Abstract
In patients with von Hippel–Lindau (VHL) disease, renal cysts and clear cell renal cell carcinoma (ccRCC) arise from renal tubular epithelial cells containing biallelic inactivation of the VHL tumour suppressor gene. However, it is presumed that formation of renal cysts and their conversion to ccRCC involve additional genetic changes at other loci. Here, we show that cystic lesions in the kidneys of patients with VHL disease also demonstrate activation of the phosphatidylinositol-3-kinase (PI3K) pathway. Strikingly, combined conditional inactivation of Vhlh and the Pten tumour suppressor gene, which normally antagonises PI3K signalling, in the mouse kidney, elicits cyst formation after short latency, whereas inactivation of either tumour suppressor gene alone failed to produce such a phenotype. Interestingly, cells lining these cysts frequently lack a primary cilium, a microtubule-based cellular antenna important for suppression of uncontrolled kidney epithelial cell proliferation and cyst formation. Our results support a model in which the PTEN tumour suppressor protein cooperates with pVHL to suppress cyst development in the kidney.
Keywords: kidney cyst, primary cilium, PTEN, VHL
Introduction
Inheritance of a mutant allele of the von Hippel–Lindau tumour suppressor gene (VHL) predisposes affected individuals to develop diverse tumours, including renal cysts and clear cell renal cell carcinoma (ccRCC) (Lonser et al, 2003; Kim and Kaelin, 2004). VHL mutation, deletion or silencing also frequently occurs in sporadic forms of these tumours. As a subset of cysts in VHL patients contain micro-foci of ccRCC, kidney cysts may, at least for a subgroup of cases, represent precursor lesions for ccRCC (Solomon and Schwartz, 1988; Choyke et al, 1992). However, small regions of ccRCC can also be found that are not associated with cysts, implying that a cystic precursor is not a prerequisite for ccRCC formation in all cases.
pVHL, the product of the VHL locus, has been ascribed several distinct biochemical activities and implicated in the regulation of diverse cellular processes, almost all of which could be envisaged to contribute to its function as a tumour suppressor protein. These include targeting the hypoxia-inducible factor α (HIFα) transcription factors for oxygen-dependent degradation, regulation of microtubule stability, maintenance of the primary cilium, activation of p53, control of neural apoptosis, suppression of epithelial to mesenchymal transition, secretion of extracellular matrix components (reviewed in Frew and Krek, 2007) and regulation of NFκB activity (Yang et al, 2007).
Although pVHL clearly regulates multiple signalling pathways, loss of pVHL function alone appears to be insufficient for tumour initiation in the kidney. Kidneys of patients with familial VHL disease typically contain many thousands of sites at which pVHL function has been lost; however, the vast majority of these lesions are single cells (Mandriota et al, 2002). Cysts occur relatively infrequently and carcinoma less frequently still. Vhlh+/− mice (Haase et al, 2001) or mice in which Vhlh was mosaically deleted under the control of the ubiquitously expressed β-actin promoter (Ma et al, 2003) are not predisposed to develop cysts or ccRCC. Cre-mediated deletion of Vhlh in the liver and in renal proximal tubule epithelial cells under the control of the PEPCK-Cre transgene likewise failed to give rise to a highly penetrant cystic phenotype, although approximately 20% of mice older than 1 year developed kidney cysts (Rankin et al, 2006). These observations suggest that multiple genetic mutations are required to cause progression from VHL mutant single-celled lesions to cysts and/or carcinomas in kidneys in VHL disease. These genetic alterations may either enhance the operation of signalling pathways already engaged upon pVHL inactivation and/or disrupt additional pathways that function cooperatively to promote tumour cell evolution.
Mutations that impair the structure or function of the primary cilium, a microtubule-based cellular sensory organelle, lead to kidney cysts in humans and in mice (reviewed in Nauli and Zhou, 2004; Pan et al, 2005). In cell culture systems, we have recently identified a cooperative function of pVHL and phosphatidylinositol-3-kinase (PI3K) and glycogen synthase kinase 3β (GSK3β) signalling in the maintenance of the primary cilium (Thoma et al, 2007a). Although pVHL-deficient primary cells form cilia normally under conditions of serum starvation, loss of pVHL sensitises cells to lose their primary cilia in response to inhibition of GSK3β or to serum-induced activation of phosphatidylinositol-3-kinase (PI3K) signalling (Thoma et al, 2007a). As PI3K-mediated activation of protein kinases including AKT (Cross et al, 1995) and p70S6K (Zhang et al, 2006) leads to the phosphorylation and inactivation of GSK3β, it is noteworthy that GSK3β was subjected to inhibitory phosphorylation in VHL mutant cysts in VHL patients, but was not phosphorylated in lesions containing only a few VHL mutant cells (Thoma et al, 2007a). On the basis of these results, it is tempting to speculate that in vivo cyst formation in VHL disease requires secondary mutations in VHL mutant cells that lead to the activation of the PI3K pathway, at least one consequence of which would be the inactivation of GSK3β and the resulting failure to maintain the primary cilium.
Aberrant activation of the PI3K signalling pathway through loss of negative regulators, such as the PTEN tumour suppressor protein, or oncogenic activation of positive mediators, such as PI3K and AKT kinases, are frequent events in many human tumours. Importantly, loss of heterozygosity of PTEN (Velickovic et al, 2002) and reduction of expression of PTEN protein (Shin Lee et al, 2003), as well as activation of AKT (Horiguchi et al, 2003; Pantuck et al, 2007), correlate with poor patient outcome in sporadic cases of ccRCC. These observations suggest that there may be a cooperative effect of loss of pVHL and PTEN functions in kidney tumorigenesis.
In this study, we demonstrate that cysts in human VHL patients exhibit hyperactivation of several components of the PI3K and mTOR signalling pathways. Indeed, deletion of Vhlh and Pten in distal tubules of the kidney in mice causes the formation of cysts at young age and with high penetrance. Importantly, these cysts are characterised by a reduced frequency of primary cilia. We propose that hyperactivation of the PI3K pathway, for example through loss of PTEN, represents one cooperating mechanism that leads to disease progression in familial and sporadic VHL-associated kidney disease.
