Abstract
Purpose
To detect the underlying genetic defect in a family with three members in two generations affected with bilateral congenital cataract.
Methods
Detailed family history and clinical data were recorded. Mutation screening in the candidate genes, αA-crystallin (CRYAA), βA1-crystallin (CRYBA1), βB2-crystallin (CRYBB2), γA–γD-crystallins (CRYGA, CRYGB, CRYGC, and CRYGD), connexin-46 (GJA3), and connexin-50 (GJA8), was performed by bidirectional sequencing of the amplified products.
Results
Affected individuals had “balloon-like” cataract with prominent Y-sutural opacities. Sequencing of the candidate genes showed a heterozygous c.262C>A change in the gene for connexin 50 (GJA8), which is localized at 1q21, that resulted in the replacement of a highly conserved proline by glutamine (p.P88Q). This sequence change was not observed in 96 ethnically matched controls.
Conclusions
We report a p.P88Q mutation in GJA8 associated with Y-sutural cataract in a family of Indian origin. Mutations of the same codon have previously been described in British families with pulverulent cataract, suggesting that modifying factors may determine the type of cataract.
Introduction
Congenital cataract is one of the most significant causes of visual impairment and childhood blindness worldwide. The prevalence of non-syndromic congenital cataract is estimated to be 1–6 cases per 10,000 live births [1,2]. It is a clinically and genetically heterogeneous ocular lens disorder. Interfamilial and intrafamilial phenotypic variation is quite significant in congenital cataract, and various types and sub-types have been reported [3-5]. Approximately one-third of the cases show a positive family history [6]. All three Mendelian modes of inheritance exist for congenital cataract. However, an autosomal dominant mode of transmission is reported to be the most common [5]. At least 27 loci have been mapped. The identified genes encode proteins such as crystallins, the major structural lens proteins (CRYAA, CRYAB, CRYBA1/A3, CRYBB1, CRYBB2, CRYBB3, CRYGC, CRYGD, and CRYGS), gap junctional proteins, the connexins (GJA3 and GJA8), major intrinsic protein (MIP/MIP26), lens integral membrane protein 2 (LIM2), beaded filament proteins (BFSP1, BFSP2), and heat shock protein (HSF4) as reviewed by Reddy et al. [2] and Hejtmancik [7].
Pulverulent cataract was the first autosomal disease locus mapped. In 1963, Renwick and Lawler described linkage to the Duffy blood group locus [8], which was found to cosegregate with an uncoiler element of chromosome 1q by Donahue in 1968 [9]. Thirty years later, Shiels analyzed the same cataract family and reported a missense mutation in codon 88 of the connexin 50 (GJA8) gene at 1q21 [10], leading to the substitution of proline by serine (p.P88S). Here, we report a cataract family of Indian origin with three affected members in two generations with a proline to glutamine mutation of the same codon (p.P88Q) but with balloon-like cataract with prominent Y-sutural opacities. Now, more than a dozen different mutations in GJA8 associated with different cataract phenotypes have been identified (Table 1).
Table 1. Mutations identified in connexin 50 in association with different cataract phenotypes in different congenital cataract families.
