphorylation of H3 at inflammatory gene promoters
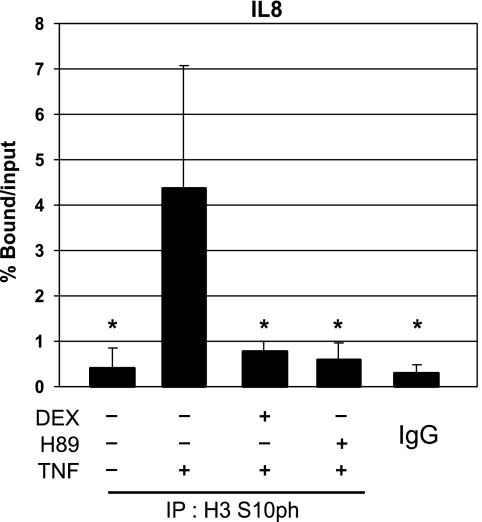
It has already been shown that phosphorylation of H3 S10 at inflammatory gene promoters, ultimately leading to relaxation of the local chromatin, is a hallmark for the induction of inflammatory gene transcription. In this respect, MSK1 has been identified as a kinase of H3 S10 (Soloaga et al, 2003). Therefore, using chromatin immunoprecipitation (ChIP) analysis, we measured endogenous histone H3 S10 phosphorylation in L929sA and A549 cells in the promoter region surrounding the κB-site of various pro-inflammatory genes during TNF treatment in the absence or presence of DEX or the MSK1 inhibitor H89. Interestingly, TNF-induced H3 phosphorylation of S10 at the IL8 gene promoter is blocked by DEX (Figure 1). As expected, phosphorylation of H3 S10 was inhibited by the MSK1 inhibitor H89 (Figure 1). Similar results were obtained for the IL6 and RANTES gene promoters in L929sA cells (data not shown). The specificity of the H3 S10ph signal was verified through analysis of aspecific plaque to the constitutive H4 promoter in mouse cells (data not shown) or binding to IgG in human cells (Figure 1).
Figure 1.
Effect of DEX and H89 on TNF-induced histone H3 phosphorylation at the NF-κB-driven gene promoter IL8. A549 cells, serum-starved for 48 h in 0% DMEM, were pretreated with solvent, DEX (1 μM), or H89 (10 μM) for 2 h. Ensuing the indicated stimulation with TNF (2000 IU/ml) for 30 min, cells were lysed and total cell extracts were subjected to ChIP analysis and subsequent qPCR, detecting phosphorylated H3 S10 (H3 S10ph) at the IL8 promoter. qPCR signal of immunoprecipitated IL8 fragments is presented relative to input data. Statistical analysis (ANOVA with Tukey's multiple comparison post-test) was performed to show significant difference with the TNF condition (*P<0.05). These results are representative for two independent experiments.
The phosphorylation of activated p65 at S276 is affected by GCs
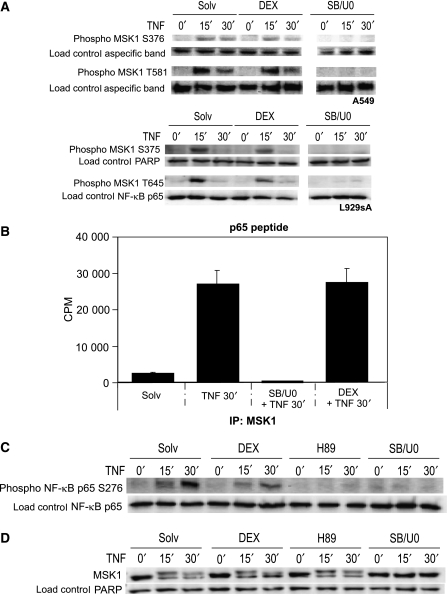
Because we observed a reduction in H3 S10 phosphorylation in the presence of DEX at inflammatory gene promoters, we decided to further investigate the potential negative influence of GCs on the H3-targeting kinase MSK1. As the phosphorylation of MSK1 S376 and MSK1 T581 serve as hallmarks for activated human MSK1 protein (Markou and Lazou, 2002; McCoy et al, 2005), we explored whether GC treatment can affect endogenous MAPK signaling to MSK1. As shown in Figure 2A, DEX influences only slightly TNF activated MSK1 phosphorylation at S376 and T581 in human A549 cells and at S375 and T645 in murine L929sA cells whereas, as expected, inhibition of the upstream MAPKs p38 and ERK with a combination of the inhibitors SB203580 and U0126 blocks all MSK1 phosphorylations completely.
Figure 2.
Influence of DEX and MSK1 inhibitors on the phosphorylation status and the kinase activity of MSK1 (A) A549 and L929sA cells, serum-starved for 48 h in 0% DMEM, were pretreated with solvent (Solv), DEX (1 μM) or SB203580 (10 μM) and U0126 (10 μM) together (SB/U0), for 2 h. Ensuing the indicated stimulation with TNF (2000 IU/ml), cells were lysed and total cell protein extracts were subjected to western blot analysis detecting human MSK1 S376 or T581 and murine MSK1 S375 and T645 phosphorylations. The displayed bands were detected from one single membrane. (B) L929sA cells, starved for 24 h in 0% DMEM, were pre-incubated for 2 h with DEX (1 μM) or a combination of SB203580 (10 μM) and U0126 (10 μM). After 30 min of TNF (2000 IU/ml) induction, endogenous MSK1 was immunoprecipitated and subjected to an in vitro kinase assay, with a p65 peptide as substrate. (C, D) A549 cells were treated as indicated in (A). Two hours pre-induction with H89 (10 μM) was also included. (C) An immunoblot was set up to detect phosphorylated NF-κB S276. (D) Western blot analysis with a pan α-MSK1 Ab was performed to detect total MSK1 protein. Detection of PARP, NF-κB p65 or aspecific bands served as loading controls. The data shown are representative for three independent experiments.
To investigate whether GCs affect MSK1 activity itself, we assayed the kinase potential of the endogenous, TNF-activated MSK1 in L929sA cells in an immunoprecipitation (IP) kinase assay with a p65 peptide as substrate, examining the ability of endogenous MSK1 to phosphorylate a p65-derived peptide at S276. Figure 2B shows that DEX-induced activation of GR does not change the kinase activity of endogenous TNF-activated MSK1. In contrast, the MAPKs p38 and ERK inhibitors SB203580 and U0126 completely block the MSK1 kinase activity.