Results
Hyperactivation of the PI3K signalling pathway in VHL mutant cysts
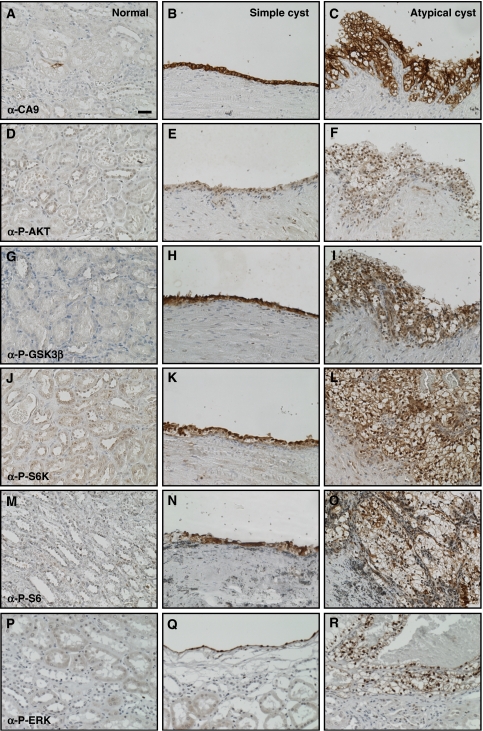
To investigate whether hyperactivation of the PI3K signalling pathway occurs in cysts in VHL disease, we immunohistochemically stained a set of serial sections derived from four previously described kidney biopsies from three VHL patients (Thoma et al, 2007a) using antibodies against CA9, phospho-Ser473 AKT, phospho-Ser9 GSK3β, phospho-Thr389 p70S6K and phospho-Ser240/244 S6 ribosomal protein to investigate the activation state of downstream components of the PI3K signalling pathway. Histologically normal regions of kidneys that contained only isolated VHL mutant cells, as identified by immunohistochemical staining for the HIFα target gene CA9 (Figure 1A), contained only occasional cells that stained for the above-mentioned markers. In contrast, 33 of 33 VHL mutant cystic lesions, which were mostly lined with a single-layered epithelium (simple cysts; Figure 1B, E, H, K and N), but in some cases contained micro-foci of ccRCC (atypical cysts; Figure 1C, F, I, L and O), all displayed staining for the tested antibodies. No staining was observed when primary antibodies were omitted and primary antibodies against other proteins (including phospho-PKCζ and active-β-catenin) did not reveal specific upregulation in cystic cells (data not shown), demonstrating the specificity of the depicted staining.
Figure 1.
PI3K pathway and ERK signalling are upregulated in kidney cysts in VHL patients. Immunohistochemical analysis of sections from a single VHL patient showing (A, D, G, J, M, P) histologically normal kidney tissue, (B, E, H, K, N, Q) a cyst lined by a single epithelial layer (simple cyst) and (C, F, I, L, O, R) a cyst lined with multilayered and disorganised epithelial cells (atypical cyst). Sections were stained with antibodies against (A–C) carbonic anhydrase 9, (D–F) phopho-Ser473 AKT, (G–I) phospho-Ser9 GSK3β, (J–L) phospho-Thr389 p70S6K, (M–O) phospho-Ser240/244 S6 ribosomal protein and (P–R) phospho-Thr202/Tyr204 ERK1/2. All panels are the same magnification, bar=20 μm. Images shown in (B, E, H, K, N, Q) and in (C, F, I, L, O, R) are derived from an equivalent region in adjacent (but not serial) sections and are representative of staining seen in multiple cysts in each of four separate kidney biopsies.
Although we cannot formally exclude that isolated VHL mutant cells may display activation of the PI3K pathway (due to a lack of sensitivity of the phospho-specific antibodies in immunofluorescence staining that prevented co-staining experiments), we quantified the staining frequency of three antibodies that gave the strongest signals in immunohistochemistry. In regions of normal kidney tissue from these biopsies, the anti-CA9 antibody stained 303 individual cells, whereas the anti-phospho-Ser240/244 S6 ribosomal protein antibody stained only 86 individual cells and the anti-phospho-Ser9 GSK3β antibody stained only 46 individual cells. These cells were morphologically identical to surrounding cells. As many fewer cells display markers of activation of PI3K signalling than loss of pVHL function, these results suggest that VHL mutant cells do not automatically display activation of PI3K signalling and imply that hyperactivation of the PI3K pathway may potentially be an early event and contributing factor in the formation of cystic and tumorigenic lesions in VHL patients.
Combined Vhlh and Pten mutation causes kidney cysts
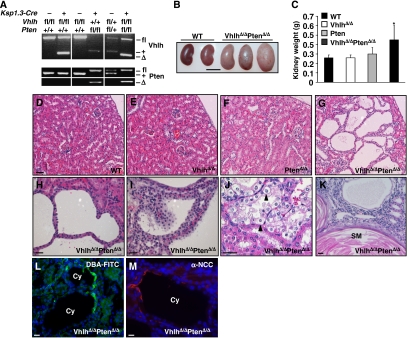
Although the precise cell type of origin of ccRCC remains controversial, VHL mutant multicellular lesions arise almost exclusively in the distal tubules in human VHL patients (Mandriota et al, 2002). To investigate the physiological effect of Vhlh deletion in distal tubule epithelial cells of the kidney, we crossed mice with a loxP-flanked Vhlh allele (Vhlhfl/fl) (Haase et al, 2001) to Ksp1.3-Cre transgenic mice (Shao et al, 2002). This transgene induces Cre-mediated recombination widely throughout the renal epithelium, including in distal tubules, ascending and descending loops of Henle and collecting ducts, but rarely in proximal tubules.
Deletion of Vhlh in kidneys of Ksp1.3-Cre; Vhlhfl/fl mice (hereafter referred to as VhlhΔ/Δ) was confirmed by PCR-mediated detection of the recombined Vhlh allele (Figure 2A) (Haase et al, 2001). Cohorts of VhlhΔ/Δ mice were analysed at 2–3 (n=8), 3–5 (n=6) and 11–13 months (n=9) of age. VhlhΔ/Δ kidneys were of normal external appearance (not shown) and parenchymal mass (Figure 2C) but displayed fully penetrant hydronephrosis, characterised by an expansion of the renal pelvis (Supplementary Figure 1B). Blockage of urinary flow induces hydronephrosis in various mammals, including mice (Ophascharoensuk et al, 1999). However, VhlhΔ/Δ mice did not display histological abnormalities in the urothelium of the renal pelvis (Supplementary Figure 1F) or the urether (data not shown), tissues in which Cre activity is also present, suggesting that blockage of urinary flow does not account for the hydronephrosis. Importantly, at all ages examined, VhlhΔ/Δ mice did not display histological abnormalities in the structure of the tubules in the kidney cortex (Figure 2E) or medulla (data not shown). These findings are consistent with the previously published mouse and human data that loss of pVHL alone does not automatically lead to cysts or ccRCC (Mandriota et al, 2002; Rankin et al, 2006).
Figure 2.
Analysis of VhlhΔ/Δ, PtenΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice. (A) PCR analysis of recombination at the Vhlh and Pten loci in mice with combinations of the Ksp1.3-Cre transgene and Vhlh and Pten floxed and wild-type alleles. The positions of the bands representing the Vhlh and Pten floxed (fl), wild type (+) and recombined (Δ) alleles are indicated. (B) Comparison of the size and external morphology of WT and VhlhΔ/ΔPtenΔ/Δ kidneys (bar=1 cm). (C) Mass of kidneys from WT, VhlhΔ/Δ, PtenΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice (n⩾9, aged 8–21 weeks). *P<0.01, Student's t-test. (D–G) Histological appearance of renal cortex from littermate 8-week-old WT, VhlhΔ/Δ, PtenΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice (bar=100 μm). Examples of (H) simple and (I) atypical cysts, (J) clear cell morphology (arrowheads) and (K) a region of squamous metaplasia (SM) characterised by keratinocyte-like cells surrounding a core of keratin (pink staining), in VhlhΔ/ΔPtenΔ/Δ renal cortex. Immunofluorescence staining for (L) the collecting duct marker FITC-tagged Dolichos biflorus agglutinin and (M) the distal tubule marker NaCl cotransporter in cysts in VhlhΔ/ΔPtenΔ/Δ renal cortex. Bars in (H–M)=10 μm.