|
Amino acid change |
Location/GJA8 Domain |
Cataract type |
Phenotype description |
Origin of family |
References |
| p.R23T |
Cytoplasmic NH2-terminal |
Congenital nuclear |
Progressive, dense nuclear (fetal/embryonal) |
Iranian |
[23] |
| p.V44E |
First transmembrane domain (M1) |
Congenital cataract and microcornea |
Total lens opacification |
Indian |
[24] |
| p.W45S |
First transmembrane domain (M1) |
Jellyfish-like cataract and microcornea |
Axial lens opacity with finger-like projections extended in all directions |
Indian |
[25] |
| p.D47N |
First extracellular loop (E1) |
Nuclear pulverulent |
Opacities confined to the fetal and embryonal nucleus |
English |
[26] |
| p.D47Y |
First extracellular loop (E1) |
Autosomal dominant congenital cataract |
Autosomal dominant congenital cataract |
Chinese |
[27] |
| p.E48K |
First extracellular loop (E1) |
Zonular nuclear pulverulent |
Non-progressive, fine dust-like opacities, more dense throughout the nucleus. Several cortical riders in the zonular region |
Pakistani |
[28] |
| p.V64G |
First extracellular loop (E1) |
Congenital nuclear |
Congenital nuclear cataract |
Chinese |
[29] |
| p.V79L |
Second transmembrane domain (M2) |
Full moon like with Y-sutural opacities |
Stationary cataract both Y-sutures affected. No opacities in the embryonal nucleus, fine granular white opacity outside the embryonal nucleus in the fetal nuclear region |
Indian |
[22] |
| p.P88Q |
Second transmembrane domain (M2) |
Lamellar
pulverulent |
Pulverulent opacities in the fetal nucleus, embryonal nucleus clear |
English |
[20] |
| p.P88Q |
Second transmembrane domain (M2) |
Balloon-like cataract with Y-sutural opacities |
Fetal nucleus and Y-sutures affected. In between the Y-sutures, feathery opacities are present. Three riders present at the perimetry of opaque fetal nucleus. No “pulverized” dust-like opacities in the lens |
Indian |
present study |
| p.P88S |
Second transmembrane domain (M2) |
Zonular pulverulent |
Non-progressive innumerable powdery opacities located in the nuclear and lamellar zones. Affects both the embryonic and fetal nucleus: “total nuclear cataract” |
English |
[10] |
| p.P189L |
Second extracellular loop (E2) |
Congenital cataract and microcornea |
Star shaped nuclear opacity with a whitish central core |
Danish |
[30] |
| p.R198Q |
Second extracellular loop (E2) |
Cataract and microcornea |
Posterior subcapsular |
Indian |
[24] |
| p.203fs |
Second extracellular loop (E2) |
Cataract and nystagmus |
Total cataract and nystagmus |
Indian |
[31] |
| p.I247M |
Cytoplasmic COOH-terminus |
Zonular pulverulent |
Progressive, non homogeneous opacity consisting of opaque particles of different sizes, most of these very small, distributed unequally in a disc of 5 mm in diameter in the center of the lens. Also a slightly cloudy inhomogeneous area of 2 mm at posterior pole |
Russian |
[32] |
| p.S276F | Cytoplasmic COOH-terminus | Pulverulent nuclear | White granular opacities in fetal and embryonal nucleus | Chinese | [33] |
This table provides information on specific mutations identified in different domains and regions of connexin 50 in association with congenital cataract in families belonging to different ethnic groups. Most of these mutations are missense mutations, and the cataract phenotypes are zonular/nuclear pulverulent type with powdery dust like opacities. However, the phenotype balloon-like cataract with Y-sutural opacities linked with p.P88Q substitution observed in present family had no pulverized dust like opacities in the lens. The feathery opacities in between the Y-sutural opacities and three riders at the perimetry of the opaque fetal nucleus are very much prominent in the affected lenses in the present family.
Methods
Family description
The proband, a seven-year-old male child, was diagnosed with bilateral cataract. The family history revealed three affected members in two generations (Figure 1). The ophthalmologic examination, including slit lamp examination, was performed on a total of four members of this family; the father (Figure 1; II:3), who had a history of cataract extraction in childhood, and two of his bilaterally affected children (Figure 1; III:1 and III:2). The proband’s mother (Figure 1; II:4) was diagnosed as unaffected.
Figure 1.
Pedigree of a family with individuals affected by bilateral congenital cataract. The pedigree of an autosomal dominant congenital cataract (ADCC) family shows three members in two generations as affected (filled squares and circle). The proband (III:2), who was diagnosed as bilaterally affected with congenital cataract at the age of seven years, is indicated with an arrow. His elder sister (III:1) was diagnosed with bilateral cataract when she was six years old. The affected father (II:3) of these children underwent cataract extraction in both of his eyes in the first decade of his life.
