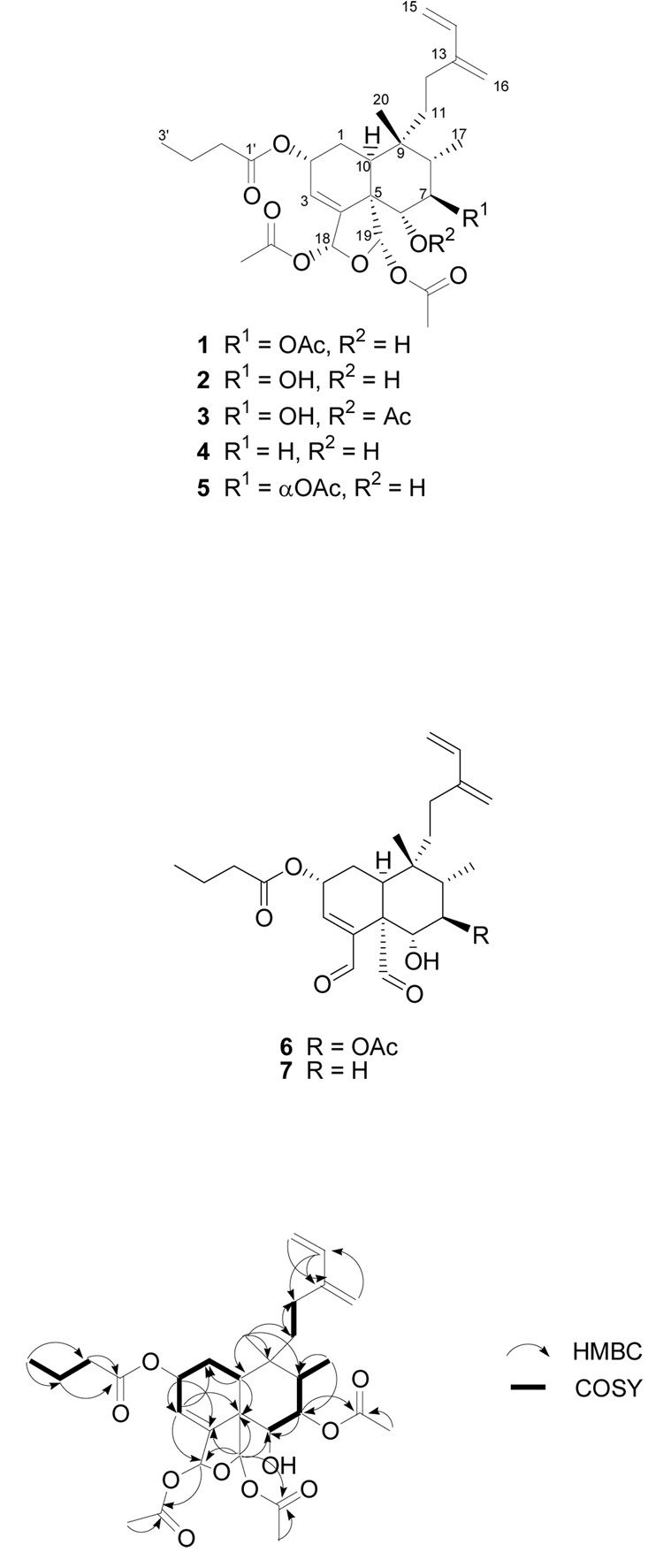
Bioassay-guided fractionation of the cytotoxic leaf and flower extract of Casearia nigrescens led to the isolation of four new clerodane diterpenoids, designated caseanigrescens A–D (1–4). These compounds were subject to hydrolysis to dialdehydes when stored in CDCl3. The structures of compounds 1–4 were determined using 1D and 2D NMR spectroscopy. All four compounds showed moderate cytotoxicity to the A2780 human ovarian cancer cell line, with an IC50 range of 0.83–1.4 µM.
