Abstract
Fatvg is a localized maternal transcript that translocates to the vegetal cortex of Xenopus laevis oocytes through both the METRO and Late RNA localization pathways. It is a member of a gene family that functions in vesicular trafficking. Depletion of the maternal store of fatvg mRNA results in a dual phenotype in which embryos are ventralized and also lack primordial germ cells. This complex fatvg loss of function phenotype is the result of stabilization of the dorsalizing factor β-catenin at the vegetal pole and the inability of the germ cell determinants to move to their proper locations. This is coincident with the inhibition of cortical rotation and the abnormal aggregation of the germ plasm. Fatvg protein is located at the periphery of vesicles in the oocyte and embryo, supporting its proposed role in vesicular trafficking in the embryo. These results point to a common fundamental mechanism that is regulated by fatvg through which germ cell determinants and dorsalizing factors segregate during early development.
Keywords: Xenopus, oocytes, vegetally localized RNA, axis specification, primordial germ cell formation, antisense oligonucleotide depletion, host transfer
Introduction
An important strategy used by a variety of organisms to initiate development is to synthesize large amounts of maternal gene products. These gene products not only provide the energy source and building blocks for rapid cell growth and division but, more importantly, they also serve as the maternal cues for setting up regional- and cell lineage-specific identities. Many of these maternal gene products are deposited as localized RNA transcripts in specific regions of oocyte, presumably to allow an efficient production of high levels of newly translated proteins (Kloc et al., 2002).
Localized maternal gene products in Xenopus play an important role in early embryogenesis. Numerous studies clearly demonstrated the presence of localized maternal determinants required for dorsal/ventral patterning (Elinson and Pasceri, 1989; Fujisue et al., 1993; Holowacz and Elinson, 1993; Kofron et al., 2007; Tao et al. 2005). The vegetal cytoplasm of oocytes or unfertilized eggs has the ability to induce the formation of a secondary axis when transplanted to a different embryo. Such dorsalizing activity is sensitive to UV irradiation. Embryos deriving from the oocytes that have been exposed to UV irradiation vegetally are deficient in dorso-anterior development, and the vegetal cytoplasm can no longer induce secondary axis when transplanted. The dorsal determinants function as part of a complex system requiring the transport of a group of molecules during cortical rotation toward the future dorsal side of the embryo. These include molecules such as disheveled and GSK-3 binding protein (GBP) that are transported via the parallel array of microtubules set-up after fertilization (Domingez and Green, 2001; Farr et al., 2001; Miller et al., 1999; Weaver et al., 2003; Yost et al., 1998). The mechanism by which these components are transported is still unclear, but it involves the movement of numerous small vesicles as well as the protein, kinesin light chain (KLC) (Weaver et al., 2003), a component of the microtubule motor kinesin.
Maternal transcripts localized to the vegetal region of the oocytes are required for the establishment of the primary germ layers. The mRNA transcripts of Vg1 (Rebagliati et al., 1985; Weeks and Melton, 1987) or VegT (Horb and Thomsen, 1997; Lustig et al., 1996; Stennard et al., 1996; Zhang and King, 1996;) are localized to a broad region in the vegetal hemisphere of the oocyte, following what is known as the Late pathway of RNA localization (Kloc and Etkin, 1995). Vg1 belongs to the TGF-β growth factor family. By the overexpression of a dominant negative Vg1 mutant in embryos, it has been shown that the maternal Vg1 protein is required for normal development of the endoderm and the dorsal mesoderm (Joseph and Melton, 1998; Birsoy et al. 2006). The depletion of the VegT transcripts in oocytes has demonstrated that the maternally expressed T-box transcription factor VegT plays a role in the establishment of the primary germ layers (Zhang et al., 1998). Embryos depleted of VegT are deficient in the expression of mesodermal and endodermal genes. During normal development, maternal VegT activates the expression of zygotic growth factors required for mesoderm induction (Kofron et al., 1999), and furthermore, VegT regulates endodermal gene expression to regulate normal development of the endodermal germ layer (Xanthos et al., 2001).
The vegetal cytoplasm also contains maternal determinants to specify the germ cell lineage in Anuran amphibians. In Xenopus, either exposure of the vegetal egg surface to UV irradiation (Holwill et al., 1987; Ijiri, 1977; Zust and Dixon, 1975) or removal of the vegetal cytoplasm (Buehr and Blackler, 1970) caused a reduction in the number of germ cells formed. The effects of UV irradiation in Rana can be reversed by the transfer of vegetal cytoplasm from an unirradiated fertilized egg (Smith, 1966). The possible candidates of the molecular components of the germ cell determinants are among a group of vegetally localized RNAs (Houston and King, 2000b), which follow the METRO pathway of RNA localization and occupy a more restricted area of the vegetal cortex than do the Late pathway RNAs. In previtellogenic oocytes, the early RNAs are localized to the mitochondrial cloud, which is enriched in mitochondria and electron dense germinal granules (al-Mukhtar and Webb, 1971; Coggins, 1973; Heasman et al., 1984). In embryos, the RNAs are associated with the germ plasm, a yolk-free area of the vegetal cytoplasm that also contains electron dense germinal granules (Blackler, 1958; Czolowska, 1969; Czolowska, 1972). The localization of METRO RNAs to the mitochondrial cloud and the germ plasm suggests a possible role of these RNAs in regulating germ cell formation (Kloc and Etkin, 1995). By electron microscopic analysis, Xcat2 mRNA has been shown to directly associate with the germinal granules (Kloc et al., 1998), and is likely to be a cell fate-specific determinant required for specifying the germ cell lineage. Houston and King, (2000a) showed by the depletion study of Xdazl, another METRO RNA, that maternally expressed Xdazl is required not for early germ plasm aggregation or formation, but specifically for a later migration step of the primordial germ cells when the cells undergo differentiation in the tadpole endoderm.
We previously reported the identification of fatvg, an mRNA that is localized at the vegetal cortex through both the METRO and Late pathways in Xenopus oocytes (Chan et al., 1999). Thus, a fraction of fatvg mRNA that localizes through the METRO pathway is associated with the germ plasm, whereas that fraction localizing through the Late pathway is associated with the entire vegetal cortex (Chan et al.2001). In the present study, we analyzed the function of the localized fatvg transcripts by depleting the maternal pool of fatvg mRNA using antisense oligodeoxynucleotides (ODN). Our findings suggest that maternally expressed fatvg plays a dual role in specifying both the body axis and the germ cell lineage. Our data also show that this dual effect occurs through the inability of maternal dorsalizing factors and germ cell determinants to segregate properly in fatvg-depleted embryos. This is the first example of a coding RNA playing an important role in both the specification of germ cell lineage and the dorsal/ventral axis.