Mutation analysis
Informed consent was obtained for each individual studied. This study was approved by the ethics review board of the Guru Nanak Dev University (Amritsar, Punjab, India), consistent with the provisions of the Declaration of Helsinki. Blood was drawn and DNA isolated using method of Adeli and Ogbonna [11]. Mutation screening was performed in the exonic regions of the following candidate genes: CRYAA (21q22.3; GenBank NM_000394), CRYBA1 (17q11-q12; GenBank NM_005208), CRYBB2 (22q11.2; GenBank NM_000496), CRYGA (2q33-q35; GenBank NM_014617), CRYGB (2q33-q35; GenBank NM_005210), CRYGC (2q33-q35; GenBank NM_020989), CRYGD (2q33-q35; GenBank NM_006891), GJA3 (13q11-q13; GenBank NM_021954), and GJA8 (1q21-q25; GenBank NM_005267). The coding regions and exon-intron boundaries of the candidate genes were amplified using previously published primer sequences [10,12-16]. Genomic DNA from all three affected and one unaffected individual was amplified. Purified polymerase chain reaction (PCR) products were sequenced bidirectionally with ABI BigDyeTM Terminator Cycle Sequencing Ready Reaction Kit version 3.1 (Applied Biosystems, Foster City, CA) for a 10 µl final volume containing 5.0 µl of purified PCR product, 4.0 µl of BigDye Terminator ready reaction mix, and 3.2 pmol of primer. Cycling conditions were: 95 °C for 2 min, 25 cycles at 95 °C for 30 s, 52 °C for 15 s, and 60 °C for 4 min. The sequencing reaction products were purified by the isopropanol precipitation method (ABI protocol; Applied Biosystems), resuspended in 10 µl of loading buffer (5:1 ratio of deionized formamide and 25 mM EDTA with blue dextran [50 mg/ml]), denatured at 95 °C for 5 min, and electrophoresed on 4% denaturing polyacrylamide gels on the DNA sequencer (ABI-Prism 377; Applied Biosystems). Sequencing results were assembled and analyzed using the SeqMan II program of the Lasergene package (DNA STAR Inc., Madison, WI).
Results
Phenotype description
Slit lamp examination of the lenses in affected individuals (III:1, III:2) showed that the cataract affected the fetal nucleus with additional changes on its surface. The fetal nucleus appeared semi-opaque. The Y-sutures on the anterior surface seem sharp and narrow (Figure 2A). In between the Y-sutures, there appeared feathery opacities extending up to three-fourths of the length of the Y-sutures. Along the perimeter of the fetal nucleus, three prominent riders were present. The posterior Y-sutures were not clearly seen through the anterior central feathery opacification, but its presence is in no doubt. Inside the semi-opaque fetal nucleus, there appeared no opacities.
Figure 2.