In our continuing search for bioactive molecules from the rainforests of Madagascar as part of an International Cooperative Biodiversity Group (ICBG) program,2 an extract of the leaves and flowers of Casearia nigrescens Tul. (Flacourtiaceae) showed moderate cytotoxicity against the A2780 human ovarian cancer cell line. This extract was selected for fractionation based on its cytotoxicity and the lack of phytochemical data for this species. The crude extract was purified by liquid–liquid partition, reversed phase chromatography, and reversed phase HPLC to yield the four new clerodane diterpenoids 1–4, designated caseanigrescens A–D. These compounds were subject to hydrolysis to dialdehydes when stored in CDCl3.
The genus Casearia is a member of the Saliciaceae. It consists of about 180 species and occurs throughout the tropics. Five species are found in Madagascar.3,4 The Flacourtiaceae family, and Casearia in particular, are well known for the production of clerodane diterpenoids.5–8 Such compounds have been reported to possess a wide range of biological activities including cytotoxic, antimalarial, antimycobacterial, DNA-damaging, trypanocidal, immunosuppressive, antifungal, and apoptosis inducing activity.5–7, 9–15 C. nigrescens is known locally as Hazondrano.
Results and Discussion
Compound 1 was isolated as a colorless amorphous solid, with a molecular formula of C30H42O10 as determined by HRFABMS. The 1H and 13C NMR data (Table 1) indicated that it was a clerodane diterpenoid analogue. Correlations obtained from HMBC and COSY experiments were used to establish the 2-D structure (Figure 1). The assignment of the 2D structure was straightforward with the exception of the butyrate group, which did not correlate to any of the oxymethine signals in the HMBC experiment. However, as all of the acetates had been accounted for via HMBC correlations, only two possibilities remained, the C-2 oxymethine that resonated at δC 66.4 (δH 5.47) and the C-6 oxymethine that resonated at δC 75.0 (δH 3.65). Based on the chemical shifts in the 1H NMR spectrum it was clear that the butyrate group was located at the C-2 position.
Table 1.
1H and 13C NMR data for caseanigrescen A (1), B (2), and C (3).
| Caseanigrescen A (1)c |
Caseanigrescen B (2)c |
Caseanigrescen C (3)c |
||||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.62a | 27.1 | 1.84a | 27.2 | 1.72a | 27.1 |
| 1.77, m | ||||||
| 2 | 5.47, m | 66.4 | 5.53, brs | 66.5 | 5.47, m | 66.2 |
| 3 | 6.10, d (3.7) | 121.9 | 6.15, d (2.8) | 122.0 | 6.02, dd (1.4, 3.9) | 123.6 |
| 4 | 145.7 | 145.4b | 144.0 | |||
| 5 | 54.3 | 53.5 | 53.1 | |||
| 6 | 3.65, d (10.7) | 75.0 | 3.64, m | 77.1 | 5.13, d (10.6) | 77.0 |
| 7 | 5.35, dd (10.7, 11.0) | 75.6 | 3.55, m | 72.8 | 3.11, dd (10.6, 11.0) | 71.2 |
| 8 | 1.64a | 41.9 | 1.70a | 43.3 | 1.46a | 43.2 |
| 9 | 39.1 | 38.8 | 38.6 | |||
| 10 | 2.46, dd (3.5, 13.8) | 36.4 | 2.46, dd (5.6, 11.6) | 36.5 | 2.38, dd (6.3, 10.9) | 37.3 |