To genetically test the effect of constitutive activation of PI3K signalling in the kidney, we utilised mice with a loxP-flanked Pten allele (Ptenfl/fl) (Lesche et al, 2002) to generate Ksp1.3-Cre; Ptenfl/fl (hereafter referred to as PtenΔ/Δ) and Ksp1.3-Cre; Vhlhfl/fl; Ptenfl/fl (hereafter referred to as VhlhΔ/ΔPtenΔ/Δ) mice, in which the Pten tumour suppressor, which normally opposes the activity of PI3K, was deleted in a wild-type or Vhlh mutant background, respectively.
Deletion of Pten in kidneys of PtenΔ/Δ mice was confirmed by PCR-mediated detection of the recombined Pten allele (Figure 2A) (Lesche et al, 2002). Cohorts of PtenΔ/Δ mice were analysed at 2–3 (n=3), 3–5 (n=5) and 11–13 months (n=6) of age. Kidneys of PtenΔ/Δ mice were not significantly different to controls in mass (Figure 2C), and tubules within the cortex and medulla appeared histologically normal at all ages examined (Figure 2F and Supplementary Figure 1C). The urothelium of the renal pelvis (Supplementary Figure 1G), urether and bladder (not shown) displayed a similar phenotype to that previously published for the deletion of Pten in the bladder and urether (Tsuruta et al, 2006; Yoo et al, 2006), namely hyperproliferation and enlarged epithelial cells. Unlike in VhlhΔ/Δ mice, kidneys from PtenΔ/Δ mice did not display hydronephrosis (Supplementary Figure 1C).
Combined deletion of Vhlh and Pten in VhlhΔ/ΔPtenΔ/Δ mice (Figure 2A) resulted in a similar hydronephrosis phenotype (Supplementary Figure 1D) to that seen in VhlhΔ/Δ kidneys and a more pronounced hyperproliferation of the urothelium (Supplementary Figure 1H) than that of PtenΔ/Δ mice. Kidneys from mice with combinations of homozygous and heterozygous Vhlh and Pten deletion (VhlhΔ/ΔPtenΔ/+ (n=8) and VhlhΔ/+PtenΔ/Δ (n=5)) were indistinguishable from those of VhlhΔ/Δ and PtenΔ/Δ mice, respectively. Strikingly, male and female VhlhΔ/ΔPtenΔ/Δ mice had enlarged and heavier kidneys (Figure 2B and C) due to the formation of multiple epithelial tubule cysts in the cortex and medulla (Figure 2G and Supplementary Figure 1D). Cysts were present in kidneys from 14 of 14 mice and were detected as early as 6–8 weeks of age. The majority of cysts were lined by a single layer of epithelial cells (simple cysts) (Figure 2H), whereas approximately 8% of cysts (21 of 269 cysts from three mice) were lined by multilayered epithelial cells with occasional papillary projections (atypical cysts) (Figure 2I). Cells lining all cysts displayed clear cell morphology (Figure 2J), mimicking the histological changes seen upon pVHL loss in human kidneys (Mandriota et al, 2002). Three of 14 kidneys displayed isolated regions of benign squamous metaplasia within the cortex (Figure 2K). VhlhΔ/ΔPtenΔ/Δ mice were killed within 3–6 months of birth to avoid renal failure. No overt tumours or even small areas of adenoma or carcinoma were observed within this time frame in the kidney. Staining with Dolichos biflorus agglutinin (Figure 2L) and with an antibody against the Na-Cl co-transporter (Figure 2M) demonstrated that cysts arose in collecting ducts and distal tubules, respectively. Interestingly, cysts often contained only a few cells that stained positively for these markers (perhaps reflecting cells in which Cre-mediated recombination had not taken place) and in many cysts there was no staining at all, consistent with a possible de-differentiation of these cells, as postulated for the cysts that develop in PEPCK-Cre; Vhlh mutant mice (Rankin et al, 2006). A similar phenomenon was seen in human VHL patients where VHL mutant cells within a histologically normal distal kidney tubule lost expression of Tamm-Horsfall protein, a distal tubule marker (Mandriota et al, 2002). Consistent with the expression pattern of the Cre transgene, we observed no cysts that stained positively for the proximal tubule marker Lotus tetragonolobus lectin.
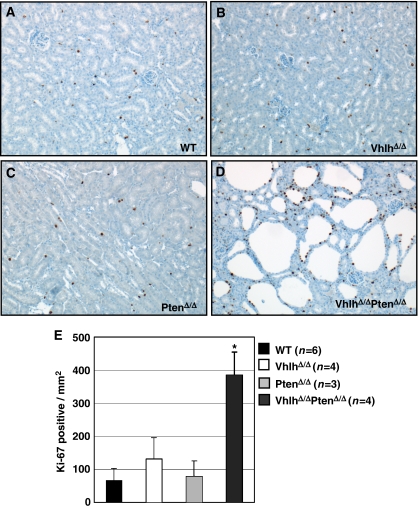
Immunohistochemical staining for the proliferation marker Ki-67, which is expressed by proliferating, but not by resting cells, demonstrated that although epithelial cell proliferation was not significantly increased by deletion of Vhlh (Figure 3B) or Pten (Figure 3C) alone, cysts in VhlhΔ/ΔPtenΔ/Δ mice displayed an approximately 3- to 6-fold increase in staining for Ki-67 (Figure 3D and E). In summary, simple and atypical cystic proliferative lesions that resemble the lesions seen in distal tubules in VHL patients arise in the kidneys of VhlhΔ/ΔPtenΔ/Δ mice.
Figure 3.
Increased proliferation in kidneys of VhlhΔ/ΔPtenΔ/Δ mice. (A–D) Immunohistochemical analysis of kidney cortex from WT (A), VhlhΔ/Δ (B), PtenΔ/Δ (C) and VhlhΔ/ΔPtenΔ/Δ (D) mice using an antibody against the proliferation marker Ki-67. (E) Quantification of the number of epithelial cells per mm2 of the kidney cortex that express Ki-67. One field per kidney section (between three and six mice of each genotype, aged between 8–12 weeks) was counted and the data represent mean±standard deviation. *Statistically significant (P<0.001, Student's t-test) difference between VhlhΔ/ΔPtenΔ/Δ and all other genotypes.