Results
Depletion of maternal fatvg mRNA by antisense oligo injection
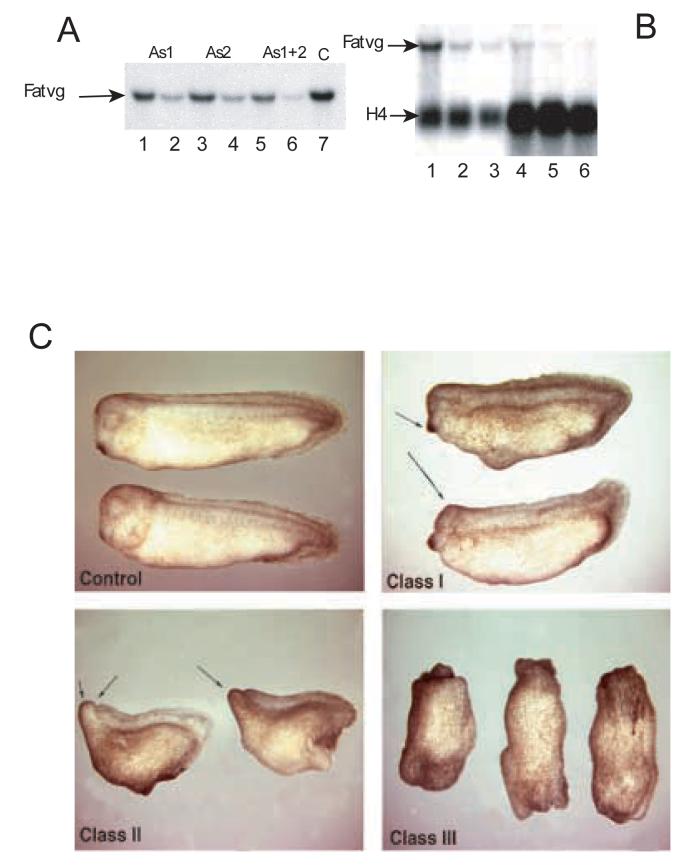
To determine the function of fatvg during early development, we depleted the maternal store of fatvg mRNA using antisense ODNs. Four antisense ODNs with phosphorothioate modification were injected separately into defolliculated stage VI oocytes, to select for those that can deplete the maternal pool of fatvg transcript. Injection of either fv-as1 or fv-as2 resulted in the partial depletion of the fatvg transcripts (Figure 1A). Figure 1B shows that fatvg mRNA is not re-expressed zygotically at the gastrula stage (stage 10) in depleted and control embryos, indicating that the depletion of the maternal mRNA would have an effect throughout early development.
Figure 1.
Northern blot analysis of antisense oligo-injected oocytes. (A) The level of endogenous fatvg mRNA was analyzed after oocytes were injected, singly or with a mixture of two antisense ODNs, in two doses. Lane 1, 2.5 ng of fatvg antisense 1 (fatvgAS1); Lane 2, 5ng fatvgAS1; Lane 3, 2.5 ng fatvgAS2; Lane 4, 5ng fatvgAS2; Lane 5, total 2.5ng fatvgAS1 and 2; Lane 6, total 5ng fatvgAS1 and 2; Lane 7 Control. (B) Oocytes and gastrula stage embryos derived from oocytes injected with a total of 2.5 ng fatvg antisense ODN were analyzed by Northern analysis. Lane 1, levels of fatvg mRNA in uninjected stage VI oocytes; Lane 2 levels of fatvg mRNA in fatvgAS1 injected oocytes; Lane 3, level of fatvg mRNA in fatvgAS1 and2 injected oocytes; Lane 4, Level of fatvg mRNA in uninjected stage 10 embryos; Lane 5, Levels of fatvg mRNA in fatvgAS 1 injected embryos at stage 10; Lane 6, Levels of fatvg mRNA in fatvgAS1 and 2 injected embryos at stage 10. The level of fatvg mRNA in embryos derived from depleted oocytes did not increase, indicating that there was no detectable zygotic transcription of fatvg. The histone gene H4 was used as a loading control and was zygotically activated.
Fatvg-depleted embryos display axial defects. (C) Axis formation in embryos derived from uninjected oocytes is normal. Class I embryos have reduced anterior development and a shortened axis, but the most anterior structure, the cement gland (arrows), is still formed. Class II embryos have anterior truncation of head structures (arrows) and are lacking the cement gland and the eyes. Dorsal-ventral polarity can be distinguished from the formation of the dorsal fin. Class III embryos show a complete loss of axial identity and these embryos appear as an elongated mass of tissue with a highly ruffled pigmented end and a blastopore formed at the other end.
Fertilization of oocytes injected with fatvg-antisense ODN was carried out by the host transfer procedure (Zuck et al., 1999). At the tadpole stage (stage 33/34), about 50% of the antisense ODN injected embryos displayed axial defects to varying degrees (Figure 1C). We classified the phenotype into three classes (Table 1 and Figure 1C). Class I embryos displayed mild microcephaly. Their heads were of reduced size and they had shortened anteroposterior axes with the presence of cement glands (Figure 1C). Class II embryos showed a truncation of the head and anterior structures, including loss of the cement glands and the eyes (Figure 1C). The dorsoventral polarity in these embryos was apparent as indicated by the formation of the dorsal fin. Class III embryos displayed the most severely ventralized phenotype with complete loss of all axial structures (Figure 1C). Alone, both fv-as1 and fv-as2 generated between 50-70% ventralized embryos; however, this increased to 90% when fv-as1 and fv-as2 were injected together. Also, the percentage of class III embryos increased from 9% to 50% with injection of the two ODNs. Specificity of the phenotype was shown by rescuing the phenotype by overexpressing fatvg mRNA in oocytes 24 hours after ODN injection (Table 2). Therefore, we conclude that the axial defects resulted from specific depletion of the endogenous fatvg transcripts by the fatvg ODNs.
Table 1. Effect of fatvg depletion on axial patterning.
The embryos that have undergone early cleavages are compared with injected oocytes in five independent experiments. The numbers of embryos showing each class of phenotype are expressed as a percentage of the total number of tadpole stage embryos for each oligo injection. About 10% of embryos surviving to the tadpole stage display the most severe class III phenotype in single oligo injection. When two oligos were injected in combination, the percentage of class III embryos increased to 50%. 2.5ng of single oligo and total 2.5ng of oligos in combination were injected Control represents embryos derived from host-transferred uninjected oocytes, which behave like the embryos derived from oocytes injected with water i.e do not show any axial defects.
 |
Table 2. Injection of fatvg mRNA rescues the phenotype.
Embryos that have undergone early cleavages are compared with injected oocytes in three independent experiments. In the rescued embryos, the percentage of embryos showing a phenotype reduced to half of that observed in embryos injected with the oligo alone. There is also a doubling of the percentage of “normal” embryos among the rescued embryos. Control represents embryos derived from host-transferred uninjected oocytes, which behave like the embryos derived from oocytes injected with water i.e do not show any axial defects
 |
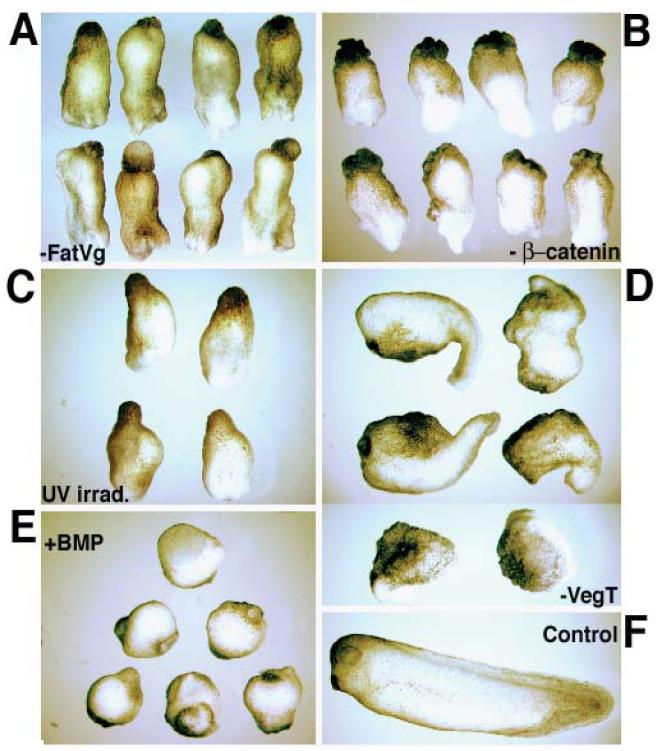
The fatvg ventralized embryo (Figure 2A) was a phenocopy of that produced by β-catenin depletion (Figure 2B) and strongly resembled that produced by UV irradiation (Figure 2C), yet differed from that produced by loss of VegT (figure 2D) and overexpression of BMP (Figure 2E) (Heasman et al., 1994, 2000; Zhang et al., 1998). These data are consistent with the loss of the anterior marker engrailed (Figures 3A, 3B) and the decreased expression of the dorsal mesodermal marker 12/101 in fatvg-depleted embryos (Figures 3C, 3D) as well as UV-treated embryos (Figures 3E, F) and BMP-4-injected embryos (Figure 3G). The fatvg-depleted phenotype is also consistent with the finding of elevated levels of the ventral mesodermal marker α-T4 globin in fatvg-depleted embryos (Figure 4A, 4B) and UV-treated embryos (Figure 4C). These data suggest that there was an increase in the amount of ventral mesoderm at the expense of the dorsal mesoderm in fatvg-depleted embryos, which is supported by the increased α-T4 globin in β–catenin depleted embryos (Figure 4D) and BMP-4 injected embryos (Figure 4E). All of these data support the conclusion that fatvg plays an important role in dorsal-ventral axis specification.