| 11 | 1.49a | 29.2 | 1.37a | 29.5 | 1.14, ddd (3.0, 12.8, 12.8) | 29.3 |
| 1.64a | 1.69a | 1.48a | ||||
| 12 | 2.04, m | 24.2 | 2.08, m | 24.3 | 2.01, m | 24.2 |
| 2.17, m | 2.19, m | 2.12, m | ||||
| 13 | 144.8 | 145.3b | 145.3 | |||
| 14 | 6.34, dd (10.8, 17.7) | 140.9 | 6.37, dd (11, 17.4) | 140.9 | 6.34, dd (10.9, 17.7) | 140.7 |
| 15 | 4.86, d (10.8) | 112.0 | 4.88, d (11) | 112.1 | 4.87, d (10.9) | 112.3 |
| 5.07, d (17.7) | 5.10, d (17.4) | 5.07, d (17.7) | ||||
| 16 | 5.00, brs | 116.6 | 5.09, s | 116.2 | 5.02, s | 115.9 |
| 5.23, s | 5.05, s | |||||
| 17 | 0.83, d (6.7) | 11.3 | 1.12, d (6.4) | 11.3 | 0.85, d (6.7) | 11.2 |
| 18 | 7.15a | 96.1 | 7.11, s | 96.2 | 6.79, dd (1.6, 1.6) | 95.1 |
| 19 | 6.98, s | 98.7 | 6.76, s | 99.0 | 6.69, s | 98.6 |
| 20 | 0.67, s | 25.6 | 0.76, s | 25.7 | 0.64, s | 25.7 |
| 1′ | 172.5 | 172.4 | 172.3 | |||
| 2′ | 1.89, m | 36.2 | 1.88, m | 36.2 | 1.85, m | 36.1 |
| 3′ | 1.46, m | 18.8 | 1.45, m | 18.8 | 1.43, m | 18.7 |
| 4′ | 0.73, t (7.4) | 13.5 | 0.72, t (7.4) | 13.5 | 0.70, t (7.3) | 13.5 |
| 6-O2CCH3 | 170.6 | |||||
| 6-O2CCH3 | 1.92, s | 20.9 | ||||
| 7-O2CCH3 | 171.2 | |||||
| 7-O2CCH3 | 1.93, s | 20.8 | ||||
| 18-O2CCH3 | 169.7 | 170.3 | 169.6 | |||
| 18-O2CCH3 | 1.63, s | 20.8 | 1.64, s | 20.7 | 1.53, s | 20.6 |
| 19-O2CCH3 | 168.9 | 169.2 | 168.9 | |||
| 19-O2CCH3 | 1.72, s | 21.4 | 1.70, s | 21.4 | 1.68, s | 21.5 |
Signals were in overlapped regions of the spectrum.
Signals are interchangeable.
Spectra collected in C6D6.
Figure 1.
Selected HMBC correlations observed for 1.
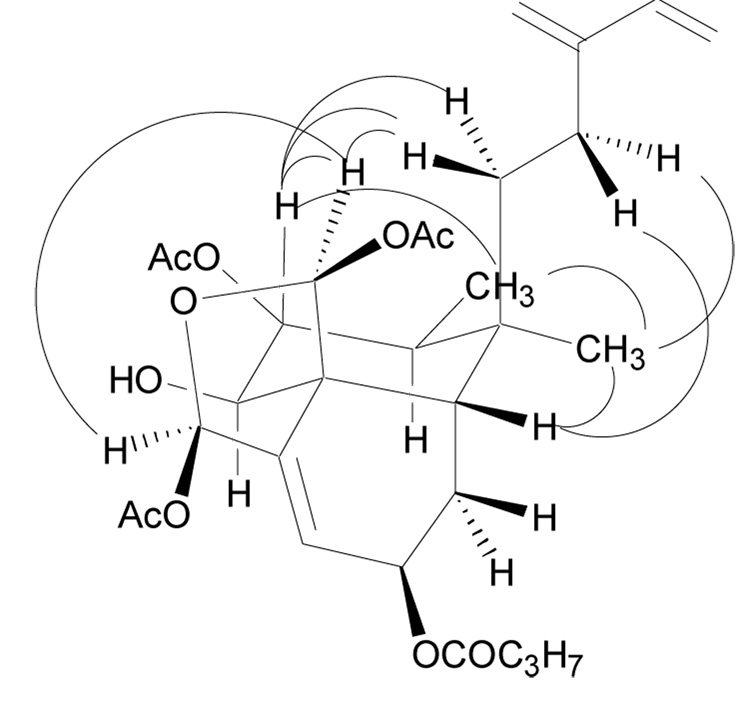
The relative configuration of 1 was determined using coupling constants and NOE data (Figure 2) and was supported by comparison of the optical rotation to literature values for similar compounds. A NOE enhancement from the C-19 proton to the C-18 proton indicated that the acetate groups attached to the hemiacetal ring were in a cis configuration. Two 1,3-diaxial NOE enhancements of the C-7 proton and one of the C-11 protons (δH 1.49) were also observed when the C-19 proton was irradiated. Thus, a chair conformation with the C-19 and C-11 groups in axial positions and the C-7 acetate in an equatorial position was indicated for the B-ring. A NOE enhancement of the C-17 protons when the C-7 proton was irradiated indicated that the C-17 methyl group was equatorial. No enhancement of was observed for the C-6 proton indicating that it was axial and trans from the C-7 proton and the C-19 group. This was supported by the observed coupling constants of the C-6 proton (δH 3.65, d, J = 10.7 Hz) and the C-7 proton (δH 5.35, dd, 10.7, 11.0 Hz), both of which indicated 1,2-diaxial couplings. Upon irradiation of the C-10 proton an enhancement was observed for the protons of the C-12 and C-20 groups, which indicated that the C-10 proton was equatorial and that the rings were cis fused. The final position to be assigned was the C-2 position. The lack of any significant NOE enhancements when the C-2 proton was irradiated