HIFα activation is not sufficient for cyst formation
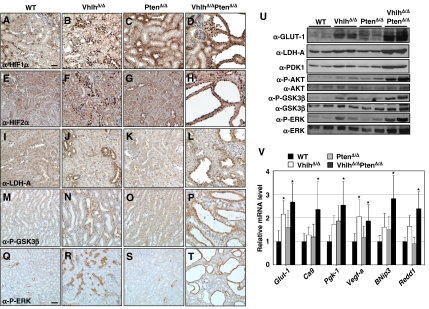
As activation of the PI3K pathway has been shown to enhance the translation of HIFα, and as pVHL regulates the proteolytic degradation of HIFα, we investigated the activation status of the HIFα proteins in Vhlh and Pten mutant kidneys. Immunohistochemical staining revealed that mutation of Vhlh but not of Pten leads to strong, primarily nuclear accumulation of HIF1α (Figure 4B and C) and HIF2α (Figure 4F and G) in epithelial cells in tubule types that are known to express Cre. Epithelial cells lining cysts in VhlhΔ/ΔPtenΔ/Δ kidneys also exhibited strong nuclear accumulation of HIF1α (Figure 4D) and HIF2α (Figure 4H), demonstrating that cystic cells had arisen from cells in which Cre-mediated recombination had occurred. High levels of the HIFα target protein LDH-A were also observed in tubules of kidneys from VhlhΔ/Δ (Figure 4J) and VhlhΔ/ΔPtenΔ/Δ (Figure 4L) mice, demonstrating that HIFα was transcriptionally active.
Figure 4.
Analysis of HIFα activation and PI3K signalling in kidneys of WT, VhlhΔ/Δ, PtenΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice. Immunohistochemical analysis of kidney sections from WT (A, E, I, M, Q), VhlhΔ/Δ (B, F, J, N, R), PtenΔ/Δ (C, G, K, O, S) and VhlhΔ/ΔPtenΔ/Δ (D, H, L, P, T) mice. Sections were stained with antibodies against (A–D) HIF1α, (E–H) HIF2α, (I–L) LDH-A, (M–P) phospho-Ser9 GSK3β and (Q–T) phospho-Thr202/Tyr204 ERK1/2. (A–P) are the same magnification, bar=20 μm, (Q–T) are the same magnification, bar=40 μm. (U) Whole kidney protein lysates from two mice of each indicated genotype were analysed by western blotting using antibodies against GLUT-1, LDH-A, PDK1, phospho-Ser473 AKT (P-AKT), AKT, phospho-Ser9 GSK3β (P-GSK3β), GSK3β, phospho-Thr202/Tyr204 ERK1/2 (P-ERK) and anti-ERK1/2 (ERK). (V) cDNA prepared from whole kidney RNA extracts was analysed by real-time quantitative PCR using specific primers for Glut-1, Ca9, Pgk-1, Vegf-a, BNip3 and Redd1. Fold expression changes were normalised against the expression level of the 18S ribosomal RNA. Data represent mean±standard deviation of four or five mice of each genotype, each sample analysed in triplicate. *Statistically significant (P<0.05, ANOVA followed by Dunnett's two-tailed test) differences in gene expression in comparison to wild type. There were no statistically significant differences between VhlhΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice.
Western blotting of whole kidney lysates for phospho-Ser473 AKT confirmed activation of the PI3K signalling pathway in VhlhΔ/ΔPtenΔ/Δ kidneys (Figure 4U). To quantitatively assess whether loss of HIFα degradation due to loss of pVHL combined with potentially enhanced HIFα translation due to loss of PTEN might lead to an overall increase in HIFα activity, we analysed the mRNA and protein abundance of various known HIFα target genes in mutant kidneys. Western blotting for the HIFα-inducible proteins GLUT-1, LDH-A and PDK-1 (Figure 4U) and real-time PCR analysis of the HIFα target genes Glut-1, Ca9, Pgk-1, Vegf-a, BNip3 and Redd1 (Figure 4V) revealed increased HIFα target gene expression in VhlhΔ/Δ and VhlhΔ/ΔPtenΔ/Δ kidneys. Although there was a trend towards higher HIFα target gene expression in kidneys of VhlhΔ/ΔPtenΔ/Δ mice than in kidneys of VhlhΔ/Δ or PtenΔ/Δ mice, this trend is most likely attributable to the greater proportion of Vhlh-negative cells in whole kidney RNA extracts from VhlhΔ/ΔPtenΔ/Δ kidneys due to the presence of an expanded population of Vhlh-negative cells in cysts. Although we cannot exclude that Pten mutation may contribute to cyst formation by selectively altering the pattern or magnitude of expression of a subset of HIFα target genes, collectively these findings demonstrate that activation of HIF1α and HIF2α following Vhlh mutation alone are not sufficient to cause kidney pathology and suggest that the cooperative suppression of cyst formation by Vhlh and Pten likely involves mechanisms that are independent of, or additional to, HIFα.
Reduced frequency of primary cilia in VhlhΔ/ΔPtenΔ/Δ cysts
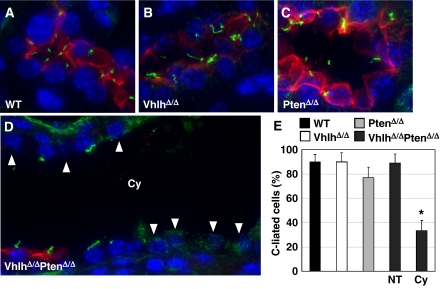
Defects in function or structure of the primary cilium cause kidney cyst formation (reviewed in Nauli and Zhou, 2004; Pan et al, 2005) and cystic epithelial cells in VHL patients frequently lack a primary cilium (Thoma et al, 2007a). As loss of pVHL function sensitises cells to lose their primary cilia in response to PI3K-dependent signalling (Thoma et al, 2007a), we analysed cysts in kidneys of VhlhΔ/ΔPtenΔ/Δ mice for the presence of primary cilia by immunofluorescence staining for acetylated tubulin. In contrast to distal tubules in wild-type (Figure 5A), VhlhΔ/Δ (Figure 5B) and PtenΔ/Δ (Figure 5C) kidneys, in which 80–90% of epithelial cells were ciliated (Figure 5E), distal tubule cysts in VhlhΔ/ΔPtenΔ/Δ kidneys displayed only approximately 30% ciliated cells (Figure 5E; non-ciliated cells indicated by arrowheads in Figure 5D). In comparison, non-cystic distal tubules in VhlhΔ/ΔPtenΔ/Δ kidneys contained normal frequencies of ciliated epithelial cells (Figure 5E), further emphasising the connection between loss of primary cilia and cyst formation. Combined with our previous findings (Thoma et al, 2007a), these data suggest that loss of Vhlh and Pten leads to failure of kidney epithelial cells to maintain their primary cilia, resulting in uncontrolled proliferation and cyst formation.
Figure 5.
Analysis of primary cilia in distal tubules of WT, VhlhΔ/Δ, PtenΔ/Δ and VhlhΔ/ΔPtenΔ/Δ mice. Kidney sections from (A) WT, (B) VhlhΔ/Δ, (C) PtenΔ/Δ and (D) VhlhΔ/ΔPtenΔ/Δ mice were stained with antibodies against NaCl cotransporter (red) and acetylated tubulin (green) and with DAPI to label distal tubules, cilia and nuclei, respectively. Arrowheads in (D) depict cells that lack primary cilia. (E) Quantitation of cilia frequency in distal tubule epithelial cells in WT, VhlhΔ/Δ and PtenΔ/Δ kidneys and in normal (NT) and cystic (Cy) distal tubules in VhlhΔ/ΔPtenΔ/Δ kidneys. Data represent mean±standard deviation of analysis of between 6 and 16 distal tubules (representing 100–730 cells) of each genotype. *P=0.002, Student's t-test.