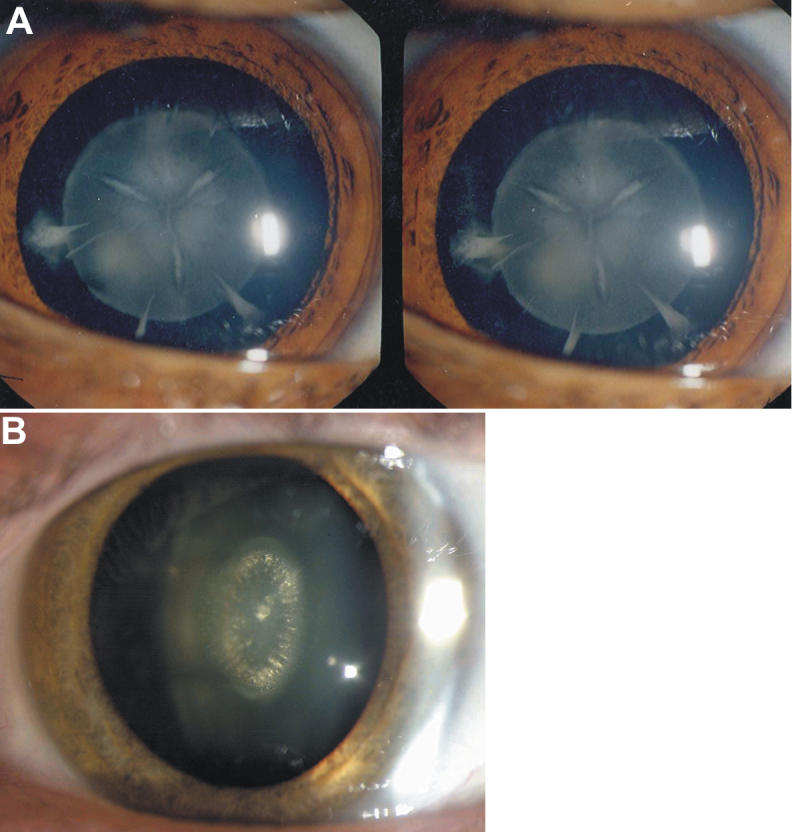
Photograph of cataract phenotypes observed in the present Indian family and in the British family having the identical mutation (p.P88Q) in GJA8. A: The slit lamp photograph (three-dimensional lens) of a patient shows very prominent feather-like sutural opacities. Apart from sutural opacities, riders are prominent. The inside of the semi-opaque fetal nucleus appeared optically empty. B: The photograph from the affected lens of an individual from the British autosomal dominant congenital cataract family with zonular pulverulent cataract [20] shows linear dense vertical opacities inside the fetal nucleus with embryonic nucleus remaining clear and without sutural opacities.
Mutation screening
Bidirectional sequencing of the coding regions of the candidate genes showed a heterozygous change, C>A (Figure 3), at position 262 (c.262 C>A) in GJA8 in all three affected individuals. This substitution was not seen in the unaffected mother or in 96 unrelated control subjects (192 chromosomes) from the same North Indian population as tested by bidirectional sequence analysis (data not shown). The substitution replaces an evolutionarily highly conserved proline by glutamine at amino acid position 88 (p.P88Q) in the second α-helical transmembrane domain 2 (M2) of connexin 50.
Figure 3.
Partial DNA sequence of GJA8 from an unaffected and an affected individual. The wild type C in the sequence of the unaffected individual and the heterozygous c.262C>A change, which results in the substitution of proline by glutamine at amino acid position 88 (p.P88Q), in the affected individual are indicated by arrows. F=forward strand.
Discussion
Connexins are integral membrane proteins with four transmembrane domains, two extracellular loops, and an intracellular loop with both NH2- and COOH-termini localized in the cytoplasm. In humans, at least 20 connexins classified into three families are known [17,18]. Connexin 46 and connexin 50 are responsible for joining the lens cells into a functional syncytium. In addition, connexin 50 is also important for lens growth [19]. The observed p.P88Q substitution is centrally located within the second α-helical transmembrane domain (M2) of connexin 50 and replaces the highly conserved hydrophobic proline by the non-polar glutamine at position 88, in association with the congenital cataract in the present family.
The same mutation was seen in a British family. However, this mutation was associated with lamellar pulverulent cataract [20]. In another family of English descent, a mutation of the same codon lead to the substitution of proline by serine (p.P88S), which is associated with nuclear pulverulent cataract, characterized by innumerable powdery opacities located in the nuclear (central) and perinuclear (lamellar) zones of the lens [8,10]. Further, functional analyses revealed that mutants p.P88S and p.P88Q act as dominant negative inhibitors and significantly decreases the activity of co-expressed wild type connexin 50 [20,21].