suggested that the C-2 proton was equatorial. This was confirmed by an examination of its chemical shift (δC 66.4); a chemical shift of approximately δC 66.0 indicates that the C-2 ester group is of the same relative configuration as C-11, C-17, and C-19, but a chemical shift of approximately δC 70.0 indicates that C-2 is of the opposite configuration.3,9 The specific rotation of 1 was + 59.6, which was consistent with values for previously published compounds of similar structure. The relative configuration of 1 has been depicted in the same configuration as similar compounds whose absolute configuration has been determined.7,9,16 Compound 1 is a new clerodane diterpenoid and has been given the trivial name caseanigrescen A.
Figure 2.
Selected NOE enhancements observed for 1.
A recent paper reported the structure of caseamembrin N (5), which differed from 1 only in the configuration of C-7.17 The 1H and 13C NMR data for 1 (CDCl3) are very similar to those reported for caseamembrin N, but the two compounds differ significantly in their specific rotations, with that for caseamembrin N reported as + 9.8.
Compound 2 was isolated as a colorless amorphous solid, with a molecular formula of C28H40O9 as determined by HRFABMS. The 1H and 13C NMR data (Table 1) for 2 were similar to those of 1 and indicated that it was the C-7 deacetate of 1. This assignment was confirmed by analysis of the 2D NMR spectra of 2. Compound 2 is a new clerodane diterpenoid and has been given the trivial name caseanigrescen B.
Compound 3 was isolated as a colorless amorphous solid. Its HRFABMS indicated a molecular formula of C30H42O10, which was the same as that of 1. The 1H and 13C NMR data (Table 1) for 3 were virtually identical to those of 1. The only significant differences were that in the 1H NMR spectrum of 3 the oxymethine doublet (J = 10.6 Hz) resonated at δH 5.13 and the oxymethine doublet of doublets (J = 10.6, 11.0 Hz) resonated at δH 3.11. Based on HMBC and COSY experiments the doublet at δH 5.13 was assigned to the C-6 oxymethine and the doublet of doublets at δH 3.11 to the C-7 oxymethine. These values were reversed from the corresponding values for 1, which were δH 3.65 and 5.35, respectively, and indicated that the acetate was located on the C-6 hydroxyl group in 3. Compound 3 is a new clerodane diterpenoid and has been given the trivial name caseanigrescen C.
Compound 4 was isolated as a colorless amorphous solid. Its HRFABMS indicated a molecular formula of C28H40O8. Its 1H and 13C NMR spectra (Table 2) indicated that it had one less acetate than 1 and 3, and its MS indicated that it also contained one less oxygen than 2. Because the remaining oxymethine (δH 3.41, dd, J = 3.7, 11.9 Hz) was a doublet of doublets it must be adjacent to two protons, indicating that 4 is the C-7 deoxy analogue of compound 2. This assignment was confirmed by HMBC and COSY experiments. Thus, 4 is a new compound and has been given the trivial name caseanigrescen D.
Table 2.