In an in vitro IP kinase assay, the endogenous, TNF-induced MSK1 kinase activity toward a p65 peptide was not affected by DEX (Figure 2B); however, western blot analysis of A549 total cell lysates showed that DEX administration lowers the endogenous phosphorylation grade of NF-κB p65 S276 in vivo (Figure 2C). As expected, H89 as well as a combination of SB203580 and U0126 abolished MSK1 kinase activity completely and thus also NF-κB p65 phosphorylation at S276. Similar data were obtained in L929sA cells (data not shown). Band density quantifications of Figure 2A and C were added as Supplementary data S1.
Finally, none of the inhibitors affect the total amount of MSK1 protein present in A549 (Figure 2D) or L929sA cells (data not shown). The observed doublets are due to differences between non-phosphorylated and activated, phosphorylated MSK1, the latter of which is of a higher molecular weight.
Taken together, the results of Figure 2 demonstrate that DEX slightly interferes with MSK1 phosphorylation, whereas p38 and ERK MAPK inhibitors completely block MSK1 phosphorylation and activation. Notwithstanding the minor change in MSK1 phosphorylation, the kinase activity of MSK1 to phosphorylate a presented p65 peptide is not affected by DEX. However, in vivo DEX does decrease the endogenous phosphorylation of NF-κB p65 S276.
Recruitment of MSK1 at TNF-activated cytokine promoters is blocked by GCs
Despite the fact that neither activation nor kinase activity of MSK1 seems to be abolished by GCs (Figure 2A, B, D), these steroids efficiently block MSK1-mediated phosphorylation of H3 S10 at gene promoters involved in inflammation (Figure 1) and partly inhibit the phosphorylation of endogenous NF-κB p65 S276 (Figure 2C).
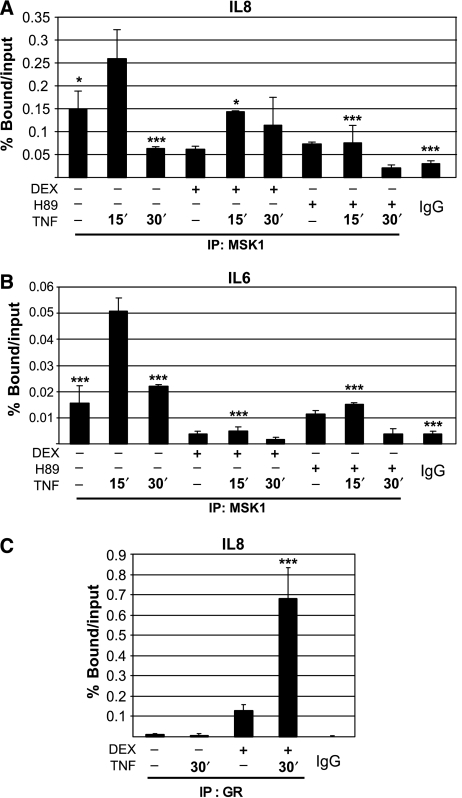
These apparently contradictory findings led us to hypothesize that possibly the physical recruitment of MSK1 to the NF-κB p65-containing enhanceosome could be influenced by GCs. Indeed, ChIP analysis revealed that activation of GR diminishes the detection of TNF-induced recruitment of endogenous MSK1 to the pro-inflammatory promoters of IL8 and IL6 (Figure 3A, B), whereas the addition of GCs to TNF-stimulated A549 cells has a negligible effect on the recruitment of p65 (data not shown). As expected, H89 inhibits recruitment of MSK1 to both IL6 and IL8 gene promoters (Figure 3A, B). Furthermore, we also show that the administration of DEX in combination with TNF results in a strong recruitment of GR to the IL8 inflammatory gene promoter (Figure 3C). These experiments demonstrate that the recruitment dynamics of MSK1 to pro-inflammatory promoters differ upon treatment of the cells with GCs. The specificity of the MSK1 and GR signal was verified through analysis of aspecific plaque to the β-actin coding region (data not shown) and binding to IgG (Figure 3).
Figure 3.
Recruitment of MSK1 to TNF-activated inflammatory gene promoters. (A, B) Following serum starvation for 48 h, A549 cells were incubated with Solvent (Solv), DEX (1 μM) or H89 (10 μM) for 2 h after which TNF (2000 IU/ml) was added for the indicated time. Crosslinked and sonicated cell lysates were subjected to ChIP analysis against MSK1. qPCR, in triplicate, was used to assay MSK1 recruitment to the IL8 and IL6 gene promoters. MSK1 recruitment on the IL6 and IL8 gene promoters was determined by correction of the qPCR signal of the bound fraction to that of the respective input fraction and presented as % bound/input. Statistical analysis (ANOVA with Tukey's multiple comparison post-test) was performed to show significant difference with the TNF 15′ condition (*P<0.05; ***P<0.001) for selected conditions. These data are representative for four independent experiments. (C) A549 cells, serum-starved for 48 h, were incubated with Solv or DEX (1 μM) for 2 h after which TNF (2000 IU/ml) was added for 30 min. The subsequent ChIP procedure against GR was performed as described in (A and B). qPCR of the IL8 promoter region −121/+61 reveals promoter occupancy of GR. Immunoprecipitated bound signal was corrected for input and presented as % bound/input. Statistical analysis (ANOVA with Tukey's multiple comparison post-test) was performed to show significant difference with the Solv condition (***P<0.001). These data are representative for four independent experiments. The IgG controls indicate aspecific binding.
Endogenous MSK1 effuses to the cytoplasm upon GC administration
Many authors have previously reported on signal-dependent subcellular redistribution of various transcription factors. In this regard, we explored whether the GC-provoked recruitment dynamics of MSK1 (Figure 3A, B) might be reflected in a subcellular redistribution of MSK1.
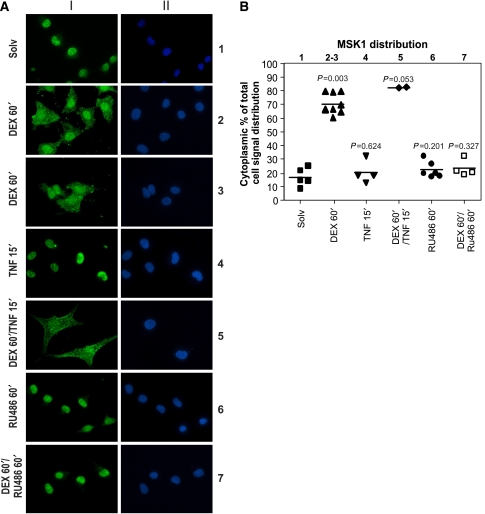
Hereto, we examined through indirect immunofluorescence analysis whether GCs could influence the nucleocytoplasmic localization of endogenous MSK1. Upon induction of A549 cells with DEX for 60 min, a clear nucleocytoplasmic shift of a fraction of the nuclear MSK1 pool becomes visible (Figure 4: lane 2–3). DEX-instigated export of endogenous MSK1 was also observed in L929sA cells (Supplementary data S2).