Figure 2.
Ventralization caused by fatvg-depletion is reminiscent of UV irradiation and β-catenin-depleted embryos but different than VegT depleted embryos. (A) fatvg-depleted embryos showing a class III phenotype with a complete loss of axial identity. (B) β-catenin-depleted embryos showing a similar phenotype. (C) Embryos that had been irradiated at the vegetal pole after fertilization. (D) VegT-depleted embryos are defective in endoderm and mesoderm formation. The two bottom embryos show the most severe phenotype of VegT depletion in which only epidermal development is apparent. (E) Embryos injected with 2ng BMP-4 at 2-cell stage. (F) Control embryo at stage 39.
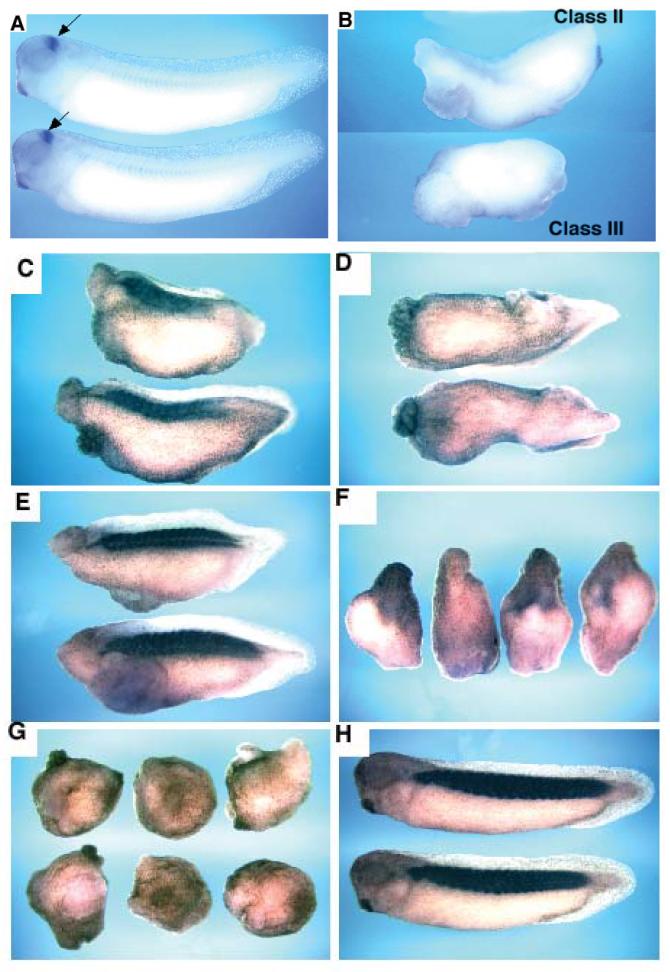
Figure 3.
The anterior marker engrailed is not expressed in fatvg-depleted embryos. (A) Embryos derived from uninjected oocytes express engrailed mRNA (arrows) in the midbrain-hindbrain boundary as analyzed by in situ hybridization. (B) The upper embryo is a class II fatvg-depleted embryo and the lower one is a class III embryo. Niether express engrailed.
Muscle differentiation is deficient in fatvg-depleted embryos. (C) Class II fatvg-depleted embryos showed a reduced amount of 12/101 immunostaining which is a specific marker for muscle differentiation. (D) No 12/101 staining was detected in class III fatvg-depleted embryos. (E) A reduced amount of 12/101 staining is obtained in embryos exposed to a low dose of UV irradiation. (F) High dose of UV irradiation generated ventralized embryos that show no staining. Dark shadows are regions of the embryo concentrated with pigments. (G) No staining is detected in BMP-4-injected embryos. (H) Control embryos showing 12/101 staining in the somites.
Figure 4.
The ventral mesodermal marker α-T4 globin expression is enhanced in fatvg-depleted class III embryos. (A) α-T4 globin is expressed at a normal level in class II embryos as shown by in situ hybridization. (B) Class III embryos have an increase amount of α-T4 globin expression close to one end of the embryo. (C) UV irradiated embryos have elevated levels of expression of α-T4 globin. Embryos on the left are treated with a low UV dose. A higher dose was used for the embryos on the right. (D) β-catenin depleted embryos have increased α-T4 globin expression. (E) α-T4 globin is highly expressed in BMP-4 injected embryos. (F) A control embryo showing α-T4 globin expression in the developing blood islands derived from the ventral mesoderm. Arrows point to the expression domain of α-T4 globin. Open arrows point to the position of the blastopore.
Dorsal accumulation of β-catenin is inhibited in fatvg-depleted embryos
In fertilized embryos, a maternal β-catenin pathway is utilized to activate dorsal gene expression in the prospective dorsal side of the embryo. β-catenin is preferentially accumulated in the subcortical cytoplasm in the dorsal sector of early cleavage stage embryos (Larabell, et al., 1997). The β-catenin levels continue to increase dorsally and, at the 32-cell stage, β-catenin translocates to the nucleus of the dorsal blastomeres (Larabell et al., 1997). Since we observed that fatvg-depleted embryos were a phenocopy of the β–catenin-depleted embryos, we analyzed the β-catenin protein distribution in these embryos using immunostaining with anti-β-catenin antibody and confocal microscopy at the 8-cell stage. Control embryos that underwent the host transfer procedure showed β-catenin localized to the peripheral cytoplasm of the dorsal blastomeres (Figure 5A). In contrast, the dorsal accumulation of β-catenin was disrupted in fatvg-depleted embryos (Figures 5B, 5C), and β-catenin accumulated either at the vegetal pole region (Figure 5B, 50% n=6, total n=12) or was slightly displaced towards the dorsal side (Figure 5C, 50% of n=6, total n=12). The varying degree of displacement of β–catenin towards the dorsal side may account for the varying degrees of ventralization observed in different fatvg-depleted embryos. These data indicate that fatvg depletion disrupts cortical rotation in a way that alters the normal accumulation of β-catenin in the dorsal sector of the embryo.
Figure 5.