1H and 13C NMR data for caseanigrescen D (4), caseanigrescen A-2 (6), and caseanigrescen D-2 (7).
| Caseanigrescen D (4)b |
Caseanigrescen A-2 (6)c |
Caseanigrescen D-2 (7)c,d |
||||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.50a | 27.1 | 1.65-2.00e | 25.8 | 1.92a | 25.8 |
| 1.73a | ||||||
| 2 | 5.45, m | 66.5 | 5.55, m | 64.5 | 5.52, m | 64.8 |
| 3 | 6.04, d (4.1) | 121.4 | 7.04, d (4.5) | 147.3 | 7.05, d (5.1) | 148.6 |
| 4 | 146.4 | 147.1 | 148.5 | |||
| 5 | 54.1 | 56.4 | 55.9 | |||
| 6 | 3.41, dd (3.7, 11.9) | 72.8 | 3.71, d (9.8) | 74.8 | 3.86, dd (6.6, 10.5) | 71.8 |
| 7 | 1.10, m | 36.8 | 5.36, dd (9.8, 11.5) | 75.6 | 1.87a | 36.7 |
| 1.20a | ||||||
| 8 | 1.23 | 36.8 | 38.8 | 1.73a | 35.4 | |
| 9 | 37.4 | 39.4 | 37.8 | |||
| 10 | 2.36, dd (3.4, 13.7) | 37.1 | 2.12, dd (3.5, 13.5) | 40.3 | 2.34a | 40.5 |
| 11 | 1.23a | 28.3 | 1.19, m | 33.5 | 1.14, m | 32.4 |
| 1.35, ddd (4.5, 12.9, 12.9) | 1.63, m | 1.50, ddd (3.6, 12.8, 14.7) | ||||
| 12 | 2.03, m | 24.2 | 1.65-2.00e | 23.6 | 1.85a | 23.4 |
| 2.16, m | 1.98, ddd (3.7, 13.4, 13.4) | |||||
| 13 | 145.6 | 145.7 | 146.3 | |||
| 14 | 6.36, dd (10.9, 17.5) | 140.8 | 6.31, dd (11.0, 17.5) | 138.8 | 6.32, dd (10.8, 17.5) | 138.9 |
| 15 | 4.89, d (10.9) | 112.2 | 5.02, d (11.0) | 112.9 | 5.02, d (10.8) | 112.9 |
| 5.11, d (17.5) | 5.15, d (17.5) | 5.17, d (17.5) | ||||
| 16 | 5.06, s | 115.9 | 4.99, brs | 116.5 | 4.97, s | 116.2 |
| 5.08, s | 4.99, s | |||||
| 17 | 0.60, d (6.7) | 15.6 | 0.86, d (7.0) | 10.7 | 0.89, d (6.7) | 15.2 |
| 18 | 7.15a | 96.1 | 9.40, s | 194.7 | 9.41, s | 196.4 |
| 19 | 6.61, s | 98.3 | 10.24, s | 200.4 | 10.30, s | 202.0 |
| 20 | 0.63, s | 25.2 | 1.00, s | 26.4 | 0.97, s | 26.0 |
| 1′ | 172.4 | 172.8 | 172.9 | |||
| 2′ | 1.89, m | 36.2 | 2.35, t (7.0) | 36.1 | 2.35, t (7.5) | 36.2 |
| 3′ | 1.46, m | 18.8 | 1.67a | 18.5 | 1.68a | 18.5 |
| 4′ | 0.73, t (7.3) | 13.5 | 0.97, t (7.5) | 13.6 | 0.97, t (7.4) | 13.7 |
| 7-O2CCH3 | 171.2 | |||||
| 7-O2CCH3 | 2.09, s | 21.0 | ||||
| 18-O2CCH3 | 169.6 | |||||
| 18-O2CCH3 | 1.57, s | 20.7 | ||||
| 19-O2CCH3 | 169.1 | |||||
| 19-O2CCH3 | 1.72, s | 21.5 | ||||
Signals were in overlapped regions of the spectrum.
Spectra collected in C6D6.
Spectra collected in CDCl3.
Assignments based on chemical shift and comparison to other fully elucidated compounds.