Multiple signalling pathways control cilia in pVHL-deficient cells
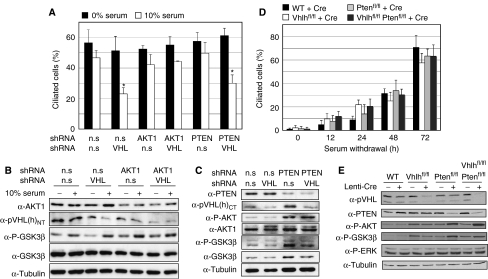
To investigate whether there is a cell-autonomous requirement for pVHL and PTEN in cilia formation or cilia maintenance, we performed cilia assays using cultured cells. h-TERT-immortalised human retinal pigment epithelium (RPE-1) were chosen for analysis as they represent a non-transformed human epithelial cell line that readily forms primary cilia and can be easily manipulated genetically. Consistent with our previous findings in other cell systems (Thoma et al, 2007a), knockdown of VHL sensitised RPE-1 cells to lose their already established primary cilia in response to serum re-addition for 4 h (Figure 6A). Knockdown of AKT1 rescued the effect of serum on VHL knockdown cells (Figure 6A), demonstrating genetically that AKT1 activity contributes to cilia loss and supporting earlier data that suggested the requirement of a wortmannin-sensitive signalling pathway for serum-induced cilia loss in VHL-negative cells (Thoma et al, 2007a). However, although knockdown of PTEN led to elevated AKT activity (Figure 6C), VHL/PTEN double knockdown cells were still able to form cilia normally following serum starvation and lost their cilia equivalently to VHL knockdown cells following serum re-stimulation (Figure 6A). These findings suggest that although AKT activity is necessary, constitutive AKT activity alone is not sufficient to cooperate with loss of pVHL to cause cilia loss. Supporting this conclusion, Vhlh and/or Pten were deleted from primary mouse embryo fibroblasts (MEFs) derived from wild-type, Vhlhfl/fl, Ptenfl/fl or Vhlhfl/flPtenfl/fl mice by infection with a non-genotoxic Cre-expressing lentivirus and the cells were subjected to cilia formation assays. There were no differences among the genotypes in the frequency of ciliated cells following serum starvation for 12, 24, 48 or 72 h (Figure 6D), despite the maintenance of AKT activity in Pten and Vhlh/Pten mutant cells even after 72 h of growth in the absence of serum (Figure 6E). Vhlh/Pten double mutant cells lost their primary cilia in response to serum restimulation equivalently to Vhlh single mutant cells (data not shown).
Figure 6.
Loss of PTEN does not cause cilia loss in pVHL-deficient cells. (A) Quantitation of frequency of ciliated RPE-1 cells following short hairpin-mediated gene knockdown using vectors expressing non-silencing (n.s) or combinations of VHL, AKT1 or PTEN hairpins. Following 3 days of serum starvation, cells were re-stimulated for 4 h with 0 or 10% serum. Data represent mean±standard deviation of two or three independent experiments, each assayed in triplicate assays. *P<0.01, Student's t-test. (B) Western analysis of RPE-1 cells after control knockdown (n.s) or knockdown of VHL and/or AKT1. Blots were probed with antibodies against AKT1, pVHL, phospho-Ser9 GSK3β (P-GSK3β), GSK3β and α-tubulin. (C) Western analysis of RPE-1 cells after control knockdown (n.s) or knockdown of VHL and/or PTEN. Blots were probed with antibodies against PTEN, pVHL, phospho-Ser473 AKT (P-AKT), AKT1, phospho-Ser9 GSK3β (P-GSK3β), GSK3β and α-tubulin. (D) Quantitation of frequency of ciliated primary MEFs derived from wild-type, Vhlhfl/fl, Ptenfl/fl and Vhlhfl/flPtenfl/fl embryos following infection with Lenti-Cre. Subconfluent cells were subjected to serum starvation for the indicated times. Data represent mean±standard deviation of triplicate assays and are representative of data seen in one (12, 24 and 48 h time points) or three (72 h time point) independent experiments. (E) Western analysis of cells from (D) that were grown for 3 days in the absence of serum prior to harvesting. Blots were probed with antibodies against PTEN, pVHL, phospho-Ser473 AKT (P-AKT), phospho-Ser9 GSK3β (P-GSK3β), GSK3β, α-tubulin and phospho-Thr202/Tyr204 ERK1/2 (P-ERK).
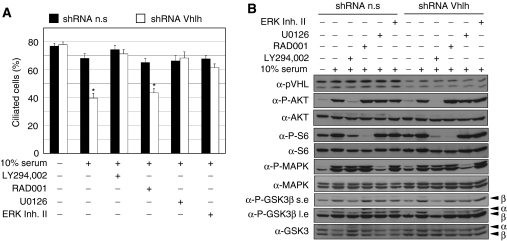
Although AKT activation is a necessary but not sufficient component of a serum-induced signalling pathway that causes loss of cilia in pVHL-deficient cells, we reasoned that additional signalling pathways might be required for this response and therefore attempted to pharmacologically define the molecular requirements for cilia loss. Immortalised MEFs expressing control non-silencing or short hairpin RNA interference against Vhlh (Thoma et al, 2007a) were serum starved for 3 days to induce cilia formation and pretreated with inhibitors against PI3K (LY294,002), mTOR (RAD001), MEK (U0126), ERK (ERK inhibitor II), PKCζ (myristoylated PKCζ pseudosubstrate) or JNK (JML), prior to serum re-stimulation in the presence of inhibitor for 4 h. The PKCζ and JNK inhibitors alone altered microtubule and cilia structures and were therefore not useful in this setting. Western blotting confirmed the specific effects of the remaining inhibitors upon their respective signalling pathways (Figure 7B). Consistent with our previous results (Thoma et al, 2007a), inhibition of PI3K signalling by LY294,002 blocked cilia loss caused by serum (Figure 7A). This response is independent of mTOR activation as RAD001 failed to block cilia loss in Vhlh-deficient cells (Figure 7A). Interestingly, both the MEK and ERK inhibitors also potently blocked serum-induced cilia loss (Figure 7A), implicating this signalling cascade in controlling cilia loss in pVHL-deficient cells. Thus, serum-induced activation of AKT as well as of MEK-ERK appears to be required for the loss of cilia when pVHL is not present. As deletion of Vhlh and Pten causes AKT activation but not ERK activation in serum-starved cultured cells (Figure 6E), this finding may explain why Vhlh/Pten mutant cells in culture do not display defects in cilia formation in response to serum starvation.
Figure 7.
Multiple pathways control cilia loss in pVHL-deficient cells. (A) Quantitation of frequency of ciliated immortalised MEFs following short hairpin-mediated gene knockdown using vectors expressing non-silencing (n.s) or Vhlh knockdown hairpins. Cells were starved of serum for 3 days, treated for 70 min with indicated inhibitors prior to stimulation with 0 or 10% serum for 4 hours in the presence of the inhibitor. Data represent mean±standard deviation of three independent experiments, each assayed in triplicate. *P<1 × 10−8, Student's t-test. (B) Western analysis of cells from (A). Blots were probed with antibodies against pVHL, phospho-Ser473 AKT (P-AKT), AKT, phospho-Ser240/244 S6 ribosomal protein (P-S6), S6 ribosomal protein (S6), phospho-Thr202/Tyr204 ERK1/2 (P-ERK), anti-ERK1/2 (ERK), phospho-Ser9 GSK3β (P-GSK3β) and GSK3. The phospho-Ser9 GSK3β antibody cross-reacts with phosphorylated GSK3α and the positions of the respective proteins are indicated by α and β.