Confocal analysis of β-catenin protein distribution in 8-cell stage fatvg-depleted embryos showing abnormal accumulation of β-catenin at the vegetal pole of the embryos. Embryos were bisected along animal/vegetal axis into halves before the immunostaining procedure was carried out. (A) In embryos derived from uninjected oocytes that had undergone the host transfer procedure β-catenin protein is detected in an arc region in the outer region of the dorsal blastomeres. (B) In fatvg-depleted embryos an accumulation of β-catenin is seen at the vegetal pole region, rather than in the future dorsal blastomeres of the embryo (C) In some fatvg-depleted embryos, low levels of β-catenin are seen in a vegetal blastomere slightly displaced from the vegetal pole. Arrows point to β-catenin staining.
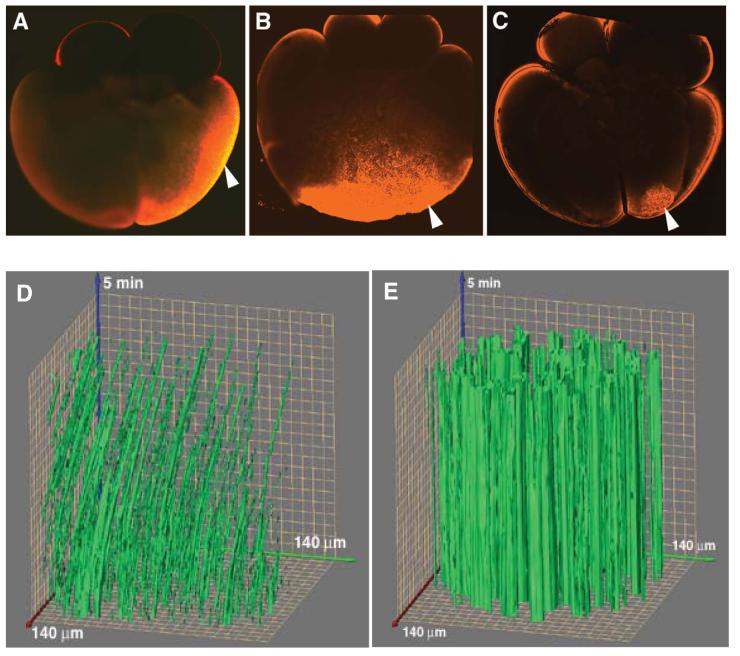
Organelle transport and cortical rotation are inhibited in fatvg-depleted embryos. Displacement of DiOC(6)3-labeled organelles located in the egg periphery was monitored over time during cortical rotation. The DiOC(6)3-labeled organelles in an optical section (140 μm × 140 μm field of view) are plotted along the x-y axis. Images of this field of view were collected at 3-sec intervals for 5 min, and the movement of organelles across this field, from frame to frame, is plotted along the z-axis. Movement of the organelles in the control egg (D) is seen as an arcing of the green line to the right; discontinuous green lines indicate organelles that enter or leave the plane of focus over time. (E) In fatvg antisense ODN injected eggs in which β-catenin accumulated at the vegetal pole, the large clusters of DiOC(6)3-labeled organelles remain in the same position over time, as indicated by the perfectly straight green columns along the z-axis.
Cortical rotation and organelle transport are inhibited in fatvg-depleted embryos
One possible mechanism by which fatvg could cause the abnormal distribution of β–catenin and subsequent ventralization is by affecting the degree of cortical rotation and/or movement of dorsalizing factors to the future dorsal side of the embryo. To test this we injected fatvg antisense ODN into oocytes, which were subsequently matured by addition of progesterone and activated by pricking with a glass needle. Artificial activation induces all of the changes associated with normal development after fertilization such as cortical granule breakdown, cortical rotation, and movement of components needed to stabilize β–catenin in the dorsal region of the embryo (Rowning et al., 1997). To measure the degree of cortical rotation, we incubated prick-activated mature oocytes in DiOC6(3), which stains the mitochondria that move along the microtubules during cortical rotation. In some activated oocytes, there was a complete lack of organelle transport; whereas in others oocytes, organelles were observed to move randomly, in multiple different directions, indicating the presence of randomly oriented microtubules. The extent of displacement of these stained organelles is a direct measure of the degree of cortical rotation (Rowning et al., 1997). By plotting organelle displacement over time, it became very clear that the degree of cortical rotation was greatly reduced or completely inhibited in fatvg-depleted vs. control embryos (Figures 5D, 5E). This suggests that the ventralization caused by the stabilization of β–catenin at the vegetal pole was a consequence of the inhibition of cortical rotation, which is directly linked to the movement of the DiOC6(3) staining organelles and dorsalizing components. Interestingly, different embryos (total 20 embryos were monitored) differed in the degree of cortical rotation, which is consistent with the range in severity of ventralized phenotype seen in the fatvg-depleted embryos.
Embryos depleted of fatvg are defective in PGC formation
We previously reported that maternal fatvg transcripts are associated with the germ plasm in cleavage stage embryos (Chan et al., 2001). To address the function of fatvg in the germ cell lineage we analyzed germ cell formation in fatvg-depleted embryos using the germ cell specific markers, Xpat and Xcat2. Figures 6A, 6B and Table 3 show that in Fatvg depleted embryos there was a dramatic decrease or complete absence of germ cells (controls average 12-13, whereas fatvg-depleted average 4 germ cells/embryo). In the embryos shown there is minimal perturbation of the dorsal axis yet the germ cell numbers are affected. These were selected since we wanted to illustrate that the loss of germ cells was independent of the axial defects. The loss of germ cells also occurs in the severely ventralized embryos (data not shown). Although we do not know if the germ cell phenotype can be rescued, this result does suggest; however, that while both germ cell formation and axial patterning are affected by loss of fatvg they exhibit different sensitivities. This may be due to different extents to which fatvg is depleted. This is supported by the results showing that in different embryos the accumulation of β–catenin at the future dorsal side of the embryo is either partially (Figure 5C) or completely (Figure 5B) disrupted.
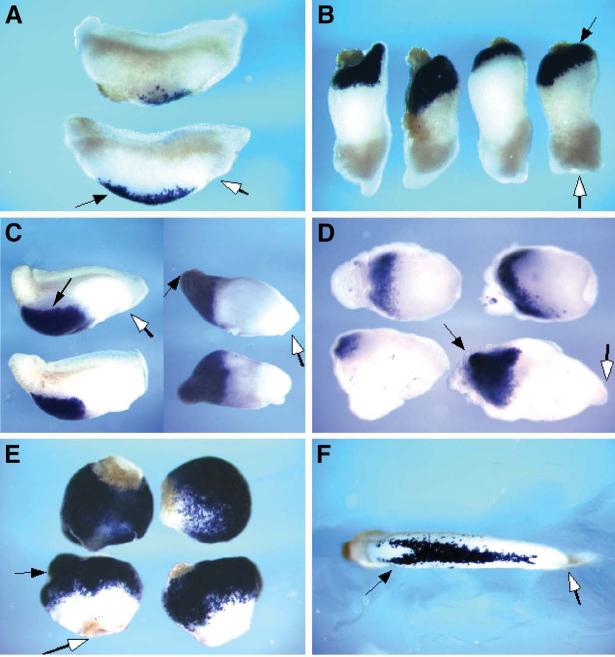
Figure 6.