Mutations in connexin 46 and connexin 50 have been reported to be linked with cataractogenesis in humans as well as in mice. In humans, over a dozen mutations in each connexin 46 and connexin 50 gene have so far been detected in association with congenital cataract [2,7] and significant interfamilial phenotypic variability. The phenotype in most cases with mutations in connexin 46 and connexin 50 has been described as zonular/nuclear pulverulent cataract [3,5,7]. The cataract phenotype in the present family differs from these as no “pulverized” dust-like opacities are seen in the lens (Table 1). It also differs from the British family with the identical mutation [20], which showed linear dense vertical opacities inside the fetal nucleus with the embryonic nucleus remaining clear and without sutural opacities (Figure 2B). In the present family, Y-sutural opacities are very prominent, comparable with the p.V79L mutation in the second transmembrane domain (M2) of connexin 50, which is linked with “full moon” like cataract with Y-sutural opacities (Table 1) in another Indian family having 15 affected members in three generations reported previously by us [22].
In summary, we describe a heterozygous p.P88Q mutation in connexin 50 that showed marked phenotypic differences to previously reported cases affecting the same codon. Thus, variants in other genes might act as modifiers of the cataract phenotype.
Acknowledgments
We wish to thank the patients and their relatives for their cooperation. We are grateful to Dr. P.N. Robinson for critically reading the manuscript. This work was in part supported by grant number DBT/BT/IC/71/89/Pt from the Department of Biotechnology, Ministry of Science and Technology, Government of India sanctioned to J.R.S. and INI 331 from Bundesministerium für Bildung und Forschung, Federal Ministry of Education and Research, Bonn, Germany to K.S.
References
- 1.Lambert SR, Drack AV. Infantile cataracts. Surv Ophthalmol. 1996;40:427–58. doi: 10.1016/s0039-6257(96)82011-x. [DOI] [PubMed] [Google Scholar]
- 2.Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004;49:300–15. doi: 10.1016/j.survophthal.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Scott MH, Hejtmancik JF, Wozencraft LA, Reuter LM, Parks MM, Kaiser-Kupfer MI. Autosomal dominant congenital cataract: Interocular phenotypic variability. Ophthalmology. 1994;101:866–71. doi: 10.1016/s0161-6420(94)31246-2. [DOI] [PubMed] [Google Scholar]
- 4.Vanita. Genetical investigations in congenital cataract cases. 1998; Ph.D. thesis, Guru Nanak Dev University, Amritsar, India.
- 5.Amaya L, Taylor D, Russell-Eggitt I, Nischal KK, Lengyel D. The morphology and natural history of childhood cataracts. Surv Ophthalmol. 2003;48:125–44. doi: 10.1016/s0039-6257(02)00462-9. [DOI] [PubMed] [Google Scholar]
- 6.Vanita, Singh JR, Singh D. Genetic and segregation analysis of congenital cataract in the Indian population. Clin Genet. 1999;56:389–93. doi: 10.1034/j.1399-0004.1999.560507.x. [DOI] [PubMed] [Google Scholar]
- 7.Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19:134–49. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renwick JH, Lawler SD. Probable linkage between a congenital cataract locus and the Duffy blood group locus. Ann Hum Genet. 1963;27:67–84. doi: 10.1111/j.1469-1809.1963.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 9.Donahue RP, Bias WB, Renwick JH, Kusick VA. Probable assignment of the Duffy blood group locus to chromosome 1 in man. Proc Natl Acad Sci USA. 1968;61:949–55. doi: 10.1073/pnas.61.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–32. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeli K, Ogbonna G. Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem. 1990;36:261–4. [PubMed] [Google Scholar]
- 12.Vanita V, Singh JR, Hejtmancik JF, Nürnberg P, Hennies HC, Singh D, Sperling K. A novel fan-shaped cataract microcornea syndrome caused by a mutation of CRYAA in an Indian family. Mol Vis. 2006;12:518–22. [PubMed] [Google Scholar]