Cystic cells display activating phosphorylation of AKT and ERK and inhibitory phosphorylation of GSK3β
Given the requirement for both AKT and ERK signalling in causing cilia loss in pVHL-deficient cells in culture, we investigated the activation status of these signalling pathways in kidney cysts in vivo. Kidney lysates from VhlhΔ/ΔPtenΔ/Δ mice display increased expression of phospho-Ser473 AKT, phospho-Thr202/Tyr204 ERK1/2 and of phospho-Ser9 GSK3β (Figure 4U), markers of activation (AKT and ERK) or inhibition (GSK3β) of these protein kinases. Immunohistochemical staining confirmed that both phospho-Thr202/Tyr204 ERK1/2 (Figure 4P) and phospho-Ser9 GSK3β (Figure 4T) are expressed within cystic cells. Interestingly, Vhlh deletion alone led to an increase in expression of phospho-Ser473 AKT (Figure 4U), phospho-Ser9 GSK3β (Figure 4N and U) and of phospho-Thr202/Tyr204 ERK1/2 (Figure 4R and U), possibly attributable to the HIFα-mediated upregulation of growth factors such as TGFα or PDGF that signal through these pathways. Whereas mutation of Pten alone caused a mild upregulation of phospho-Ser473 AKT, the combined mutation of Vhlh and Pten resulted in the enhancement of activating phosphorylation of AKT and inhibitory phosphorylation of GSK3β (Figure 4U), possibly due to the increased sensitivity of Pten mutant cells to dysregulated growth factor signalling following Vhlh mutation. In cysts from human VHL patients, which display activation of the PI3K signalling cascade, 25 of 33 cysts also stained positively for phospho-Thr202/Tyr204 ERK1/2 (Figure 1Q and R). Thus, in two different physiological settings of VHL-associated cyst formation there is activation of two distinct signalling pathways that are necessary for cilia loss in pVHL-deficient cells. We suggest that, at the minimum, the combination of loss of pVHL function and activation of PI3K and ERK signalling is required to induce loss of cilia and subsequent cyst formation in vivo.
Discussion
Despite intense investigations, the molecular genetics underlying renal pathology in VHL disease remains incompletely understood. Studies of kidneys from VHL patients revealed a low frequency of multicellular, cystic lesions compared with single cell lesions, implying that the early proliferative advantage conveyed by biallelic VHL inactivation may be relatively modest (Mandriota et al, 2002). Consistent with this view, conditional inactivation of Vhlh in the mouse liver and kidney elicits renal cysts only after long latency with low penetrance (Rankin et al, 2006). Moreover, although renal cysts of VHL patients display a reduced frequency of primary cilia, consistent with the role of this sensory organelle as a suppressor of uncontrolled proliferation and cyst formation, cell biological evidence suggests that pVHL is dispensable for cilia formation in primary cells and that additional pathway(s) need to be inactivated for cells to lose their primary cilia (Frew and Krek, 2007; Thoma et al, 2007a, 2007b). Here, we identify the PTEN tumour suppressor as at least one potential critical suppressor of the conversion of pVHL-deficient renal tubular epithelial cells to cysts, an initial step of kidney cancer progression.
We show that VHL mutant cystic lesions in VHL patients display hyperactivation of the PI3K signalling pathway and that mimicking these conditions by combined mutation of Vhlh and Pten causes kidney cysts in mice. Consistent with the role of the primary cilium as a suppressor of kidney epithelial cell proliferation, and with our previous findings that PI3K signalling in pVHL-defective cells causes loss of the primary cilium (Thoma et al, 2007a), cysts in VhlhΔ/ΔPtenΔ/Δ mice displayed a reduced frequency of primary cilia and increased proliferation. Interestingly, cysts in VhlhΔ/ΔPtenΔ/Δ mice developed later (6–8 weeks) than cysts that develop in other mutant mice with defects in cilia structure or function (Nauli and Zhou, 2004; Pan et al, 2005). Notably, deletion of Kif3a under the control of the Ksp1.3-Cre transgene, the same that was used in this study, resulted in the complete absence of cilia and caused kidney cyst formation within days of birth (Lin et al, 2003). The later onset of cyst formation and slower disease progression in VhlhΔ/ΔPtenΔ/Δ mice are consistent with our model that loss of pVHL sensitises cells to lose cilia in response to external signals (Thoma et al, 2007a, 2007b), rather than being absolutely required for the formation of cilia.
We have previously shown that pVHL maintains primary cilia through its microtubule-stabilising function and that acute inhibition of GSK3β in pVHL-deficient cells causes them to lose their established primary cilia (Thoma et al, 2007a). Now we identify other potential components of this network and show that serum-induced activation of PI3K-AKT1 and of MEK-ERK signalling are both necessary for pVHL-deficient cells to lose their cilia. Both of these signalling pathways are activated in human VHL mutant cysts and in cystic kidneys of VhlhΔ/ΔPtenΔ/Δ mice, suggesting that the combination of PI3K activation and ERK activation may cause pVHL-mutant kidney cells to lose their primary cilia, causing proliferation and cyst formation. As ERK has been shown to phosphorylate GSK3β at Thr43, priming it for subsequent inhibitory phosphorylation at Ser9 (Ding et al, 2005) one possible point of intersection of these pathways is at the level of inhibition of GSK3β. We attempted to test this idea by analysing the effect of ERK inhibition on the levels of phospho-Ser9 GSK3β (Figure 7B); however, changes in total levels of GSK3β phosphorylation induced by serum were too subtle to allow a conclusion to be drawn. Further elucidation of the signalling events that cooperate with pVHL deficiency would be aided by the identification of the component(s) in serum that induces cilia loss. None of the stimuli tested, including PDGF-AA, IGF-1, insulin, FGF, TGFβ1, EGF and soluble fibronectin, were able to mimic the effects of serum on cilia loss in Vhlh-deficient cells, suggesting that a combination of these or other factors, in conjunction with loss of pVHL function, is required to disassemble the primary cilium in cultured cells.