Signals were not assignable due to the presence of multiple signals in the region.
During the course of NMR analysis compounds 1 – 4 were stored in CDCl3 for varying periods of time, and it was noted that all four compounds slowly hydrolyzed to their corresponding dialdehydes, as indicated by the disappearance of the hemiacetal resonances and the appearance of resonances for aldehydes.8 These dialdehydes were not stable, but partial NMR data could be obtained for two of the compounds. The hydrolysis was presumably due to the presence of traces of acid in the CDCl3 used on this occasion, a conclusion supported by the fact that hydrolysis did not occur when the compounds were allowed to stand in a fresh sample of CDCl3.
The hydrolysis product of 1 (6) was a colorless amorphous solid. HRFABMS indicated a molecular formula of C26H36O7 (m/z 461.2575 [M + H]+), which was consistent with the loss of the two hemiacetal acetates and the formation of the dialdehyde from 1. This conclusion was supported by both 1H and 13C NMR data (Table 2). This hydrolysis product has not previously been reported and has been given the trivial name caseanigrescen A−2 (6).
The hydrolysis product of 4 was a colorless amorphous solid. No MS data was obtained prior to its decomposition. However, 1H and 13C NMR as well as HMBC and COSY experiments all confirmed that the hydrolysis product of 4 was the dialdehyde 7. This hydrolysis product has not previously been reported and has been given the trivial name caseanigrescen D−2 (7).
The remaining two hydrolysis products were not stable enough to provide any spectroscopic data beyond 1H NMR spectra, but they are presumably the dialdehydes corresponding to 2 and 3.
Caseanigrescen A–D (1–4) and the two hydrolysis products (6 and 7) were found to exhibit good activity against the A2780 human ovarian cancer cell line, with IC50 values of 1.4, 0.83, 1.0, and 1.0 µM, respectively for the natural products, and IC50 values of 3.5 and 1.5 µM, respectively for the hydrolysis products.
Experimental Section
General Experimental Procedures
Optical rotations were recorded on a Perkin-Elmer 241 polarimeter. IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. NMR spectra were obtained on a JEOL Eclipse 500 or a Varian Inova 400 spectrometer in C6D6 or CDCl3. Mass spectra were obtained on a JEOL JMS-HX-110 instrument. The chemical shifts are given in δ (ppm) with the residual C6D6 solvent peak referenced to δH 7.15 and δC 128.0, and the residual CDCl3 solvent peak referenced to δH 7.24 and δC 77.0 as the internal reference; coupling constants are reported in Hz. HPLC was performed on a Shimadzu LC-10AT or an LC-8A instrument with a C18 Varian Dynamax column (5 µm, 100 Å, 250 × 10 mm, or 8 µm, 100 Å, 250 × 21.4 mm, respectively).
Plant Material
The leaves and flowers of Casearia nigrescens were collected in the Zahamena region of Madagascar, in the province of Toamasina, 3 km northeast of Nosivola (17°41′01″S, 48°38′28″) under the vernacular name Hazondrano in November, 2001. It was originally identified as an Erythroxylum sp. but was later re-identified as C. nigrescens Tul. (Saliciaceae) by G. McPherson (MO), 2003, and C. Birkinshaw (MO), 2004. Duplicates of the voucher specimen (Ratovoson.F 589) were deposited at the Missouri Botanical Garden, the Muséum National d’Histoire Naturelle, Paris, the Département des Recherches Forestières et Pisicoles, Madagascar, and the Centre National d’Application des Recherches Pharmaceutique, Madagascar. The shrub had a height of 3 m and was collected in a secondary humid forest at an altitude of 900 m.
Extract Preparation
Dried leaves and flowers of C. nigrescens (305 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 h at rt at the Centre National d’Applications des Recherches Pharmaceutique and evaporated to give the crude extract MG 1071 (19.6 g). A portion of the extract was shipped to VPISU for bioassay and isolation chemistry.