Figure 4.
Indirect immunofluorescence of endogenous MSK1 in A549 cells in the presence of DEX. (A) A549 cells were treated with DEX (1 μM) and/or RU486 (1 μM) and/or TNF (2000 IU/ml) for the indicated times. Through indirect immunofluorescence using an α-MSK1 Ab, endogenous MSK1 (I) was visualized (green) and DAPI staining (II) indicates the nuclei of the cells (blue). (B) ImageJ integrated density analysis of the MSK1 distribution in the cells displayed in (A) is depicted as a scatter dot plot. Statistical analysis (Mann–Whitney U-test) was performed to show the P-value of each condition compared with the Solv condition. These images are representative for six independent experiments.
In contrast to DEX, TNF alone has no influence on the distribution of endogenous MSK1 (Figure 4: lane 4). Furthermore, TNF administration, subsequent to GC pretreatment, does not drive MSK1 back to the nucleus (Figure 4: lane 5). Figure 4B represents the quantification analysis of the corresponding signal from Figure 4A.
Lastly, we have performed statistical analysis on all A549 and L929sA cell-derived immunofluorescence data, which were semiquantitatively scored in a dichotomic data structure (0=no export, 1=export). This analysis shows a significant difference (P<0.0001) in MSK1 distribution between the DEX-treated condition and solvent control (Supplementary data S3A). The dichotomic semiquantitative scoring in itself was analysed through ROC analysis, and this type of scoring was found to have an almost perfect fit with digital quantification image analysis, as the ROC curve had an area under the curve (AUC) of 0.994 for cytoplasmic MSK1 and an AUC of 0.992 for nuclear MSK1 (Supplementary data S3B). In A549 cells, 85.0% of DEX-treated cells showed export, in contrast to 4.3% of Solv-treated cells. In L929sA cells, 95.1% of DEX-treated cells showed export, in contrast to 12.7% of Solv-treated cells. Analysis with a GEE model showed a significant treatment effect (odds ratio=128.79; 95%; confidence interval=58.90–281.58, P<0.0001) without significant interaction terms, showing a statistically significant export of MSK1 to the cytoplasm under the influence of DEX.
GC-instigated export of MSK1 is GR-dependent
Next, we wondered whether the GC-induced translocation of MSK1 is a GR-dependent phenomenon. Hereto, we used RU486, an antagonist of GR that can bind the LBD of GR with a higher affinity than DEX and thus block the function of GR. It was reported earlier that RU486-bound GR can migrate to the nucleus and can bind onto DNA (Pariante et al, 2001; Schulz et al, 2002; Garside et al, 2004). Our results (Figure 4: lane 7) show that RU486 blocks the GC-mediated effects and consequently MSK1 is not exported to the cytoplasm.
Secondly, we also implemented a knockdown of GR in A549 cells through the transfection of siRNA targeting GR mRNA. Knockdown of GR protein clearly impedes GC-induced translocation of MSK1 from the nucleus to the cytoplasm (Supplementary data S4).
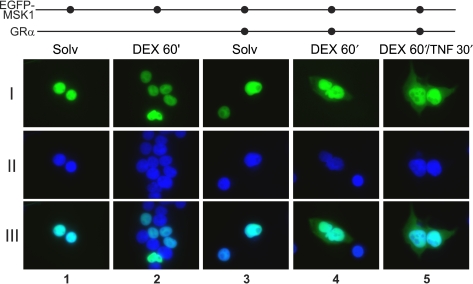
As a third line of evidence, we transiently transfected EGFP-MSK1 into HEK293T cells, which lack functional endogenous GR protein (Figure 8: lane 2). When no pSVhGRα was cotransfected, EGFP-MSK1 protein is exclusively localized in the nucleus, and addition of DEX in these cells does not alter the nuclear localization of MSK1 (Figure 5: lane 1–2). Upon cotransfection of pSVhGRα, stimulation of the cells with DEX triggers an effusion of EGFP-MSK1 into the cytoplasm (Figure 5: lane 4). In contrast to DEX, TNF alone has no influence on the localization of EGFP-MSK1, which was as expected (data not shown). Similar to endogenous MSK1 (Figure 4), EGFP-MSK1 relocalization still occurs upon combined treatment with DEX and TNF (Figure 5: lane 5).
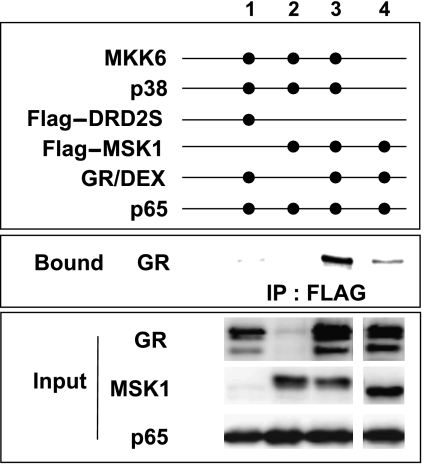
Figure 8.
Effect of GCs on the interaction of MSK1 and GR. HEK293T cells, transiently transfected with the relevant expression constructs as indicated, were treated with DEX (1 μM) for 2 h. Flag-tagged precipitated complexes were analysed through western blotting for the presence of GR protein. The input fraction of GR, MSK1 and p65 visualizes the transfection efficiency. Background signal was determined through Flag-immunoprecipitation of the irrelevant protein Flag-DRD2S. The displayed bands were blotted onto one single membrane. These results are representative for two independent experiments.
Figure 5.
Immunofluorescence microscopy of EGFP-MSK1, transfected in HEK293T cells. HEK293T cells were transiently transfected with pEGFP-hMSK1 together with empty vector or pSVhGRα. After 24 h serum starvation and subsequent to the indicated inductions with DEX (1 μM) and/or TNF (2000 IU/ml), cells were prepared for immunofluorescence microscopy. Green signal represents EGFP-MSK1 protein (I), whereas blue signal visualizes the Hoechst-stained nuclei (II). Overlays of both pictures are presented in (III). These images are representative for two independent experiments.