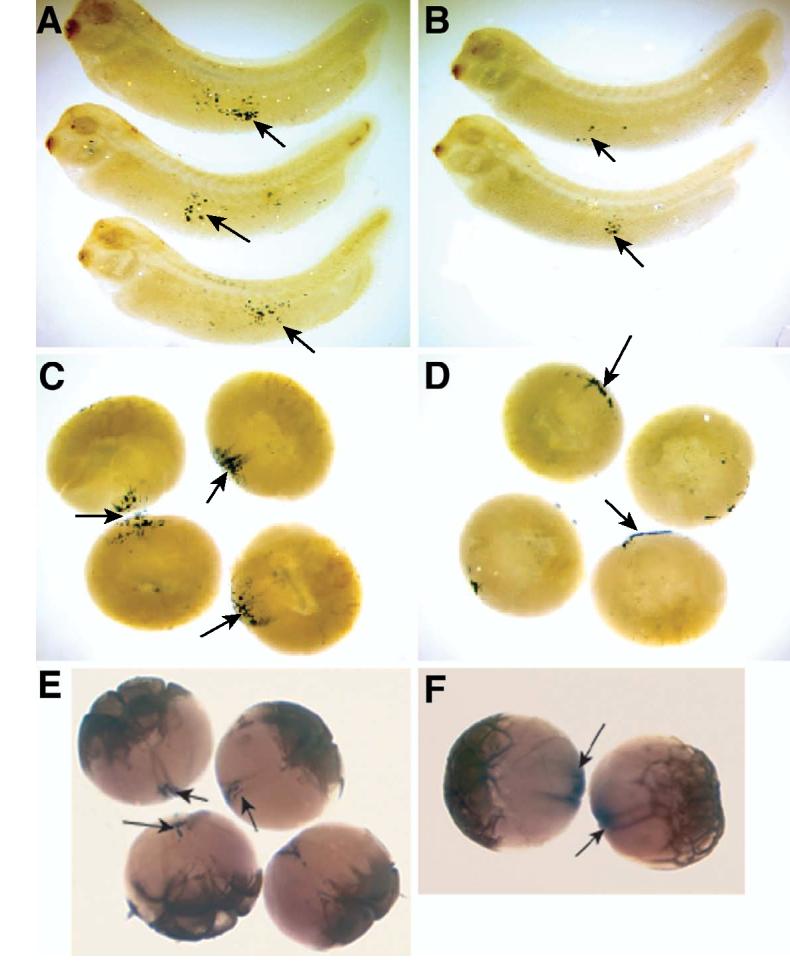
Fatvg-depleted embryos are deficient in primordial germ cells.
Xpat mRNA is detected in the germ plasm and primordial germ cells as shown by in situ hybridization. Germ cells can be detected in the endoderm by Xpat in situ hybridization signal (arrows) in control embryos at stage 40 (A). The number of Xpat-expressing cells is reduced in fatvg-depleted embryos (B).
Germ plasm islands as detected by Xpat mRNA signal can be observed in control embryos at early cleavage stage. Ingression of the germ plasm material into the interior of control early cleavage embryos is apparent (C, arrows). In fatvg-depleted embryos, Xpat mRNA did not ingress, but remained at the vegetal cortex (D, arrows).
Xcat2 is associated with the germ plasm that has ingressed into the interior of the blastomeres. Xcat2 (Forristall et al., 1995) expression in control cleavage stage embryos (E, arrows). In fatvg-depleted embryos, Xcat2 mRNA remains close to the cortical region and does not undergo ingression (F, arrows).
Table 3. Depletion of fatvg mRNA results in a reduction or loss of primordial germ cells.
Number of cells expressing Xpat was counted at the tadpole stage in Class I embryos. Fatvg-depleted and UV irradiated embryos have an average of 4 Xpat-positive primordial germ cells. In control embryos derived from uninjected and water-injected oocytes, there is an average of 12.5 Xpat-positive primordial germ cells. Standard deviation is shown together with the average mean.
 |
We next investigated the mechanism of germ cell loss as a result of fatvg depletion by monitoring the markers Xcat2 and Xpat. After fertilization, the germ plasm normally undergoes aggregation at the vegetal cortex followed by ingression along the cleavage planes to move deep into the interior of the embryo (Figures 6C, 6E). When we examined the distribution of the germ plasm in fatvg-depleted embryos, we found that these mRNAs remained tightly affixed to the vegetal cortex and that no ingression took place (Figure 6D, 6F). We also analyzed the ability of the germ plasm to aggregate in embryos that lacked fatvg. This was performed by analyzing the aggregation of the germ plasm in eggs that were prick activated and stained with DiOC6(3) as described above. Savage and Danilchik (1993) showed that in prick-activated eggs the germ plasm remains scattered as small islands throughout the vegetal pole region; however after several hours it aggregates into large masses. Figure 7 shows that during the first few minutes after activation the fatvg-depleted eggs show an abnormal and precocious aggregation of DiOC6(3) staining material that we interpret as being the germ plasm. This phenomenon occurs at the same time that we detect the retention of the Xcat2 and Xpat mRNAs at the cortex and concomitant with the inhibition of cortical rotation. These results suggest that fatvg affects germ cell formation by interfering with the proper segregation of the germ cell determinants during the process of PGC specification.
Figure 7.
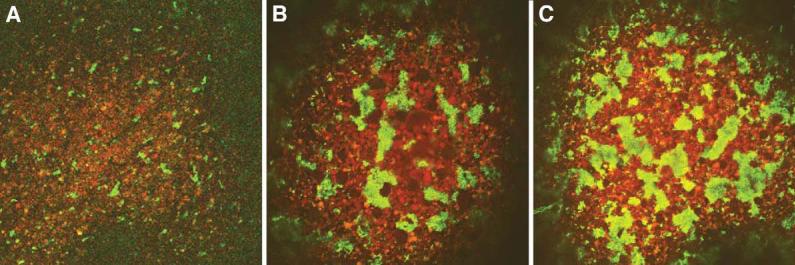
Effects of fatvg depletion on germ plasm aggregation.
Control and fatvg antisense ODN injected oocytes were matured in progesterone and prick activated with a glass needle. Eggs were stained with DiOC(6)3, and the behavior of the germ plasm was followed during the first 30 minutes. Full aggregation normally required several hours in prick activated eggs (Savage and Danilchik, 1993; Robb et al., 1999) (A) Control activated eggs showing the beginning stages of aggregation; (B) and (C) are examples of precocious aggregation of germ plasm in fatvg-depleted embryos.
Fatvg protein is located at the periphery of vesicles at the vegetal cortex
Based on the known function of other members of the protein family to which fatvg belongs, one possible function of fatvg is in vesicular transport. EM analysis of stage VI oocytes and early embryos using immunogold with fatvg antibody shows clearly that the fatvg protein is located at the periphery of vesicles located at the vegetal cortex (Figure 8). These vesicles are located at the vegetal cortex among the mitochondria and small organelles typical of germ plasm and the cytoplasm at the vegetal cortex. Their size fits well with the vesicles described by Miller et al. (1999) that are involved in the transport of dorsal determinants to the future dorsal side of the embryo. Although we have not been able to demonstrate that these vesicles are involved in the transport of dorsalizing or germ cell determinants, their location and proximity to the organelles that we observe to be affected by loss of fatvg support this model.
Figure 8.
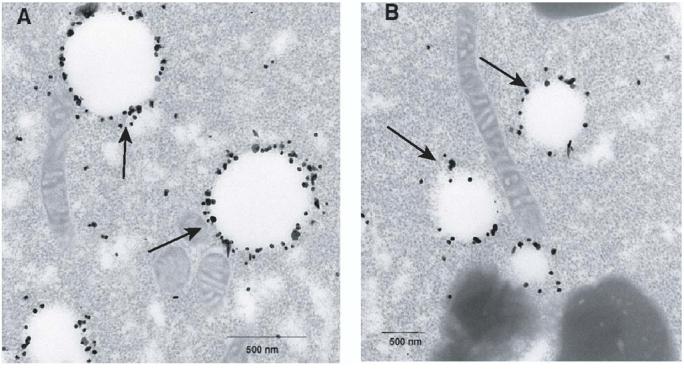
Distribution of fatvg protein on vesicles at the vegetal cortex.