The primary cilium-based model of cyst formation that we propose here needs to be viewed in light of the fact that pVHL has multiple biochemical activities. Thus, it is possible that mutation of Pten may also influence signalling pathways or cellular responses, such as HIFα-induced responses, NF-κB signalling, cell cycle control or epithelial to mesenchymal transition, that are dysregulated as a result of Vhlh mutation. Such cooperative effects of Vhlh and Pten mutation could be independent of cilia but may nonetheless contribute to dysregulated cell proliferation and cyst formation. In addition, we cannot formally exclude the possibility that alterations in urinary flow due to hyperproliferation of the urothelium in the renal pelvis and urether may also, in part, contribute to the cystic phenotype in VhlhΔ/ΔPtenΔ/Δ mice. Partial or complete blockage of urinary flow due to experimental manipulation or congenital abnormality can lead to a variety of kidney abnormalities, including cysts, in humans and in other mammals (Peters, 2001). Arguing against the idea that alteration in urinary flow could wholly account for the cystic phenotype in VhlhΔ/ΔPtenΔ/Δ mice is the fact that PtenΔ/Δ mice also show hyperproliferation of the urothelium, albeit to a lesser extent, but do not develop kidney cysts. Additionally, cystic lesions in VhlhΔ/ΔPtenΔ/Δ mice uniformly display accumulation of HIFα, demonstrating that Cre-mediated recombination has occurred in these cells. If cysts arose simply due to changes in pressure within the kidney it would be expected that cysts would also occur in tubules in which Cre was not active.
One question that arises from our study is why cysts in VHL patients display hyperactivation of PI3K and ERK signalling. HIFα activation following loss of pVHL function may contribute to this effect by inducing autocrine and paracrine signalling via the upregulation of growth factors such as PDGF, VEGF or TGFα that signal via these signal-transduction pathways. It is also possible that secondary mutations, for example inhibitory mutations in PTEN or activating mutations in growth factor receptors, PI3K, AKT, RAS or RAF family genes, may be required to further sensitise pVHL-deficient cells to hyperactivate PI3K and ERK signalling and thereby lose cilia following growth factor signalling. An important corollary of this model is that pharmacological inhibition of the PI3K and/or ERK signalling pathways may represent therapeutic strategies to prevent the loss of primary cilia in pVHL-deficient cells and thereby inhibit cyst formation and the initial steps of ccRCC formation in VHL patients.
Materials and methods
Mouse genetics
C57BL/6J Ksp1. 3-Cre mice (Shao et al, 2002) were obtained from Peter Igarashi (UT Southwestern, USA), BALB/c;129S mixed background Vhlhfl/fl mice (Haase et al, 2001) were obtained from Jackson Labs and BALB/c Ptenfl/fl mice (Lesche et al, 2002) were obtained from Andreas Trumpp (ISREC, Switzerland). Ksp1.3-Cre/+ mice were intercrossed with Vhlhfl/fl; Ptenfl/fl mice to generate Ksp1.3-Cre/+; Vhlhfl/+; Ptenfl/+ and +/+; Vhlhfl/+; Ptenfl/+ mice. These mice were subsequently intercrossed to provide genetic background-matched sets of parents from which Ksp1.3-Cre/+; Vhlhfl/fl; Pten+/+ (VhlhΔ/Δ), Ksp1.3-Cre/+; Vhlh+/+; Ptenfl/fl (PtenΔ/Δ) and Ksp1.3-Cre/+, Vhlhfl/fl; Ptenfl/fl (VhlhΔ/ΔPtenΔ/Δ) mice were generated. Littermate mice that lacked the Ksp1.3-Cre transgene (WT) were used as controls. MEFs were derived from E13.5 wild-type (C57Bl/6J background), Vhlhfl/fl, Ptenfl/fl and Vhlhfl/fl; Ptenfl/fl mice using standard techniques.
PCR
PCR-mediated detection of Cre-mediated recombination at the Vhlh and Pten loci was performed as described (Haase et al, 2001; Lesche et al, 2002).
Immunohistochemistry and immunofluorescence
Immunohistochemistry and immunofluorescence staining were performed as previously described (Thoma et al, 2007a) using the following antibodies: anti-HIF1α (Santa Cruz; sc-8711), anti-CXCR4 (Santa Cruz; sc-6279), anti-CA9 (Pastorekova et al, 1992; M75, gift of Jan Zavada), anti-phospho-Ser473 AKT (Cell Signaling Technology; no. 9277), anti-phospho-Ser9 GSK3β (Cell Signaling Technology, no. 2215), anti-phospho-Thr389 p70S6K (Santa Cruz; sc-11759), anti-phospho-Ser240/244 S6 ribosomal protein (Cell Signaling Technology; no. 2215), anti-phospho-Thr202/Tyr204 ERK1/2 (Cell Signaling Technology; no. 9101), anti-Ki-67 (DakoCytomation; clone TEC-3), anti-LDH-A (Santa Cruz; sc-27230), anti-NaCl-cotransporter (Chemicon; AB3553) and anti-acetylated tubulin (Sigma; no. T6793). Immunohistochemistry using anti-HIF1α (Novus Biologicals; NB100-105) and anti-HIF2α (Pollard et al, 2007; PM8) antibodies was performed using the DakoCytomation Catalyzed Signal Amplification System. Collecting ducts were labelled by incubating kidney sections with FITC-tagged D. biflorus agglutinin (20 μg/ml; Vector Laboratories).
Western blotting
Cultured cells were lysed in TNN buffer (25 mM Tris–HCl pH 7.2, 500 mM sodium chloride, 10% glycerol, 1% NP-40, 10 mM sodium fluoride, 1 mM Na3VO4, 1 mM PMSF, 1 mM dithiothreitol, 10 μg/ml aprotinin) and whole kidney protein extracts were prepared by powdering fresh frozen kidneys under liquid nitrogen followed by homogenisation in TNN buffer. Protein extracts (60 μg) were run on 8–15% polyacrylamide gels, transferred to nitrocellulose membranes and visualised by immunoblotting with the following antibodies: anti-GLUT-1 (Abcam; ab14683), anti-LDH-A (Santa Cruz; sc-27230), anti-PDK-1 (Stressgen; KAP-PK112), anti-phospho-Ser473 AKT (Cell Signaling Technology; no. 4058), anti-AKT (Santa Cruz; sc-1619), anti-AKT1 (2H10, Cell Signaling Technology; no. 2967), anti-phospho-Ser9 GSK3β (Cell Signaling Technology; no. 2215), anti-GSK3β (BD Transduction Labs; 610202), anti-phospho-Ser240/244 S6 ribosomal protein (Cell Signaling Technology; no. 2215), anti-S6 ribosomal protein (Cell Signaling Technology; no. 2212), anti-phospho-Thr202/Tyr204 ERK1/2 (Cell Signaling Technology; no. 9101), anti-ERK1/2 (Cell Signaling Technology; no. 9101), anti-PTEN (Santa Cruz; sc-7974) and anti-mouse pVHL(m)CT antibody (Thoma et al, 2007a).
Cilia assays
Production of lentivirus expressing Cre, MEF infections, generation of Vhlh knockdown immortalised MEFs and cilia assays were performed as described (Thoma et al, 2007a). RPE-1 cells were infected with retroviruses (pLMP) expressing short hairpin RNA interference encoding non-silencing control hairpin (n.s) (Hergovich et al, 2006) or sequences against AKT1 (Openbiosystems; RHS1764-9208187) or PTEN (Openbiosystems; RHS1764-9494281). Cells were selected with 4 μg/ml puromycin. Expression of a second hairpin was achieved by infection with lentiviruses (pCMV-nz) expressing a non-silencing control hairpin (n.s) or a hairpin against VHL. pCMV-nz was cloned from pCMV-GIN-Zeo (Openbiosystems) by removing the eGFP cassette. pCMV-GIN-Zeo was digested with Pci1 and Afe1 followed by blunting with mung bean nuclease. After separation of the fragments on an agarose gel the corresponding fragment was purified and religated to form pCMV-nz. Cloning of n.s and VHL short hairpins into pCMV-nz was performed as described (Hergovich et al, 2006). After infection, cells were selected with 0.5 mg/ml G418. LY294,002 (Sigma; 10 μM), RAD001 (Novartis; 100 nM), U0126 (Sigma; 10 μM), ERK inhibitor II (Calbiochem; 1 μM) were added for 70 min prior to stimulation with 0 or 10% FCS for 4 h.