Bioassay Guided Fractionation and Isolation of Compounds
The crude extract (820 mg) was partitioned between hexane and 90% aqueous MeOH. The MeOH fraction was adjusted to 50% aqueous MeOH and further extracted with CH2Cl2. The CH2Cl2 fraction (170 mg) was the most active and was further fractionated using a reversed phase C-18 SPE tube (1 g) eluted with 80% aq. MeOH and 100% MeOH, to generate two fractions. The 80% aq. MeOH fraction retained the activity in a preliminary fractionation, so it was further fractionated using reversed phase C-18 HPLC and elution with 65% aq. MeCN to yield HPLC fractions 1 and 6 along with compounds 2 (2.8 mg, tR 15 min), 3 (6.5 mg, tR 17.5 min), 1 (10.9 mg, tR 22 min), and 4 (3.8 mg, tR 26 min). Compounds 1–4 were responsible for the activity.
Cytotoxicity Bioassays
The A2780 human ovarian cancer cell line assay was performed at Virginia Polytechnic Institute and State University as previously reported.18 Actinomycin D was used as a positive control; it had IC50 values of 8–24 × 10−4 µM under the same conditions.
Caseanigrescen A (1)
colorless amorphous solid; [α]D30 + 59.6° (c 0.77, MeOH); UV (MeOH) λmax (log ε) 223 (4.22) nm; IR vmax 3480 br, 2968, 2930, 2880, 1729, 1597 cm−1; 1H and 13C NMR, see Table 1; HRFABMS m/z 585.2668 [M + Na]+ (calcd for C30H42O10Na, 585.2676).
Caseanigrescen B (2)
colorless amorphous solid; [α]D30 + 42.9° (c 0.21, MeOH); UV (MeOH) λmax (log ε) 223 (4.21) nm; IR vmax 3396 br, 2966, 2923, 2878, 1754, 1734, 1597 cm−1; 1H and 13C NMR, see Table 1; HRFABMS m/z 543.2547 [M + Na]+ (calcd for C28H40O9Na, 543.2570).
Caseanigrescen C (3)
colorless amorphous solid; [α]D30 + 45.3° (c 0.57, MeOH); UV (MeOH) λmax (log ε) 223 (4.28) nm; IR vmax 3450 br, 2969, 2933, 2880, 1730, 1596 cm−1; 1H and 13C NMR, see Table 1; HRFABMS m/z 585.2728 [M + Na]+ (calcd for C30H42O10Na, 585.2676).
Caseanigrescen D (4)
colorless amorphous solid; [α]D30 + 33.2° (c 0.31, MeOH); UV (MeOH) λmax (log ε) 223 (4.16) nm; IR vmax 3480 br, 2964, 2929, 2878, 1750, 1729, 1596 cm−1; 1H and 13C NMR, see Table 2; HRFABMS m/z 527.2595 [M + Na]+ (calcd for C28H40O8Na, 527.2621).
Caseanigrescen A-2 (6)
colorless amorphous solid; [α]D30 + 74.0° (c 0.50, MeOH); IR vmax 3430, 2967, 2930, 2883, 1726, 1596 cm−1; 1H and 13C NMR, see Table 2; HRFABMS m/z 461.2575 [M + H]+ (calcd for C26H37O7, 461.2539).
Caseanigrescen D-2 (7)
colorless amorphous solid; 1H and 13C NMR, see Table 2.