Oocytes were fixed, sectioned, incubated with immunogold anti-fatvg antibody, and examined using electron microscopy. A and B are two examples of the localization of the fatvg protein on vesicles (arrows) located at the vegetal cortex. These structures are located among the mitochondria containing material that contains the germ plasm and dorsal determinants at the vegetal cortex.
Discussion
These data demonstrate that maternally localized fatvg plays an important role in both cortical rotation and consequent establishment of the dorsal-ventral body axis, and probably in specification of the fate of the germ cell lineage. Importantly, it does so through a common mechanism of regulating the movement of both germ cell determinants and dorsalizing factors such as β-catenin. As a result, in fatvg-depleted embryos the intracellular movement or segregation of vegetally localized maternal determinants is affected.
The fatvg depletion ventralized phenotype is reminiscent of ventralized embryos generated by exposure to UV (Grant and Wacaster, 1972) and β-catenin depletion (Heasman et al., 1994, 2000; Chan et al., 2001). In fact, as we have demonstrated, these embryos are a phenocopy of the β–catenin-depleted embryos. However, injection of β–catenin mRNA into fatvg depleted embryos does not rescue the phenotype (data not shown). This is most likely due to the fact that the deficiency is in the transport mechanism that moves the components that stabilize β–catenin at the future dorsal side of the embryo.
In fatvg-depleted embryos, β-catenin accumulation is not blocked as it is in UV-ventralized embryos (Larabell et al, 1997); instead, it accumulates abnormally. As we show, this is a result of the disruption of the directional transport of organelles during the process of cortical rotation, an event that is known to be required for the accumulation of β-catenin at the future dorsal side of the embryo. These organelles most likely represent the vesicular structures described by Miller et al (1999) that transport disheveled as well as GSK-3 binding protein (GBP) and kinesin light chain (KLC)(Weaver et al., 2003), and other factors involved in the stabilization of β–catenin at the future dorsal side of the embryos.
Normal cortical rotation requires microtubule polymerization and transport of organelles along the microtubules (MTs) to facilitate their alignment into the parallel array. Only with an aligned array of MTs can there be a directional displacement of dorsalizing components to the future dorsal region of the embryo. Since randomized microtubule-mediated transport was observed in many of the fatvg-depleted eggs, the abnormal accumulation of β-catenin is likely due to a misalignment of microtubules rather than a lack of microtubules. However since we did not study the structure of the microtubules we can not exclude possibility that there was a defect in the structure or polymerization of microtubules. Randomized organelle transport has been reported to occur as a result of hyperpolymerization and consequent tangling of microtubules (Rowning et al., 1997), and has also been seen in response to greatly increased or decreased numbers of organelles transported along microtubules during cortical rotation (Larabell, unpublished data). This indicates the need for organized transport of a significant population of organelles to align the microtubules and produce normal cortical rotation. The fact that fatvg-depletion interferes with normal cortical rotation, but not with formation of MTs, suggests that fatvg is important for the transport of organelles but not formation of microtubules. The mammalian counterparts of fatvg, known as adipose differentiation related protein (ADRP) (Jiang and Serrero, 1992;) and adipophilin (Heid et al., 1998), as well as TIP47 are associated with the surface of lipid droplets in different cell types (Brasaemle et al., 1997;Wolins et al., 2000). TIP47 is a cargo selection protein involved in intracellular protein trafficking (Diaz and Pfeffer, 1998). Like the mammalian proteins, fatvg may be associated with membrane structures and play a role in intracellular vesicular transport. Disheveled (Dsh) and GSK-3 binding protein (GBP), components of the Wnt signalling pathway, are transported via kinesin light chain (KLC) along microtubules to the dorsal side via what appears to be small (0.25-0.50 micron diameter) organelles (Miller et al., 1999; Weaver et al., (2003). Dsh and GBP subsequently activate the signaling cascade of the Wnt pathway, leading to the nuclear localization of β-catenin and activation of target genes to specify dorsal development. Fatvg may function as a structural component of the membrane vesicles or, perhaps, a linker between the determinant carrying vesicles and molecular motors utilized in the intracellular transport of the dorsal determinants.
The role of fatvg in germ cell formation may at first appear unexpected. In fact, the requirement of fatvg in germ cell development is consistent with the localization of fatvg in oocytes. During oogenesis, a fraction of the fatvg mRNA is detected in the germ plasm at the vegetal cortex and persists associated with the germ plasm in the germ cells of the embryo (Chan et al., 2001; Kloc et al., 2001). Although we do not know if the germ cell loss can be rescued we demonstrate that fatvg depletion affects early germ cell formation, and that the reduction in germ cell number is most likely a direct consequence of a defect in germ plasm aggregation and segregation accompanied by inhibition of the transport of specific germ cell markers mRNAs Xcat2 and Xpat.
Depletion of fatvg affects ingression of the germ plasm components such as Xcat2 and Xpat mRNAs along the cleavage furrow after fertilization and during the first cleavage. Germ plasm ingression is dependent on cleavage and cytoskeletal components (Ressom and Dixon, 1988; Savage and Danichik, 1993). In activated eggs in which no cleavage takes place, germ plasm aggregates normally but no ingression occurs (Savage and Danilchik, 1993). It has also been shown that the microfilament inhibitor cytochalasin B inhibits cleavage and germ plasm ingression, but not germ plasm aggregation, whereas the microtubule inhibitor nocodazole inhibits cleavage, germ plasm aggregation and ingression (Savage and Danilchik, 1993). Since we observed normal cleavage furrows in fatvg-depleted embryos, the lack of ingression of germ plasm into the interior of the embryo is not due to defective cleavage or cytoskeletal components.
Although there is evidence that vesicular movement and proper localization of the dorsal determinants are required, little is known about the role of membrane vesicles in RNA trafficking. However, there are two examples of the role of vesicles in the transport of RNAs. The first is the observation of the role of a specialized region of the ER in the transloaction of Vg1 mRNA to the vegetal cortex through the late pathway localization machinery (Deshler et al., 1997; Etkin, 1997; Kloc et al., 1998; reviewed in Kloc et al., 2002). The second example is from recent work on the transport of retroviral genomic RNAs to the plasma membrane on endosomal vesicles (Basyuk et al., 2003).
We suggest that in the embryo, fatvg depletion affects vesicular trafficking that occurs after fertilization, which may be involved in the ingression of RNAs in the germ plasm as well as the accumulation of dorsal determinants at their proper location. These results point to a common fundamental mechanism through which germ cell determinants and dorsalizing factors segregate during early development and that fatvg plays an important role in regulating these processes.
Materials and methods
Northern blot analysis
Ten to twenty oocytes were collected by freezing in liquid nitrogen after progesterone maturation, immediately before the host transfer procedure. Total RNA was extracted using TRIZOL (GIBCO). An amount of 15μg RNA from each preparation was loaded to a formaldehyde gel. The RNA on the gel was transferred to nylon membrane (Roche) for non-radioactive detection of fatvg expression. The membrane was hybridized to unpurified digoxigenin-labelled fatvg antisense probe from a standard 20ul transcription reaction in 20ml hybridization solution, prepared from DIG Easy Hyb granules (Roche). The blot was washed according to manufacturer's protocol as follows: 2×SSC, 0.1%SDS at room temperature and 0.1×SSC, 0.1%SDS at 68°C. A 1:10,000 dilution of the anti-digoxigenin-alkaline phosphatase conjugate (Roche) was used. Chemiluminescent detection was carried out using the CDP-star substrate (NEN). After 10 mins of incubation with the CDP-star substrate, the blot was exposed to an X-ray film for a period of 30s to 5mins.