Real-time PCR analysis
Total RNA was prepared from powdered tissue using the RNeasy Mini Kit (Qiagen) and cDNA was prepared using random hexanucleotide primers and Ready-to-go you-prime first-strand beads (GE Healthcare). Real-time PCR analysis of cDNA was conducted using Absolute SYBR Green (Abgene) and the following primer pairs: Glut-1 (5′-GCTTATGGGCTTCTCCAAACT-3′, 5′-GGTGACACCTCTCCCACATAC-3′), CA9 (5′-TGCTCCAAGTGTCTGCTCAG-3′, 5′-CAGGTGCATCCTCTTCACTGG-3′), Pgk-1 (5′-TGGAGCCAACTCCGTTGTC-3′, 5′-CAGGCATTCTCGACTTCTGGG-3′), Vegf-a (5′-CTTGTTCAGAGCGGAGAAAGC-3′, 5′-ACATCTGCAAGTACGTTCGTT-3′), BNip3 (5′-GACGAAGTAGCTCCAAGAGTTCTCA-3′, 5′-CTATTTCAGCTCTGTTGGTATCTTGTG-3′), Redd1 (5′-CAAGGCAAGAGCTGCCATAG-3′, 5′-CCGGTACTTAGCGTCAGGG-3′).
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank all members of our laboratory for discussions. We are particularly grateful to Peter Igarashi and Andreas Trumpp for providing Ksp1.3-Cre and Ptenfl/fl mice, respectively. This study was supported by grants to IJF from the VHL Family Alliance and to WK from the Dr Josef Steiner Foundation and Swiss National Science Foundation.
References
- Choyke PL, Glenn GM, Walther MM, Zbar B, Weiss GH, Alexander RB, Hayes WS, Long JP, Thakore KN, Linehan WM (1992) The natural history of renal lesions in von Hippel–Lindau disease: a serial CT study in 28 patients. AJR Am J Roentgenol 159: 1229–1234 [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC (2005) Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 19: 159–170 [DOI] [PubMed] [Google Scholar]
- Frew IJ, Krek W (2007) Multitasking by pVHL in tumour suppression. Curr Opin Cell Biol 19: 685–690 [DOI] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R (2001) Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci USA 98: 1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W (2003) Regulation of microtubule stability by the von Hippel–Lindau tumour suppressor protein pVHL. Nat Cell Biol 5: 64–70 [DOI] [PubMed] [Google Scholar]
- Hergovich A, Lisztwan J, Thoma CR, Wirbelauer C, Barry RE, Krek W (2006) Priming-dependent phosphorylation and regulation of the tumor suppressor pVHL by glycogen synthase kinase 3. Mol Cell Biol 26: 5784–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi A, Oya M, Uchida A, Marumo K, Murai M (2003) Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol 169: 710–713 [DOI] [PubMed] [Google Scholar]
- Kim WY, Kaelin WG (2004) Role of VHL gene mutation in human cancer. J Clin Oncol 22: 4991–5004 [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H (2002) Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32: 148–149 [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P (2003) Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH (2003) von Hippel–Lindau disease. Lancet 361: 2059–2067 [DOI] [PubMed] [Google Scholar]
- Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC, Spence S, Lobe CG, Sharma N, Maher GW, Pack S, Vortmeyer AO, Guo C, Zbar B, Schmidt LS (2003) Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res 63: 5320–5328 [PubMed] [Google Scholar]
- Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH (2002) HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468 [DOI] [PubMed] [Google Scholar]
- Nauli SM, Zhou J (2004) Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26: 844–856 [DOI] [PubMed] [Google Scholar]
- Ophascharoensuk V, Giachelli CM, Gordon K, Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ, Alpers CE, Couser WG, Johnson RJ (1999) Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int 56: 571–580 [DOI] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ (2005) Cilium-generated signaling and cilia-related disorders. Lab Invest 85: 452–463 [DOI] [PubMed] [Google Scholar]
- Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS, Figlin RA (2007) Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer 109: 2257–2267 [DOI] [PubMed] [Google Scholar]
- Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J (1992) A novel quasi-viral agent, MaTu, is a two-component system. Virology 187: 620–626 [DOI] [PubMed] [Google Scholar]
- Peters CA (2001) Animal models of fetal renal disease. Prenat Diagn 21: 917–923 [DOI] [PubMed] [Google Scholar]
- Pollard PJ, Spencer-Dene B, Shukla D, Howarth K, Nye E, El-Bahrawy M, Deheragoda M, Joannou M, McDonald S, Martin A, Igarashi P, Varsani-Brown S, Rosewell I, Poulsom R, Maxwell P, Stamp GW, Tomlinson IP (2007) Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell 11: 311–319 [DOI] [PubMed] [Google Scholar]
- Rankin EB, Tomaszewski JE, Haase VH (2006) Renal cyst development in mice with conditional inactivation of the von Hippel–Lindau tumor suppressor. Cancer Res 66: 2576–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Somlo S, Igarashi P (2002) Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846 [DOI] [PubMed] [Google Scholar]
- Shin Lee J, Seok Kim H, Bok Kim Y, Cheol Lee M, Soo Park C (2003) Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol 84: 166–172 [DOI] [PubMed] [Google Scholar]
- Solomon D, Schwartz A (1988) Renal pathology in von Hippel–Lindau disease. Hum Pathol 19: 1072–1079 [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W (2007a) pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 9: 588–595 [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Krek W (2007b) The VHL tumor suppressor: riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle 6: 1809–1813 [DOI] [PubMed] [Google Scholar]
- Tsuruta H, Kishimoto H, Sasaki T, Horie Y, Natsui M, Shibata Y, Hamada K, Yajima N, Kawahara K, Sasaki M, Tsuchiya N, Enomoto K, Mak TW, Nakano T, Habuchi T, Suzuki A (2006) Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res 66: 8389–8396 [DOI] [PubMed] [Google Scholar]
- Velickovic M, Delahunt B, McIver B, Grebe SK (2002) Intragenic PTEN/MMAC1 loss of heterozygosity in conventional (clear-cell) renal cell carcinoma is associated with poor patient prognosis. Mod Pathol 15: 479–485 [DOI] [PubMed] [Google Scholar]
- Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X, Zhang L, Kim WY, Olumi AF, Kaelin WG Jr (2007) pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell 28: 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo LI, Liu DW, Le Vu S, Bronson RT, Wu H, Yuan J (2006) Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res 66: 1929–1939 [DOI] [PubMed] [Google Scholar]
- Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD (2006) S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell 24: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1