- 13.Lu S, Zhao C, Jiao H, Kere J, Tang X, Zhao F, Zhang X, Zhao K, Larsson C. Two Chinese families with pulverulent congenital cataracts and deltaG91 CRYBA1 mutations. Mol Vis. 2007;13:1154–60. [PubMed] [Google Scholar]
- 14.Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet. 1997;6:665–8. doi: 10.1093/hmg/6.5.665. [DOI] [PubMed] [Google Scholar]
- 15.Mackay DS, Andley UP, Shiels A. A missense mutation in the gammaD crystallin gene (CRYGD) associated with autosomal dominant “coral-like” cataract linked to chromosome 2q. Mol Vis. 2004;10:155–62. [PubMed] [Google Scholar]
- 16.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S. Connexin 46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–64. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans WH, Martin PEM. Gap junctions: structure and function. Mol Membr Biol. 2002;19:121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 18.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 19.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 20.Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol. 1999;276:C1443–6. doi: 10.1152/ajpcell.1999.276.6.C1443. [DOI] [PubMed] [Google Scholar]
- 22.Vanita V, Hennies HC, Singh D, Nuernberg P, Sperling K, Singh JR. Novel mutation in GJA8 associated with autosomal dominant congenital cataract in a family of Indian origin. Mol Vis. 2006;12:1217–22. [PubMed] [Google Scholar]
- 23.Willoughby CE, Arab S, Gandhi R, Zeinali S, Arab S, Luk D, Billingsley G, Munier FL, Heon E. A novel GJA8 mutation in an Iranian family with progressive autosomal dominant congenital nuclear cataract. J Med Genet. 2003;40:e124. doi: 10.1136/jmg.40.11.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devi RR, Vijayalakshmi P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol Vis. 2006;12:190–5. http://www.molvis.org/molvis/v12/a21/ [PubMed] [Google Scholar]
- 25.Vanita V, Singh JR, Singh D, Varon R, Sperling K. A novel mutation in GJA8 associated with jellyfish-like cataract in a family of Indian origin. Mol Vis. 2008;14:323–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Arora A, Minogue PJ, Liu X, Addison PK, Russel-Eggitt I, Webster AR, Hunt DM, Ebihara L, Beyer EC, Berthoud VM, Moore AT. A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts. J Med Genet. 2008;45:155–60. doi: 10.1136/jmg.2007.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Liu NN, Lei CT, Fan YC, Liu XQ, Yang Y, Wang JF, Liu B, Yang ZL. A novel GJA8 mutation in a Chinese family with autosomal dominant congenital cataract. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25:59–62. [PubMed] [Google Scholar]
- 28.Berry V, Mackay D, Khaliq S, Francis PJ, Hameed A, Anwar K, Mehdi SQ, Newbold RJ, Ionides A, Shiels A, Moore T, Bhattacharya SS. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum Genet. 1999;105:168–70. doi: 10.1007/s004399900094. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z, Zheng J, Yang F, Ji J, Li X, Tang X, Yuan X, Zhang X, Sun H. Two novel mutations of connexin genes in Chinese families with autosomal dominant congenital nuclear cataract. Br J Ophthalmol. 2005;89:1535–7. doi: 10.1136/bjo.2005.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen L, Yao W, Eiberg H, Kjaer KW, Baggesen K, Hejtmancik JF, Rosenberg T. Genetic heterogeneity in microcornea-cataract: five novel mutations in CRYAA, CRYGD, and GJA8. Invest Ophthalmol Vis Sci. 2007;48:3937–44. doi: 10.1167/iovs.07-0013. [DOI] [PubMed] [Google Scholar]
- 31.Ponnam SPG, Ramesha K, Tejwani S, Ramamurthy B, Kannabiran C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet. 2007;44:e85. doi: 10.1136/jmg.2007.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polyakov AV, Shagina IA, Khlebnikova OV, Evgrafov OV. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin Genet. 2001;60:476–8. doi: 10.1034/j.1399-0004.2001.600614.x. [DOI] [PubMed] [Google Scholar]
- 33.Yan M, Xiong C, Ye SQ, Chen Y, Ke M, Zheng F, Zhou X. A novel connexin 50 (GJA8) mutation in a Chinese family with a dominant congenital pulverulent nuclear cataract. Mol Vis. 2008;14:418–24. [PMC free article] [PubMed] [Google Scholar]