Oligodeoxynucleotide injection and host transfer
The sequence of two phosphorothioate-modified antisense oligos (Genosys) used were: fv-as1, c*g*a*c*atgtttccaa*c*g*c*a and, fv-as2, t*t*c*c*actgccgccg*a*c*a*t. An “*” indicates the phosphorothioate linkage. The amount of each oligo used was between 2-7 ng. A total of 2.5-5 ng was injected when the two oligos were used in combination. Oocyte preparation and the host transfer procedure were carried out according to Zuck et al. (1999). Stage VI oocytes were collected from oocyte-positive grade of Xenopus laevis females (NASCO) by manual defolliculation. Injection of oligos was carried out using a pressure injector (MPPI-2, from ASI). For fatvg depletion, a volume of 10 nl oligo solution was injected at the vegetal apex of the oocytes. The injected oocytes were cultured in OCM at 18°C. After 24 hrs incubation, the oocytes were matured with progesterone at a final concentration of 1μM for 12-15hrs. At the same time, a host frog was induced by hormone injection of 800U hCG (Sigma). In the following day, the matured oocytes were first incubated in vital dye solutions. They were then transferred into the body cavity of the host female close to the opening of the oviduct. After 2.5 to 3 hrs, eggs were collected and fertilized with sperm suspension prepared from macerated testis. The embryos were dejellied at two-cell stage and sorted into different colored groups. In rescue experiments, oocytes injected with antisense oligos were divided into two groups. One of them was injected with 1ng of full-length fatvg mRNA 24 hrs after the antisense oligo injection. The rescued embryos were always scored in parallel with oligo-injected-alone embryos in the rescue experiments.
Embryo manipulations
Ultraviolet irradiation was carried out according to Kao and Danilchik (1991). Embryos were dejellied 10 mins after fertilization. Ultra violet irradiation was carried out for a period of 6-10 mins. The embryos were raised in 1/10 MBS at 18°C. 2 ng of BMP-4 RNA was injected into two-cell embryos to generate BMP-4-ventralized embryos.
Whole mount in situ hybridization and immunostaining
Embryos were fixed in MEMFA at room temperature for 1-2 hrs and kept in methanol at −20°C. Whole mount in situ hybridization was carried out according to Ryan et al. (1996). The engrailed construct was a gift from H. Brivanlou. The α-T4 globin was a gift from R. Patient (Walmsley et al., 1994). The Xpat construct was a gift from H. Woodland (Hudson and Woodland, 1998). Antisense digoxigenin-labelled probes were detected using anti-digoxigenin alkaline phosphatase conjugate (Roche) and the BCIP/NBT substrate (Roche). Immunostaining for light microscopy was carried out with a buffer containing 1XPBS, 0.1% Triton 100, 0.2% BSA. The 12/101 monoclonal antibody was obtained from the developmental studies hybridoma bank (DSHB, University of Iowa) and β catenin antibody was gift from Randall Moon (University of Washington). Antibodies were diluted in 1:500 for whole mount staining. A secondary bridging antibody against mouse-IgG (Roche) was diluted in 1:2000. Antibody detection was amplified using the APAAP complex (Roche) and the BCIP/NBT substrate. Embryos were post-fixed in MEMFA after color reaction. Bleaching was performed by first dehydrating the embryos in methanol, followed by treatment in 10% H2O2, 70% methanol overnight. Bleached embryos were kept in 100% methanol. Clearing was carried out to visualize the interior of the embryos using Murray's Clear, which contains benzyl benzoate (Sigma) and benzyl alcohol (Sigma) in a ratio of 1 to 2. Immunostaining for electron microscopy was carried out as described in Song et al.(in press).
Confocal studies
Embryo fixation and immunostaining for confocal analysis of β-catenin distribution was performed according to Larabell et al. (1997). Embryos were demembranated and cut into halves prior to the immunostaining procedure.
Acknowledgements
The authors would like to thank Drs. A. Hemmati- Brivanlou, R. Patient, and H. Woodland for the engrailed2, α-T4 globin and Xpat constructs. This work was supported by grants from NIH and NSF. EM analysis was supported by grant CA 16672 for the electron microscopy core facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Mukhtar KA, Webb AC. An ultrastructural study of primordial germ cells, oogonia and early oocytes in Xenopus laevis. J Embryol Exp Morphol. 1971;26:195–217. [PubMed] [Google Scholar]
- Basyuk E, Galli T, Mougel M, Blanchard J-M, Sitbon M, Bertrand E. Retroviral Genomic RNAs Are Transported to the Plasma Membrane by Endosomal Vesicles. Developmental Cell. 2003;5:161–174. doi: 10.1016/s1534-5807(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg 1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. doi: 10.1242/dev.02144. [DOI] [PubMed] [Google Scholar]
- Blackler AW. Contribution to the study of germ-cells in the Anura. J Embryol Exp Morph. 1958;6:491–503. [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- Buehr ML, Blackler AW. Sterility and partial sterility in the South African clawed toad following the pricking of the egg. Journal of Embryology & Experimental Morphology. 1970;23:375–384. [PubMed] [Google Scholar]
- Chan AP, Kloc M, Bilinski S, Etkin LD. The vegetally localized mRNA fatvg is associated with the germ plasm in the early embryo and is later expressed in the fat body. Mech Dev. 2001;100:137–140. doi: 10.1016/s0925-4773(00)00517-7. [DOI] [PubMed] [Google Scholar]
- Chan AP, Kloc M, Etkin LD. fatvg encodes a new localized RNA that uses a 25-nucleotide element (FVLE1) to localize to the vegetal cortex of Xenopus oocytes. Development. 1999;126:4943–4953. doi: 10.1242/dev.126.22.4943. [DOI] [PubMed] [Google Scholar]
- Coggins LW. An ultrastructural and autoradiographic study of early oogenesis in the toad Xenopus laevis. J Cell Sci. 1973;12:71–93. doi: 10.1242/jcs.12.1.71. [DOI] [PubMed] [Google Scholar]
- Czolowska R. Observations on the origin of the ‘germinal cytoplasm’ in Xenopus laevis. J Embryol Exp Morphol. 1969;22:229–251. [PubMed] [Google Scholar]
- Czolowska R. The fine structure of the “germinal cytoplasm” in the egg of Xenopus laevis. Wilhelm Roux' Arch EntwMech Org. 1972;169:335–344. doi: 10.1007/BF00580253. [DOI] [PubMed] [Google Scholar]
- Deshler JO, Highett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by Vera protein and the endoplasmic reticulum. Science. 1997;276:1128–1131. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
- Diaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Green JB. Dorsal downregulation of GSK3beta by a non-Wnt-like mechanism is an early molecular consequence of cortical rotation in early Xenopus embryos. Develop. 2001;127:861–8. doi: 10.1242/dev.127.4.861. [DOI] [PubMed] [Google Scholar]
- Elinson RP, Pasceri P. Two UV-sensitive targets in dorsoanterior specification of frog embryos. Development. 1989;106:511–518. doi: 10.1242/dev.106.3.511. [DOI] [PubMed] [Google Scholar]
- Etkin LD. A new face for the endoplasmic reticulum: RNA localization. Science. 1997;276:1092–1093. doi: 10.1126/science.276.5315.1092. [DOI] [PubMed] [Google Scholar]
- Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J. Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristall C, Pondel M, Chen L, King ML. Patterns of localization and cytoskeletal association of two vegetally localized RNAs, Vg1 and Xcat-2. Development. 1995;121:201–208. doi: 10.1242/dev.121.1.201. [DOI] [PubMed] [Google Scholar]
- Fujisue M, Kobayakawa Y, Yamana K. Occurrence of dorsal axis-inducing activity around the vegetal pole of an uncleaved Xenopus egg and displacement to the equatorial region by cortical rotation. Development. 1993;118:163–170. doi: 10.1242/dev.118.1.163. [DOI] [PubMed] [Google Scholar]
- Grant P, Wacaster JF. The amphibian gray crescent region--a site of developmental information? Dev Biol. 1972;28:454–471. doi: 10.1016/0012-1606(72)90029-2. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Heasman J, Quarmby J, Wylie CC. The mitochondrial cloud of Xenopus oocytes: the source of germinal granule material. Dev Biol. 1984;105:458–469. doi: 10.1016/0012-1606(84)90303-8. [DOI] [PubMed] [Google Scholar]
- Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- Holowacz T, Elinson RP. Cortical cytoplasm, which induces dorsal axis formation in Xenopus, is inactivated by UV irradiation of the oocyte. Development. 1993;119:277–285. doi: 10.1242/dev.119.1.277. [DOI] [PubMed] [Google Scholar]
- Holwill S, Heasman J, Crawley CR, Wylie CC. Axis and germ line deficiencies caused by u.v. irradiation of Xenopus oocytes cultured in vitro. Development. 1987;100:735–743. [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Houston DW, King ML. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development. 2000a;127:447–456. doi: 10.1242/dev.127.3.447. [DOI] [PubMed] [Google Scholar]
- Houston DW, King ML. Germ plasm and molecular determinants of germ cell fate. Curr Top Dev Biol. 2000b;50:155–181. doi: 10.1016/s0070-2153(00)50008-8. [DOI] [PubMed] [Google Scholar]
- Hudson C, Woodland HR. Xpat, a gene expressed specifically in germ plasm and primordial germ cells of Xenopus laevis. Mech Dev. 1998;73:159–168. doi: 10.1016/s0925-4773(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Ijiri KI. Existence of ultraviolet-labile germ cell determinant in unfertilized eggs of Xenopus laevis and its sensitivity. Dev Biol. 1977;55:206–211. doi: 10.1016/0012-1606(77)90333-5. [DOI] [PubMed] [Google Scholar]
- Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci U S A. 1992;89:7856–7860. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development. 1998;125:2677–2685. doi: 10.1242/dev.125.14.2677. [DOI] [PubMed] [Google Scholar]
- Kao K, Danilchik M. Generation of body plan phenotypes in early embryogenesis. Methods Cell Biol. 1991;36:271–284. doi: 10.1016/s0091-679x(08)60282-4. [DOI] [PubMed] [Google Scholar]
- Kloc M, Zearfoss NR, Etkin LD. Mechanisms of RNA localization. Cell. 2002;108:533–544. doi: 10.1016/s0092-8674(02)00651-7. [DOI] [PubMed] [Google Scholar]
- Kloc M, Bilinski S, Chan AP, Allen LH, Zearfoss NR, Etkin LD. RNA localization and germ cell determination in Xenopus. Int Rev Cytol. 2001;203:63–91. doi: 10.1016/s0074-7696(01)03004-2. [DOI] [PubMed] [Google Scholar]
- Kloc M, Etkin LD. Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development. 1995;121:287–297. doi: 10.1242/dev.121.2.287. [DOI] [PubMed] [Google Scholar]
- Kloc M, Larabell C, Chan AP, Etkin LD. Contribution of METRO pathway localized molecules to the organization of the germ cell lineage. Mech Dev. 1998;75:81–93. doi: 10.1016/s0925-4773(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, Heasman J. Wnt11/<beta>-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Weeks DL, Harvey RP, Melton DA. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985;42:769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Ressom RE, Dixon KE. Relocation and reorganization of germ plasm in Xenopus embryos after fertilization. Development. 1988;103:507–518. doi: 10.1242/dev.103.3.507. [DOI] [PubMed] [Google Scholar]
- Robb DL, Heasman J, Raats J, Wylie C. A kinesin-like protein is required for Germ plasm aggregation in Xenopus. Cell. 1996;87:823–831. doi: 10.1016/s0092-8674(00)81990-x. [DOI] [PubMed] [Google Scholar]
- Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA. Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc. Natl. Acad. Sci. 1997;94:1224–1229. doi: 10.1073/pnas.94.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K, Garrett N, Mitchell A, Gurdon JB. Eomesodermin, a key early gene in Xenopus mesoderm differentiation. Cell. 1996;87:989–1000. doi: 10.1016/s0092-8674(00)81794-8. [DOI] [PubMed] [Google Scholar]
- Savage RM, Danilchik MV. Dynamics of germ plasm localization and its inhibition by ultraviolet irradiation in early cleavage Xenopus embryos. Dev Biol. 1993;157:371–382. doi: 10.1006/dbio.1993.1142. [DOI] [PubMed] [Google Scholar]
- Smith LD. The role of a “germinal plasm” in the formation of primordial germ cells in Rana pipiens. Dev Biol. 1966;14:330–347. doi: 10.1016/0012-1606(66)90019-4. [DOI] [PubMed] [Google Scholar]
- Song H-W, Cauffman K, Chan AP, Zhou Y, King ML, Etkin LD, Kloc M. Hermes RNA binding protein targets RNAs encoding proteins involved in meiotic maturation, early cleavage, and germline development. Differentiaition. doi: 10.1111/j.1432-0436.2006.00155.x. in press. [DOI] [PubMed] [Google Scholar]
- Stennard F, Carnac G, Gurdon JB. The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development. 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie C, Lin X, Heasman J. Maternal Wnt11 activates the canonical Wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Walmsley ME, Guille MJ, Bertwistle D, Smith JC, Pizzey JA, Patient RK. Negative control of Xenopus GATA-2 by activin and noggin with eventual expression in precursors of the ventral blood islands. Development. 1994;120:2519–2529. doi: 10.1242/dev.120.9.2519. [DOI] [PubMed] [Google Scholar]
- Weaver C, Farr GH, Pan W, Rowning BA, Wang J, Mao J, Wu D, Li L, Larabell CA, Kimelman D. GBP binds kinesin light chain and translocates during cortical rotation in Xenopus eggs. Development. 2003;130:5425–5436. doi: 10.1242/dev.00737. [DOI] [PubMed] [Google Scholar]
- Weeks DL, Melton DA. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987;51:861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2000;17:17. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development. 2001;128:167–180. doi: 10.1242/dev.128.2.167. [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–41. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
- Zuck MV, Heasman J, Wylie CC. Studying the function of maternal mRNAs in Xenopus embryos: An antisense approach. In: Richter JD, editor. A comparative methods approach to the study of oocytes and embryos. Oxford University Press; New York: 1999. pp. 341–354. [Google Scholar]
- Zust B, Dixon KE. The effect of u.v. irradiation of the vegetal pole of Xenopus laevis eggs on the presumptive primordial germ cells. J Embryol Exp Morphol. 1975;34:209–220. [PubMed] [Google Scholar]