Taken together, Figures 4, 5 and Supplementary data S4 clearly show that GC-initiated translocation of MSK1 from nucleus to cytoplasm is GR-dependent.
GC-mediated MSK1 export is CRM1-dependent
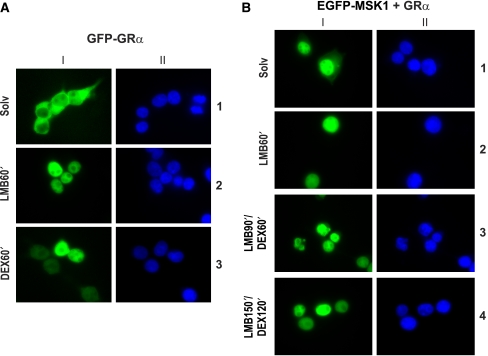
In a parallel experiment, we assayed whether the GC-induced subcellular translocation of EGFP-MSK1 from the nucleus to the cytoplasm could be classified as a nuclear export signal (NES)-mediated export, requiring the CRM1 protein. As a positive control for the functionality of the substances leptomycin B (LMB) and DEX, we included an experiment with HEK293T cells, transiently transfected with pCMX-hGR-GFP. As expected, DEX stimulation prompted the nuclear translocation of GR (Figure 6A: lane 3), and LMB, a specific inhibitor of CRM1-mediated nuclear export, blocked GR shuttling and trapped the uninduced GR in the nucleus (Figure 6A: lane 2) (Savory et al, 1999).
Figure 6.
Subcellular localization of EGFP-MSK1 and GFP-GRα in HEK293T cells in the presence of LMB. HEK293T cells were transiently transfected with pCMX-hGR-GFP and empty vector (A) or pEGFP-hMSK1 and pSVhGRα (B). Following serum starvation, cells were treated as indicated with LMB (20 ng/ml) and/or DEX (1 μM) and prepared for immunofluorescence analysis. Green signal (I) represents either GFP-GRα (A) or EGFP-MSK1 (B). Hoechst treatment (II), represented by the blue signal, allows imaging of the nuclei.
Treatment of pSVhGRα- and pEGFP-hMSK1-transfected HEK293T cells with LMB rendered all EGFP-MSK1 signal absolutely nuclear, irrespective of the presence of DEX (Figure 6B). Note that, upon cotransfection with pSVhGRα and without DEX, some cells already display an EGFP-MSK1 signal outside the nucleus. Thus, under the influence of DEX, a fraction of the EGFP-MSK1 pool is deposited in the cytoplasm in a GR-dependent manner (Figures 4, 5; Supplementary data S4), but the effects of LMB render EGFP-MSK1 absolutely nuclear, pointing to the blockade of a CRM1-mediated mechanism for its export (Figure 6B).
Finally, when assaying the nuclear export of EGFP-MSK1 in HEK293T cells through real-time cell imaging, a time-lapse-edited streaming video revealed that a clear GR-mediated translocation to the cytoplasm of EGFP-MSK1 is apparent already after 20 min of DEX stimulation (Supplementary data S5). Phase contrast imaging additionally showed that GCs do not change cell morphology (Supplementary data S6). Actually, the real-time tracking of EGFP-MSK1 started after 20 min of DEX treatment (Supplementary data S5) is in perfect time accordance with the export of endogenous MSK1 (data not shown), further supporting the validity of our findings. GC-induced cytoplasmic MSK1 was also observed after 120 min of DEX treatment, both for overexpressed EGFP-MSK1 in HEK293T cells and for endogenous MSK1 in L929sA and A549 cells (data not shown).
GR-mediated export of MSK1 can be linked to the repression of inflammatory gene expression
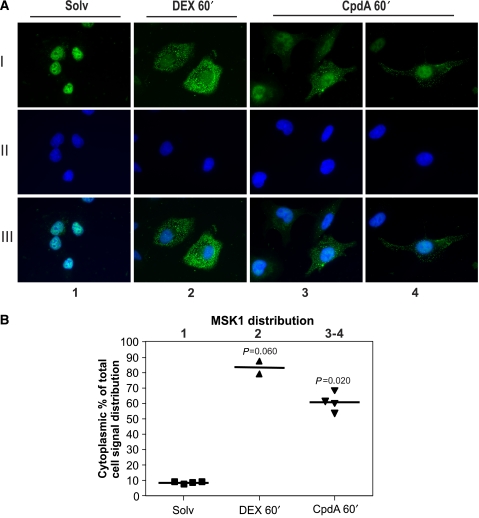
To assess whether the export of MSK1 triggered by GCs could be considered as an element of the GR-mediated transrepression mechanism, we investigated the effects of Compound A (CpdA). This plant-derived molecule is a GR modulator with dissociative properties, that is, CpdA is able to transrepress NF-κB-mediated gene expression, but cannot transactivate glucocorticoid response element (GRE)-regulated promoters (De Bosscher et al, 2005; Dewint et al, 2008). When A549 cells were induced with CpdA for 60 min, we could clearly observe a nucleocytoplasmic shift of endogenous MSK1 (Figure 7: lane 3–4). These results further allow us to directly correlate the cellular redistribution of MSK1 with the GR-mediated NF-κB-targeting, anti-inflammatory mechanism. Figure 7B represents the quantification analysis of corresponding signal from Figure 7A.
Figure 7.
Indirect immunofluorescence of endogenous MSK1 in A549 cells in the presence of CpdA. (A) A549 cells were treated with solvent (Solv), DEX (1 μM) or CpdA (10 μM) for 60 min. Through indirect immunofluorescence using an anti-MSK1 antibody, endogenous MSK1 (I) was visualized (green) and DAPI staining (II) indicates the nuclei of the cells (blue). In (III), we present an overlay of (I) and (II). (B) ImageJ integrated density analysis of the images in (A) allows us to show MSK1 distribution as a scatter dot plot. Statistical analysis (Mann–Whitney U-test) was performed to show the P-value of each condition compared with the Solv condition. These results are representative of two independent experiments.
Activated MSK1 strongly associates with liganded GR
As our results in Figures 4, 5 and Supplementary data S4 indicate the necessity of GRα in MSK1 export, we wondered whether GRα merely functions as an activator of transport through another protein or whether this receptor may act as an export vehicle and, in these conditions, can truly associate with MSK1 itself. Therefore, we immunoprecipitated Flag-tagged MSK1, transfected into HEK293T cells along with expression vectors for GRα and the MSK1 activators MKK6 and p38, as indicated. The NF-κB subunit p65 was cotransfected to obtain equimolar concentrations of the important players involved in transrepression in our system.