Supplementary Material
Acknowledgment
This project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 with the International Cooperative Biodiversity Groups, and this support is gratefully acknowledged. We thank Mr. B. Bebout for obtaining the mass spectra and Mr. T. Glass for assistance with the NMR spectra. Field work essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications et des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
Footnotes
Supporting Information Available: 1H and 13C NMR spectra for compounds 1–4 and 7; 1H NMR spectrum of compound 6. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 23. For Part 22, seeCao S, Ranarivelo L, Ratsimbason M, Randrianasolo S, Ratovoson F, Andrianjafy M, Kingston DGI. Planta Med. doi: 10.1055/s-2006-951729. in press (DOI 10.1055/s-2006-951729)
- 2.a Reynolds M, Chaturvedula VSP, Ratovoson F, Andriantsiferana R, Rasamison VE, Guza RC, Kingston DGI. Nat. Prod. Res. 2006;20:606–610. doi: 10.1080/14786410500249315. [DOI] [PubMed] [Google Scholar]; b Chaturvedula VSP, Norris A, Miller JS, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2006;69:287–289. doi: 10.1021/np050376w. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cao S, Radwan MM, Norris A, Miller JS, Ratovoson F, Andrianjafy M, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2006;69:284–286. doi: 10.1021/np050351x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Williams R, Norris A, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI. Planta Med. 2006:564–566. doi: 10.1055/s-2006-931554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y-C, Wang C-H, Cheng Y-B, Wang L-T, Guh J-H, Chien C-T, Khalil AT. J. Nat. Prod. 2004;67:316–321. doi: 10.1021/np0303658. [DOI] [PubMed] [Google Scholar]
- 4.Prakash CVS, Hoch JM, Kingston DGI. J. Nat. Prod. 2002;65:100–107. doi: 10.1021/np010405c. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y-C, Wang C-H, Cheng Y-B, Wang L-T, Guh J-H, Chien C-T, Khalil AT. J. Nat. Prod. 2004;67:316–321. doi: 10.1021/np0303658. [DOI] [PubMed] [Google Scholar]
- 6.De Carvalho PRF, Furlan M, Young MCM, Kingston DGI, Bolzani VDS. Phytochemistry. 1998;49:1659–1662. doi: 10.1016/s0031-9422(98)00249-0. [DOI] [PubMed] [Google Scholar]
- 7.Beutler JA, McCall KL, Herbert K, Herald DL, Pettit GR, Johnson T, Shoemaker RH, Boyd MR. J. Nat. Prod. 2000;63:657–661. doi: 10.1021/np990553r. [DOI] [PubMed] [Google Scholar]
- 8.Chen T-B, Wiemer DF. J. Nat. Prod. 1991;54:1612–1618. [Google Scholar]
- 9.Kanokmedhakul S, Kanokmedhakul K, Kanarsa T, Buayairaksa M. J. Nat. Prod. 2005;68:183–188. doi: 10.1021/np049757k. [DOI] [PubMed] [Google Scholar]
- 10.Espindola LS, Vasconcelos JR, Jr, de Mesquita ML, Marquié P, de Paula JE, Mambu L, Santana JM. Planta Med. 2004;70:1093–1095. doi: 10.1055/s-2004-832655. [DOI] [PubMed] [Google Scholar]
- 11.Hunter MS, Corley DG, Carron CP, Rowold E, Kilpatrick BF, Durley RC. J. Nat. Prod. 1997;60:894–899. doi: 10.1021/np970141n. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y-C, Wang L-T, Wang C-H, Khalil AT, Guh J-H. Chem. Pharm. Bull. 2004;52:108–110. doi: 10.1248/cpb.52.108. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y-C, Lee CL, Khalil AT, Cheng Y-B, Chien C-T, Kuo Y-H. Helv. Chem. Act. 2005;88:68–77. [Google Scholar]
- 14.Oberlies NH, Burgess JP, Navarro HA, Pinos RE, Fairchild CR, Peterson RW, Soejarto DD, Farnsworth NR, Kinghorn AD, Wani MC, Wall ME. J. Nat. Prod. 2002;65:95–99. doi: 10.1021/np010459m. [DOI] [PubMed] [Google Scholar]
- 15.Huang D-M, Shen Y-C, Wu C, Huang Y-T, Kung F-L, Teng C-M, Guh J-H. Eur. J. Pharmacol. 2004;503:17–24. doi: 10.1016/j.ejphar.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Jullian V, Bonduelle C, Valentin A, Acebey L, Duigou A-G, Prévost M-F, Sauvain M. Bioorg. Med. Chem. Lett. 2005;15:5065–5070. doi: 10.1016/j.bmcl.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y-C, Cheng Y-B, Ahmed AF, Lee CL, Chen S-Y, Chien C-T, Kuo Y-H, Tzeng G-L. J. Nat. Prod. 2005;68:1665–1668. doi: 10.1021/np058063o. [DOI] [PubMed] [Google Scholar]
- 18.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.