Immunoprecipitates were analysed through western blotting in search of the presence of GRα. Figure 8 demonstrates that activated MSK1 indeed interacts with GRα. Overexpressed MSK1 and ligand-activated GR show a mild interaction (Figure 8: lane 4), but when MSK1 is activated through the cotransfection of the upstream kinases MKK6 and p38, a marked increase in the amount of immunoprecipitated GR can be seen (Figure 8: lane 3 versus 4). Cotransfection of p65, however, is not a prerequisite for the association of GR and MSK1 (data not shown). The lack of endogenous GR in HEK293T cells is shown in Figure 8 (lane 2).
Discussion
To date, various models and mechanisms culminate in the explanation of GR-mediated gene repression, ranging from transcription factor occlusion to interference with upstream signaling pathways (De Bosscher et al, 2003, 2006). The present data lead us to speculate upon a completely novel aspect within the action mechanism of GC transrepression of NF-κB-driven gene expression in which GCs can modulate the chromatin environment and the functionality of the enhanceosome through targeting the kinase MSK1. We demonstrated that GC-mediated repression involves a loss of MSK1 recruitment at inflammatory gene promoters, causing inhibition of NF-κB transactivation and H3 S10 phosphorylation. Moreover, we showed that MSK1 can associate with GR and that a substantial amount of MSK1 can be exported to the cytoplasm in a GR- and CRM1-dependent manner. The shift of MSK1 in recruitment and subcellular dynamics under the influence of GCs fits in the framework of a novel GR-mediated anti-inflammatory mechanism.
Glucocorticoids target MSK1 in the GC-mediated inhibition of NF-κB-regulated gene expression
Various gene transcription models integrate huge (enzymatic) cofactor complexes, which establish a transcription-competent or transcription-deficient promoter, of which the chromatin environment depends on the coactivator/corepressor balance present in the cell (Perissi and Rosenfeld, 2005). In this respect, various histone modifications (histone code) are currently considered testimony for specific cofactor activities and read-out for gene expression levels. Moreover, it has been established that H3 modifications (phosphorylation and acetylation) coincide with inflammatory gene induction of NF-κB-driven promoters (Edelstein et al, 2003; Vermeulen et al, 2003; Yamamoto et al, 2003). Addition of an MSK1 inhibitor to TNF-activated A549 and L929sA cells, resulting in the abrogation of pro-inflammatory gene expression, also leads to a clear inhibition of H3 S10 phosphorylation at inflammatory gene promoters (Figure 1 and data not shown), which is consistent with preceding reports identifying MSK1 as the targeting kinase of H3 S10 (Saccani et al, 2002; Soloaga et al, 2003; Vermeulen et al, 2003). Together with histone acetylation, histone H3 S10 phosphorylation contributes to the conformational change of chromatin from a so-called ‘closed' to a more ‘open' configuration, thus allowing transcription factor access and formation of the pre-initiation transcription machinery.
In this respect, we were intrigued by the finding that H3 S10 phosphorylation at pro-inflammatory gene promoters could be blocked by the administration of GCs (Figure 1). To our knowledge, a similar inhibition of H3 S10 phosphorylation has not been reported before in the context of nuclear receptor-mediated transrepression mechanisms targeting NF-κB-driven gene expression of cytokines. A decline of H3 S10 phosphorylation in response to GCs has recently been reported for the promoter of Surfactant Protein A (SP-A) (Islam and Mendelson, 2008), suggesting a general mechanism. In contrast, however, GR activation was previously linked to elevated H3 S10 phosphorylation at the activated and nuclear receptor DNA-bound MMTV promoter (Vicent et al, 2006).
The activated MAPKs p38 and ERK can phosphorylate the murine or human MSK1 at the respective residues T645 or T581 (Markou and Lazou, 2002). Subsequently, MSK1 can autophosphorylate on S375 or S376 in murine or human MSK1, respectively. These phosphorylations are known as hallmarks for active MSK1 (McCoy et al, 2005). When investigating the effects of GCs, we observed that both murine MSK1 T645 and S375 and human T581 and S376 phosphorylations were inhibited slightly (Figure 2A, Supplementary data S1A). Further, we demonstrated that neither the endogenous MSK1 protein levels (Figure 2D) nor the general endogenous MSK1 kinase potential toward a p65 peptide in an in vitro IP kinase assay (Figure 2B) was greatly influenced by GC treatment. In vivo, however, the phosphorylation grade of endogenous NF-κB p65 S276 declined (Figure 2C, Supplementary data S1B), and the H3 S10 phosphorylation at inflammatory gene promoters disappeared (Figure 1) after treatment with GCs. Altogether, GCs are much less effective in inhibiting MSK1 activity, than either the ERK and p38 MAPK inhibitors or the ATP-competitive MSK1 inhibitor H89. Therefore, our data strongly suggest that a dynamic mechanism might operate here, physically separating the active kinase MSK1 and its substrates NF-κB p65 and H3.
In support of this hypothesis, ChIP analysis revealed that MSK1 detection at inflammatory gene promoters, proximal to or including the κB-site, is drastically impaired by GC stimulation (Figure 3A, B). Previous results (Vermeulen et al, 2003) already revealed that MSK1 is readily attracted to the IL6 gene promoter in response to TNF, whereas inhibiting MSK1 activity impedes the TNF-stimulated recruitment of MSK1 to the IL6 gene promoter and IL6 gene transcription. As MSK1/MSK2−/− MEF cells and p65−/− MEF cells reconstituted with p65 S276A display a critically impaired TNF activation of the IL6 gene, the functionality and presence of MSK at the IL6 gene promoter is of the utmost importance (Okazaki et al, 2003; Vermeulen et al, 2003). Because of the importance of MSK1 in inflammatory gene expression, we consequently assume that GC-induced repression of inflammatory genes involves prevention and/or loss of MSK1 recruitment to the inflammatory gene promoter, and could thus indeed explain the GC-mediated decline in NF-κB p65 S276 and H3 S10 phosphorylation (Figure 1, 2C).
In contrast, GR activation does not block recruitment of p65, confirming previous results of our group and others (data not shown) (Nissen and Yamamoto, 2000; De Bosscher et al, 2005). Furthermore, in accordance with literature (Luecke and Yamamoto, 2005), GC treatment prompted the recruitment of GR on the inflammatory promoter IL8, near the NF-κB-binding site (Figure 3C). This recruitment occurs most probably through tethering onto NF-κB and thus it is indirect, as there is no GRE present in the IL8 promoter. Moreover, GR competes with the Cdk9 and cyclinT complex, P-TEFb, for recruitment at the IL8 promoter, thus impeding the P-TEFb-mediated phosphorylation of the RNA-polymerase II C-terminal domain on the S2 residue (Luecke and Yamamoto, 2005). Taken together, these data possibly entail a dual mechanism in which GR inhibits both the transcription-facilitating phosphorylation of H3 S10 and RNA polymerase II CTD S2, by blocking the recruitment of both MSK1 and P-TEFb, respectively. These results fit in the framework of a work model in which GC-mediated inhibition of NF-κB-activated genes involves a necessary association of GR and p65 NF-κB (Nissen and Yamamoto, 2000; De Bosscher et al, 2005; Luecke and Yamamoto, 2005), whereas the TNF-instigated recruitment of the NF-κB p65 S276 and H3 S10 kinase MSK1 is counteracted by GCs (Figure 3A, B).
GCs instigate export of MSK1 in the framework of their anti-inflammatory action mechanism
Furthermore, we noticed that absence of MSK1 recruitment upon GC treatment coincides with a subcellular redistribution of a fraction of the MSK1 pool to the cytoplasm (Figures 4, 5, 7; Supplementary data S2, S3, S4, S5). Upon GC stimulation, MSK1 clearly emerges in the cytoplasm. In contrast to DEX, TNF alone has no influence on the subcellular localization of MSK1 (Figure 4). Furthermore, the GC-induced export of MSK1 is clearly GR-dependent (Figure 4, 5; Supplementary data S4). Despite the consistency in GC-provoked MSK1 translocation in overexpression and endogenous assays, a fraction of cellular MSK1 remains nuclear throughout the induction, suggesting the existence of different subcellular MSK1 pools, most probably tagged by different post-translational modifications, either complexed or not complexed with other cofactors. Indeed, it is clear that not all of the cellular MSK1 pool becomes phosphorylated upon TNF treatment (Figure 2D) and that mainly phosphorylated MSK1 can associate with GR (Figure 8).
Moreover, Figure 7 revealed that the dissociative GR modulator CpdA, which favors GR monomer formation and which is able to transrepress NF-κB-mediated genes without displaying any transactivating properties (De Bosscher et al, 2005; Dewint et al, 2008) can also drive a fraction of the nuclear MSK1 to the cytoplasm, just like classical GCs do. Therefore, the nucleocytoplasmic translocation of MSK1, as elicited either by CpdA or GCs, can be seen in a general cellular mechanism of GR-mediated transrepression of NF-κB-driven gene expression.
To explore the actual export mechanism of MSK1, we used LMB, a chemical that blocks CRM1-dependent NES-mediated translocation, while leaving nuclear import unaffected. GC-induced MSK1 is completely retained within the nucleus upon LMB treatment (Figure 6B), indicating that GC-prompted nuclear export of MSK1 occurs in a CRM1-dependent manner.
The nuclear location of MSK1 has been attributed to its putative bipartite nuclear localization signal (hMSK1 726–745) (Robbins et al, 1991; Deak et al, 1998). Although the MSK1 protein is quite Leu-rich, we have not been able to locate a conserved NES that corresponds to earlier described consensus NES motifs, that is, short hydrophobic Leu-rich sequences (Görlich and Kutay, 1999; Pierreux et al, 2000). Therefore, activated MSK1 may perhaps associate with the shuttling GR, using the export mechanism of GR to become exported. In support, CRM1-dependent and CRM1-independent nucleocytoplasmic GR shuttling has been reported (Savory et al, 1999; Liu and DeFranco, 2000; Holaska et al, 2002; Carrigan et al, 2007). In the current work model, activated GR most probably directly dissociates from MSK1 in the cytoplasm and can then quickly return to the nucleus, where it predominantly resides upon induction with GCs. This rapid back-and-forth shuttling of GR could concur with the described ‘hit and run' mechanism in GR transactivation (McNally et al, 2000; Hager et al, 2002; John et al, 2008).
In conclusion, our data demonstrate that GCs counteract MSK1 recruitment at inflammatory gene promoters, involving complex formation between activated MSK1 and GR followed by a subcellular relocalization of activated MSK1 to the cytoplasm through CRM1-dependent export (Figure 9). The GC-provoked absence of MSK1 at inflammatory gene promoters and export of MSK1 leads to impaired phosphorylation of histone and transcription factor components, resulting in a lack of activation of MSK1-dependent NF-κB-driven promoters. Taken together, our observations reveal a completely new aspect of hormone regulation, in which gene promoter recruitment dynamics and nucleocytoplasmic shuttling of kinases form part of the mechanistic basis for inflammatory gene repression and thus opens perspectives for novel therapeutic strategies.
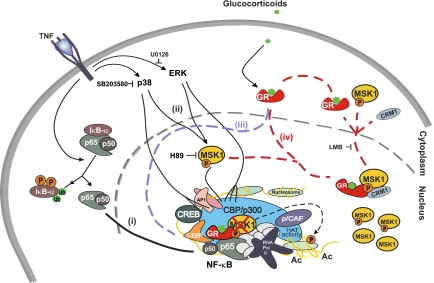
Figure 9.
Glucocorticoids target MSK1 to mediate inflammatory gene repression: a hypothetical model. (i) Release of the transcription factor NF-κB from the cytoplasm and its subsequent translocation to the nucleus, followed by DNA binding onto NF-κB-responsive sequences in genes involved in inflammation. (ii) Activation of MAPKs resulting in activation of a fraction of nuclear MSK1, which will in turn phosphorylate NF-κB p65, providing a scaffold for cofactor interaction, and will phosphorylate the H3 S10 residue, relaxing the surrounding chromatin and thus facilitating transcription. (iii) Activation of GR by GCs, leading to GR translocation to the nucleus and its recruitment to the inflammatory gene promoter, and the interaction with p65. (iv) Interaction of activated MSK1 and liganded GR, and CRM1-dependent, GC-instigated nucleocytoplasmic translocation of MSK1 concomitant with loss of MSK1 at the inflammatory gene promoter. Only a fraction of cellular MSK1 is extruded to the cytoplasm. Dissociation of the GR-MSK1-CRM1 complex in the cytoplasm and subsequent return of GR.
Materials and methods
Cell culture, cytokines and reagents
All cell lines used, A549 human lung epithelial, HEK293T human embryonic kidney and L929sA murine fibrosarcoma cells, were cultured in DMEM with 5% newborn calf serum and 5% fetal calf serum, 100 U/ml penicillin and 0.1 mg/ml streptomycin. The production and purification to 99% homogeneity of recombinant TNF has been described (Vanden Berghe et al, 1998). SB203580, U0126 and H89 were obtained from Alexis Corporation, Promega Biotech and Calbiochem, respectively. DEX, RU486 and LMB were purchased from Sigma-Aldrich. CpdA was synthesized as described (De Bosscher et al, 2005) (Alexis Biochemicals). The antibody (Ab) directed against phosphorylated H3 S10 was a gift of Dr Clayton (Oxford, UK). Besides anti(α)-MSK1, α-p65 and α-GR (Santa Cruz), the phosphospecific Abs α-MSK1 S376, α-MSK1 T581 and α-NF-κB p65 S276 (Cell Signaling) were used for western blot analysis. ChIP analysis of MSK1 and GR were carried out with α-MSK1 (Santa Cruz) and α-MSK1 (Upstate) and α-GR (Santa-Cruz). Normal rabbit, goat or sheep IgG (Santa Cruz) was used as a negative control in ChIP. In immunofluorescence analysis, we applied α-MSK1 (Santa-Cruz).
Plasmids
pSVhGRα and pCMX-hGR-GFP were gifts from Dr Rombauts (Leuven, Belgium) and Dr Tanaka (Kyoto, Japan). MSK1 or DRD2S, C-terminally fused with a Flag-tag, was provided by Dr Alessi (Dundee, UK) and Dr von Zastrow (San Francisco, CA), respectively. The plasmids expressing MKK6 and p38 were gifts of Dr Davis (Worcester, MA, USA) and Dr Cohen (Dundee, UK). The pEGFP-hMSK1 plasmid was constructed by ligating a pMSK1-E (Vermeulen et al, 2003) NcoI-cut, Klenow-filled and subsequently XbaI-restricted fragment with the XbaI–EagI and BglIII, Klenow-filled EagI fragments of pEGFP-C1 (BD Bioscience Clontech).
ChIP assay
A549 cells were starved in 0% DMEM for 48 h. After the appropriate inductions, cells were subjected to a ChIP assay against phosphorylated histone H3 S10, essentially as described (Clayton et al, 2000). For ChIP analysis of MSK1 and GR recruitment, a similar protocol was used, except for the substitution of SDS by DOC in all buffers. DNA was purified through a Nucleospin kit (Machery-Nagel).
The amount of sonicated protein–DNA complexes, present before IP, is indicated by the input controls. Purified DNA samples, enriched with the immunoprecipitated protein, were subjected to qPCR in triplicate. Subsequently, the data obtained for immunoprecipitated samples were corrected for the respective signal for input control and IP efficiencies were presented as % bound/input. The following primers were used for human IL6 (GCGCTAGCCTCAATGACGACCTAAG, CTGATTGGAAACCTTATTAAG). The primers for IL8, encompassing −121/+61, have been described earlier (Nissen and Yamamoto, 2000). Statistical analysis of qPCR data was performed with one-way analysis of variance (ANOVA) and Tukey's multiple comparison post-test on a representative experiment.
Western blot analysis
Samples separated on an SDS–PAGE gel were blotted on a Nitrocellulose membrane (Schleicher&Schuell). Western blotting was performed according to the standard protocol of Santa Cruz. Visualization was established using Western Lightning (Perkin-Elmer).
Kinase assay
After IP of MSK1, the IP-kinase assay of MSK1 was performed as described (Vermeulen et al, 2003) using the substrate p65 peptide, produced by F Jacquemotte (Brussels, Belgium).
Transfection
HEK293T cells were seeded and, the following day, transiently transfected with the calcium phosphate precipitation technique as described before (De Bosscher et al, 2000).
Immunofluorescence
A549 cells, seeded out on coverslips and incubated in serum- and phenol red-free medium for 24 h, were induced as indicated. The fixation, permeabilization and staining protocol was performed as described previously (De Bosscher et al, 2005). Endogenous MSK1 was visualized using α-MSK1 followed by Alexa Fluor 488 donkey α-goat IgG (Molecular Probes) secondary Ab. Alexa fluor 488, enhanced green fluorescent protein (EGFP) and GFP excitation was visualized on an Axiophot immunofluorescence microscope (Zeiss). DAPI staining (0.4 μg/ml) allowed visualization of nuclei upon UV illumination.
HEK293T cells, growing at 50% confluency on coverslips, were transfected with 1 μg of pEGFP-hMSK1 plasmid and 1 μg of empty vector or pSVhGRα or with 1 μg of pCMX-hGR-GFP and 1 μg of empty vector (as indicated in the figure). The next day, medium was replaced (serum-free and phenol red-free) and cells were allowed to rest for 24 h. After fixation, permeabilization and Hoechst 33342 staining (10 μg/ml), the subcellular localization of transfected EGFP- or GFP-fusion proteins was assayed through fluorescence microscopy using a Zeiss axiophot microscope.
Subcellular distribution of endogenous MSK1 was analysed using ImageJ software integrated density analysis. Cells that were not entirely captured in the image field were excluded from quantification. The distribution of MSK1 in each cell was expressed as the percentage of cytoplasmic MSK1 on a cell total of 100% MSK1, and total results of each experiment were presented as a scatter dot plot. The data sets of selected experiments were analysed through a non-parametric Mann–Whitney U-test, and the obtained asymptomatic significance P-values were displayed in the respective figures.
Co-immunoprecipitation of MSK1
HEK293T cells, transiently transfected with Flag-fusion proteins, as indicated in the figure, were left to rest for 24 h, treated with the indicated stimuli and subjected to IP using α-FLAG M2-fused beads (Sigma). Lysis and IP was performed as described previously (Adcock et al, 1999).
Supplementary Material
Supplementary data S1A
Supplementary data S1B
Supplementary data S2
Supplementary data S3
Supplementary data S4A
Supplementary data S4B
Supplementary Data S5A
Supplementary Data S5B
Supplementary Data S6
Supplementary Data - Figure Legends
Acknowledgments
We thank I Vanherpe for her excellent technical assistance. Further, the assistance of G De Wilde and D Peelman in the construction of the pEGFP-hMSK1 plasmid was appreciated. We are also grateful to E Parthoens of the Flanders Institute for Biotechnology (VIB), for helping us with the live imaging experiments. IME Beck is a Research Assistant of the Research Foundation—Flanders (FWO—Vlaanderen). K De Bosscher, W Vanden Berghe and L Vermeulen are postdoctoral fellows of the FWO-Vlaanderen. Financial support was provided by Interuniversity Attraction Poles (IAP) P5/12 and by GOA from Ghent University.
References
- Adcock IM, Nasuhara Y, Stevens DA, Barnes PJ (1999) Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targeting of NF-κB and lack of I-κB involvement. Br J Pharmacol 127: 1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Chung KF, Page CP (1998) Inflammatory mediators of asthma: an update. Pharmacol Rev 50: 515–596 [PubMed] [Google Scholar]
- Ben-Neriah Y (2002) Regulatory functions of ubiquitination in the immune system. Nat Immunol 3: 20–26 [DOI] [PubMed] [Google Scholar]
- Caelles C, Gonzalez-Sancho JM, Muñoz A (1997) Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev 11: 3351–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan A, Walther RF, Salem HA, Wu D, Atlas E, Lefebvre YA, Haché RJ (2007) An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J Biol Chem 282: 10963–10971 [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y (2002) Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 169: 6408–6416 [DOI] [PubMed] [Google Scholar]
- Clayton AL, Rose S, Barratt MJ, Mahadevan LC (2000) Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J 19: 3714–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Beck IM, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G (2005) A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA 102: 15827–15832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G (2001) Glucocorticoid repression of AP-1 is not mediated by competition for nuclear coactivators. Mol Endocrinol 15: 219–227 [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G (2003) The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev 24: 488–522 [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G (2006) Cross-talk between nuclear receptors and nuclear factor-κB. Oncogene 25: 6868–6886 [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Vermeulen L, Plaisance S, Boone E, Haegeman G (2000) Glucocorticoids repress NF-κB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc Natl Acad Sci USA 97: 3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17: 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewint P, Gossye V, De Bosscher K, Vanden Berghe W, Van Beneden K, Deforce D, Van Calenbergh S, Muller-Ladner U, Vander Cruyssen B, Verbruggen G, Haegeman G, Elewaut D (2008) A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol 180: 2608–2615 [DOI] [PubMed] [Google Scholar]
- Edelstein LC, Lagos L, Simmons M, Tirumalai H, Gelinas C (2003) NF-κB-dependent assembly of an enhanceosome-like complex on the promoter region of apoptosis inhibitor Bfl-1/A1. Mol Cell Biol 23: 2749–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside H, Stevens A, Farrow S, Normand C, Houle B, Berry A, Maschera B, Ray D (2004) Glucocorticoid ligands specify different interactions with NF-κB by allosteric effects on the glucocorticoid receptor DNA binding domain. J Biol Chem 279: 50050–50059 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Görlich D, Kutay U (1999) Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 15: 607–660 [DOI] [PubMed] [Google Scholar]
- Haché RJ, Tse R, Reich T, Savory JG, Lefebvre YA (1999) Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J Biol Chem 274: 1432–1439 [DOI] [PubMed] [Google Scholar]
- Hager GL, Elbi C, Becker M (2002) Protein dynamics in the nuclear compartment. Curr Opin Genet Dev 12: 137–141 [DOI] [PubMed] [Google Scholar]
- Holaska JM, Black BE, Rastinejad F, Paschal BM (2002) Ca2+dependent nuclear export mediated by calreticulin. Mol Cell Biol 22: 6286–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM (2002) p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol 109: 649–657 [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR (2008) Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol 22: 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Adachi M, Yasui H, Takekawa M, Tanaka H, Imai K (2002) Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol 16: 2382–2392 [DOI] [PubMed] [Google Scholar]
- John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, Voss TC, Davis SR, Meltzer PS, Stamatoyannopoulos JA, Hager GL (2008) Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell 29: 611–624 [DOI] [PubMed] [Google Scholar]
- Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC (2001) Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 20: 7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR (2002) Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22: 7802–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, DeFranco DB (2000) Protracted nuclear export of glucocorticoid receptor limits its turnover and does not require the exportin 1/CRM1-directed nuclear export pathway. Mol Endocrinol 14: 40–51 [DOI] [PubMed] [Google Scholar]
- Luecke HF, Yamamoto KR (2005) The glucocorticoid receptor blocks p-TEFb recruitment by NFκB to effect promoter-specific transcriptional repression. Genes Dev 19: 1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou T, Lazou A (2002) Phosphorylation and activation of mitogen- and stress-activated protein kinase-1 in adult rat cardiac myocytes by G-protein-coupled receptor agonists requires both extracellular-signal-regulated kinase and p38 mitogen-activated protein kinase. Biochem J 365: 757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS (2005) MSK1 activity is controlled by multiple phosphorylation sites. Biochem J 387: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JG, Muller WG, Walker D, Wolford R, Hager GL (2000) The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287: 1262–1265 [DOI] [PubMed] [Google Scholar]
- Nissen RM, Yamamoto KR (2000) The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14: 2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Sakon S, Sasazuki T, Sakurai H, Doi T, Yagita H, Okumura K, Nakano H (2003) Phosphorylation of serine 276 is essential for p65 NF-κB subunit-dependent cellular responses. Biochem Biophys Res Commun 300: 807–812 [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Su C, Miller AH (2001) The steroid receptor antagonists RU40555 and RU486 activate glucocorticoid receptor translocation and are not excreted by the steroid hormones transporter in L929 cells. J Endocrinol 169: 309–320 [DOI] [PubMed] [Google Scholar]
- Perissi V, Rosenfeld MG (2005) Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol 6: 542–554 [DOI] [PubMed] [Google Scholar]
- Pierreux CE, Nicolas FJ, Hill CS (2000) Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol 20: 9041–9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64: 615–623 [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G (2002) p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat Immunol 3: 69–75 [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Haché RJ, Lefebvre YA (1999) Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol 19: 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R (2002) RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding. J Biol Chem 277: 26238–26243 [DOI] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS (2003) MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 22: 2788–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmary Z, Garabedian MJ, Vilček J (2004) Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem 279: 43708–43715 [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G (1998) p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem 273: 3285–3290 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22: 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent GP, Ballare C, Nacht AS, Clausell J, Subtil-Rodriguez A, Quiles I, Jordan A, Beato M (2006) Induction of progesterone target genes requires activation of ERK and MSK kinases and phosphorylation of histone H3. Mol Cell 24: 367–381 [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH (2004) Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry 9: 65–75 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB (2003) Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature 423: 655–659 [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S (1998) Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1: 661–671 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data S1A
Supplementary data S1B
Supplementary data S2
Supplementary data S3
Supplementary data S4A
Supplementary data S4B
Supplementary Data S5A
Supplementary Data S5B
Supplementary Data S6
Supplementary Data - Figure Legends